Abstract
Background
Although support exists for multiple psychosocial predictors of colorectal cancer (CRC) screening, little is known about the relationships among these variables. Understanding the associations between such predictors could refine health behavior theories and inform the design of interventions.
Purpose
In addition to direct effects, we examined whether baseline perceived susceptibility was a moderator of, or was mediated by, changes in other psychosocial determinants of CRC screening intention and behavior.
Methods
Longitudinal path models were tested using data from 1001 white male automotive workers who participated in The Next Step Trial. Our sample included workers with no history of CRC who were due for CRC screening but did not complete CRC screening prior to the assessment of hypothesized mediators at year 1 follow-up.
Results
Perceived susceptibility interacted differently with four psychosocial constructs in models predicting CRC screening intention or behavior. Perceived susceptibility was independent of perceived benefits, moderated the change in perceived barriers and self-efficacy, and was mediated by the change in family influence.
Conclusions
The role of perceived susceptibility was not limited to direct effects, but involved mediating and moderating pathways of influence.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the U.S. (1). Because early detection and removal of pre-cancerous polyps may contribute to decreased incidence of CRC (2), several authoritative groups recommend CRC screening for average-risk individuals age 50 and older (1, 3, 4). However, screening rates are suboptimal (≤ 50%) (5, 6).
Sociodemographic, health status, and psychosocial factors have been associated with CRC screening (7–10). Of these, psychosocial variables are more amenable to change through behavioral interventions designed to increase CRC screening uptake. Support exists for several psychosocial correlates and predictors of CRC screening (11); however, scant attention has been paid to understanding the mechanisms underlying these associations.
Greater understanding of the longitudinal associations and causal mechanisms linking these determinants to CRC screening is needed to inform the design of effective interventions. Such mechanisms may be uncovered by exploring potential mediators and moderators. Mediators such as self-efficacy and intention may be hypothesized precursors of behavior change, and may help explain the mechanism by which distal predictors (i.e., perceived susceptibility) influence behavioral outcomes. Moderators interact with predictor variables to impact the outcome variable (i.e., direction or magnitude of effect) and specify the conditions under which the effect occurs.
Perceived susceptibility, a psychosocial construct in several health behavior theories, has been posited as an important motivating force behind precautionary behavior. However, researchers disagree on the mechanism by which perceived susceptibility affects behavior (12–14). One view is that perceived susceptibility is one of many direct causes of behavior (hypothesis 1 in the current study). The Health Belief Model reflects this view and includes only direct effects of model variables on behavioral outcomes (12). Tests of alternative models to the Health Belief Model have found support for perceived benefits as mediating the effect of perceived susceptibility on health behaviors (15, 16), thus suggesting that perceived susceptibility is a distal cause of behavior that operates through its influence on more proximal determinants (hypothesis 2 in the current study). Fishbein’s integrated model suggests the view that perceived susceptibility is expected to affect intention and behavior, but only indirectly through its effect on mediating variables such as attitudes, subjective norms, and self-efficacy (17). Empirical research to date has been limited by the use of cross-sectional data to test mediation models of perceived susceptibility and most studies have focused on perceived benefits as a mediator (15, 16, 18).
An alternative view of the relation between perceived susceptibility and behavior is that initial status of perceived susceptibility moderates the change in other psychosocial factors that influence subsequent intention and behavior (hypothesis 3 in the current study). The Precaution Adoption Process Model suggests that awareness and perceived susceptibility to a health risk are necessary but not sufficient for action (19), and that determinants of behavior change vary by stage (20). Individuals who are unengaged with a preventive behavior (Precaution Adoption Process Model stage 2) need to accept the risk as personally relevant or salient in order to move forward and decide to act (Precaution Adoption Process Model stage 5) (13). In this case, a threshold level of perceived susceptibility must be reached before one will attend to action recommendations, formulate an attitude, and ultimately increase active coping behaviors and behavior change. Then, once a decision to act is made, any barriers to performing the behavior need to be overcome. Likewise, some studies have found lower perceived susceptibility among contemplators compared with people in preparation or action stages of behavioral change (21, 22).
These health behavior theories provide different models of the structural and psychological processes that are hypothesized to influence behavior. Interventions depend on clearly delineated and empirically supported theories of behavior change; therefore, attention to theory evaluation is needed (23). Because perceived susceptibility is an important construct in many health behavior theories, and because its effect on behavior is uncertain, understanding the relative influence of perceived susceptibility and the process by which it influences behavior is important and may improve future cancer screening interventions.
The purpose of this secondary data analysis was to explore multiple hypotheses (1 to 3 above) suggested by health behavior change models about the role of perceived susceptibility to CRC on CRC screening intention and behavior at different points within a longitudinal model. We examined three pathways of influence that correspond to direct effect, mediation, and moderation hypotheses based on the theories reviewed above. Results will inform the conceptualization of the role of perceived susceptibility in models predicting intentions and behaviors.
Method
The Next Step Trial
We conducted a secondary analysis of data collected as part of The Next Step Trial, a behavioral intervention designed to improve CRC screening and healthy eating behaviors among automotive workers (24, 25), an occupational group at increased risk for CRC incidence and mortality (26, 27). Employees were offered free CRC screening through a company-sponsored program. Screening was offered during work time and employees were transported to appointments as needed. However, a majority was not being screened regularly (24). Details about The Next Step Trial are published elsewhere (24, 25, 28). Briefly, 28 worksites with a company-sponsored worksite screening program participated. Worksites were randomly assigned to intervention and control. Employees at the 15 intervention worksites received a mailed invitation to the screening program, an educational booklet tailored to the employee’s screening history, and a telephone call to reinforce messages from the booklet (24, 29). Surveys were administered at baseline (1993), year 1 follow-up (1994) and year 2 follow-up (1995). Intervention materials and telephone calls were provided post-baseline prior to each follow-up survey. Screening recommendations were based on previous medical examinations and findings and on American Cancer Society guidelines in effect at that time which recommended annual stool blood tests and digital rectal exams, and flexible sigmoidoscopy every 3–5 years. For persons at higher-risk, recommendations included referral for colonoscopy or double contrast barium enema (30, 31). Each worksite developed its own process for offering screening to employees. The research trial was approved by Henry Ford Health System’s institutional review board.
The Preventive Health Model was the conceptual framework for The Next Step Trial’s intervention and survey instrument, which drew constructs from the Health Belief Model (12), Social Cognitive Theory (32), Theory of Reasoned Action (33), and previous work by Antonovsky (34) on the sense of coherence in everyday health behavior. The Preventive Health Model posits that background, psychological and cognitive representations, social support and influence, and program factors are directly associated with both intention to take preventive action and actual preventive behavior. The framework has been used to study intention and behavior for colorectal (29, 35) and prostate cancer screening (36, 37) and includes several psychosocial variables that allowed us to test our multiple hypotheses.
Study population
Surveys were sent to all eligible workers (n = 5,042) at one-year intervals. The eligible population was 91% non-Hispanic White males. In 1993, 2,903 workers (58% of those eligible) responded to a baseline questionnaire. Of the 2,903, 95% were non-Hispanic White males so we excluded women (n = 53) and non-White males (n = 114). We excluded men with a history of CRC (n = 43) and those not due to repeat CRC screening during the trial (n = 379). In order to test our temporal hypotheses, we excluded men who engaged in the target behavior prior to assessment of the mediators at year 1 follow-up (n = 1,313). Thus, the eligible sample for this report consisted of 1,001 non-Hispanic White males with no history of CRC who responded to the baseline survey, were due for screening during the trial, but did not complete CRC screening during the first year.
Measures
Each year, data on CRC screening results were provided to The Next Step Trial by worksite staff or by the employee’s physician (with the employee’s consent) and through self-reported surveys (24, 25). All other variables were measured by scales and single items in the surveys (38). Unless otherwise noted, all items and scales were measured using the same 4-point Likert format ranging from strongly disagree (1) to strongly agree (4). High scores correspond to higher levels of the variable being measured.
Below we categorized the measured variables by their role in the statistical models. The predictor of interest was labeled the “independent variable”, whereas participant characteristics and study variables that were potential correlates of a mediator or outcome variable are labeled “covariates”. Baseline covariates were included in analyses to control for any effects (reduce variability) on the mediator and outcome variables. Baseline measures of mediator and outcome variables also were included in the models; therefore, results reflect the change (i.e., residualized change) in these variables over time and better approximate the longitudinal associations among variables.
Dependent variables
Intention to be screened for CRC was assessed by the average of responses to two survey items: “I intend to undergo CRC screening” and “I do not intend to go through CRC screening” (reverse coded). Cronbach’s alpha was 0.81 at all three time points for this subsample. Baseline (pre-intervention) and year 2 follow-up (outcome) intention measures were included in analyses.
CRC screening was recorded for any eligible employee completing at least one recommended screening examination. CRC screening status at year 2 follow-up was used as the dependent variable in these analyses (coded 0=not screened; 1 = screened).
Independent variable
Absolute perceived susceptibility to colorectal polyps or CRC was assessed at baseline (pre-intervention) by the average of three survey items: “I believe the chance that I might develop CRC is high”, “I think it is very likely that I will develop CRC or polyps”, and “I believe the chances that I will develop colorectal polyps are high”. Cronbach’s alpha was 0.80.
Psychosocial mediators
Based on previous work (15–17) and the availability of data, the psychosocial variables selected for examination in our models included: perceived benefits and barriers of CRC screening, self-efficacy, and family influence or norms. All psychosocial variables were measured at baseline (predictor, pre-intervention) and year 1 follow-up (mediator, post-intervention).
Perceived benefits of CRC screening were measured by the average of two survey items: “I think the benefits of colorectal screening outweigh any difficulty I might have in going through the tests” and “I believe that colorectal screening can help to protect my health.” Cronbach’s alpha was 0.78 at baseline and 0.72 at year 1. Perceived barriers to CRC screening were assessed with one item “I am bothered by the possibility that screening might be physically uncomfortable”.
Perceived self-efficacy related to CRC screening was assessed with one item, “Arranging my schedule to go through colorectal screening is an easy thing to do”.
Family influence on CRC screening was measured with the multiplication of two survey items consistent with recommendations for measures of subjective norms (39): “Members of my immediate family think I should go through colorectal screening” and “I want to do what members of my immediate family think I should do about colorectal screening”.
Covariates
Study group classified participants by worksite (1= intervention; 0= control). Age was assessed at baseline and was treated as a continuous variable. Family history of CRC was assessed at baseline and was coded as a dichotomous variable (0=no family history; 1=any family history).
Data Analysis
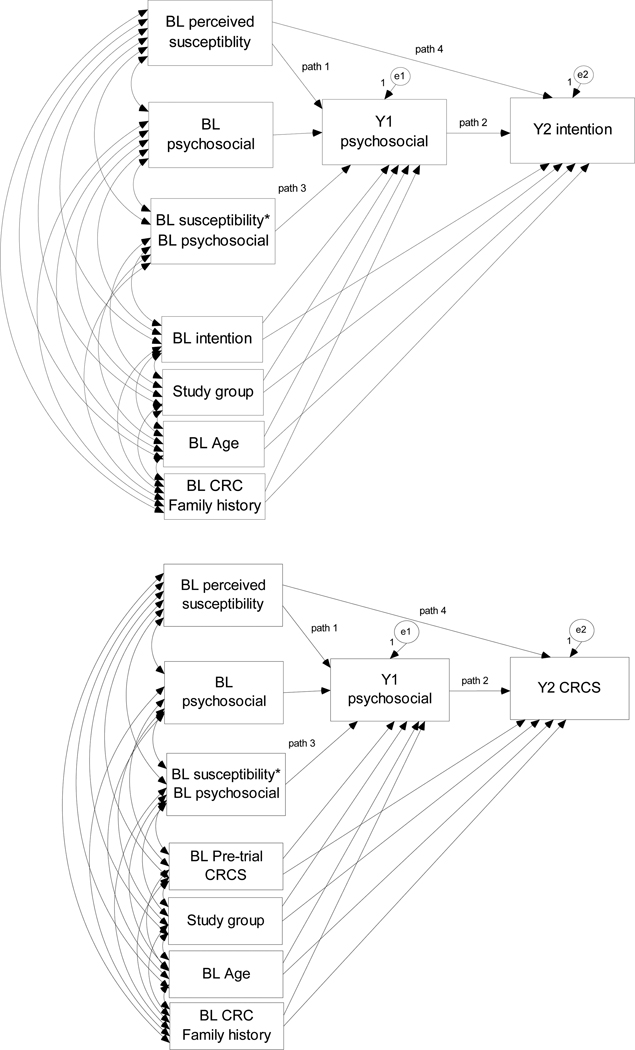
The Next Step Trial provided the necessary three waves of data (two contiguous waves of repeated measures of psychosocial constructs and a subsequent assessment of behavior) to test our theory-based hypotheses. In the same model, we examined the change in a psychosocial variable previously shown to be associated with CRC screening intention or behavior as a potential mediator of the effect of baseline perceived susceptibility, as well as examined the potential moderating effect of baseline perceived susceptibility on changes in the psychosocial variable over time while controlling for potential covariates of the mediator and outcome (Figure 1). Separate models were tested for CRC screening intention and behavior to determine to what extent the associations among these constructs are similar for these related outcomes.
Figure 1.
Models illustrating perceived susceptibility as an indirect effect of the change in a psychosocial variable on colorectal cancer screening (CRCS) intention or behavior (path 1* path 2) and as a moderator of change in a psychosocial variable (path 3) while accounting for the direct effect (path 4) and potential covariates. Models were repeated for each psychosocial (perceived benefits, barriers, self-efficacy, and family influence) and outcome variable.
Legend: BL = baseline, Y1 = Year 1, Y2 = Year 2, CRC = colorectal cancer, CRCS = CRC screening
In all models, the direct effect of baseline perceived susceptibility on the change in CRC screening intention and behavior is estimated (hypothesis 1; path 4 in Figure 1) and is expected to be attenuated when significant mediation is observed. Statistical significance of the indirect effect of path 1 by path 2 in Figure 1 would indicate that perceived susceptibility to CRC works through the change in other psychosocial constructs (i.e., benefits, barriers, self-efficacy, family influence) to influence a change in CRC screening intention and/or behavior (i.e., significant mediation; hypothesis 2). However, because of the lack of association between barriers and susceptibility reported in previous work (15, 16, 18), perceived barriers was not expected to be a significant mediator. Statistical significance in path 3 of Figure 1 would support the hypothesis for baseline perceived susceptibility as a moderator of the change in psychological constructs over time (hypothesis 3). Support for the moderator hypothesis may suggest that interventions need to address individuals’ perceived susceptibility before delivering intervention messages designed to increase positive attitudes, self-efficacy, and intentions to engage in CRC screening.
The decision to include other paths illustrated in Figure 1 was based on the Preventive Health Model. We posited associations between baseline psychosocial factors and both baseline and subsequent intention, so we included these paths in models predicting intention. Similarly, we also expected that previous behavior and related experience would subsequently influence later psychosocial factors and intention in an iterative fashion so we included the path from prior CRC screening to psychosocial mediators in models predicting behavior.
Unstandardized estimates were obtained using centered variables, whereas standardized estimates were obtained from standardized variables. Each predictor that was treated as a continuous variable was standardized by subtracting off its mean (centering) and dividing by its standard deviation. Interaction terms were created by multiplying centered or standardized baseline predictor variables (perceived susceptibility with each psychosocial variable).
Multilevel path analyses were conducted in Mplus (version 5.2) to control for participants nested within worksites. The interpretation of a parameter estimate does not depend on the respective worksite, but rather is valid for the entire sample of worksites in the study and averages the effect across worksites.
Only single mediator models were examined; therefore, only one psychosocial variable was examined in each model. We assessed significant mediation using Sobel’s Delta method (40) when both path 1 and 2 were significant. In order to compare path estimates across models, parameter estimates were determined using maximum likelihood with robust standard errors (MLR) estimation. Multiple fit indices were evaluated including the comparative fit index (CFI), and the Root Mean Square Error of Approximation (RMSEA) and its associated 95% confidence interval. CFI values 0.95 or above suggest good fit (41, 42) and RMSEA values <.06 suggest good model fit (41). Model fit indices were available for models predicting intention; all models had acceptable fit to the data. Results are not reported here because of limited space. The only model modifications involved dropping non-significant interaction terms. All covariances between exogenous variables were estimated (as shown in Figure 1), but estimates are not reported here.
Results
Preliminary Analyses
Descriptive statistics of sample characteristics are presented in Table 1. The correlation matrix of all model variables is presented in Table 2. Bivariate models were examined to determine the magnitude of the direct effect of baseline perceived susceptibility on each outcome. Both models indicated a significant effect of baseline perceived susceptibility on the residualized change from baseline to year 2 CRC screening intention (beta = .09, p = .016) and behavior (beta = .29, p = .012).
Table 1.
Sample Statistics for White Male Automotive Workers in The Next Step Trial
| Variable | Total N |
% | Meana | Standard deviation |
|---|---|---|---|---|
| Baseline | ||||
| Age | 1001 | 56.2 | 12.4 | |
| Education (total years) | 988 | 13.4 | 2.6 | |
| Marital status | ||||
| Married | 867 | 87 | ||
| Not married | 130 | 13 | ||
| Family history of CRC | ||||
| Yes 1) | 166 | 17 | ||
| No (0) | 818 | 83 | ||
| Study group | ||||
| Intervention (1) | 474 | 47 | ||
| Control (0) | 527 | 53 | ||
| Perceived susceptibility | 985 | 2.3 | 0.7 | |
| Perceived benefits | 978 | 3.3 | 0.7 | |
| Perceived barriers | 957 | 2.5 | 1.0 | |
| Self-efficacy | 970 | 2.9 | 1.0 | |
| Family influence | 885 | 9.06 | 4.5 | |
| Intention | 973 | 3.1 | 0.9 | |
| Pre-trial CRCS | ||||
| Yes (1) | 589 | 59 | ||
| No (0) | 412 | 41 | ||
| Year 1 | ||||
| Perceived benefits | 683 | 3.4 | 0.7 | |
| Perceived barriers | 680 | 3.4 | 0.7 | |
| Self-efficacy | 682 | 2.8 | 1.0 | |
| Family influence | 663 | 9.78 | 4.5 | |
| Intention | 685 | 3.1 | 0.9 | |
| Year 2 | ||||
| Intention | 702 | 3.1 | 1.0 | |
| CRCS during year 2 | ||||
| Yes (1) | 321 | 33 | ||
| No (0) | 654 | 67 |
Results are from raw data.
CRC = colorectal cancer; CRCS = CRC screening
Table 2.
Correlation Matrix of All Model Variables
| Benefits_bl | Benefits1 | Barriers_bl | Barriers1 | SE_bl | SE1 | Fam_bl | Fam1 | Int_bl | Int2 | Prior CRCS |
CRCS2 | Age | FamHx | Group | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS_bl | .29** | .19** | .01 | .01 | .11** | .07 | .27** | .19** | .32** | .22** | .15** | .14** | .01 | .11** | <.01 |
| Benefits_bl | .59** | −.23** | −.21** | .48** | .27** | .50** | .45** | .71** | .53** | .35** | .22** | −.01 | .08* | .04 | |
| Benefits1 | −.21** | −.22** | .28** | .35** | .36** | .50** | .51** | .51** | .34** | .21** | −.04 | .08* | −.01 | ||
| Barriers_bl | .50** | −.22** | −.20** | −.16** | −.17** | −.24** | −.27** | −.14** | −.10** | −.15** | −.03 | −.08* | |||
| Barriers1 | −.16** | −.21** | −.17** | −.13** | −.20** | −.21** | −.14** | −.10* | −.14** | −.04 | .01 | ||||
| SE_bl | .41** | .31** | .30** | .40** | .32** | .25** | .15** | .06 | .04 | .02 | |||||
| SE1 | .18** | .26** | .27** | .34** | .27** | .25** | .06 | .01 | .01 | ||||||
| Fam_bl | .49** | .50** | .41** | .26** | .17** | .08* | .04 | −.01 | |||||||
| Fam1 | .45** | .60** | .34** | .35** | .07 | .05 | −.08* | ||||||||
| Int_bl | .57** | .43** | .26** | −.03 | .08* | .01 | |||||||||
| Int2 | .41** | .45** | −.08* | .08* | <.01 | ||||||||||
| PriorCRCS | .32** | .04 | .09** | .05 | |||||||||||
| CRCS2 | .07 | .04 | −.02 | ||||||||||||
| Age | .01 | .13** | |||||||||||||
| FamHx | −.01 |
All non-dichotomous variables are centered at the mean. Dichotomous variables are coded 0 vs. 1; 1=screened, any family history, intervention group. PS=perceived susceptibility, bl=baseline, 1=year 1 follow-up, 2=year 2 follow-up, SE=self-efficacy, Fam=family influence, Int=intention, CRCS=colorectal cancer screening, FamHx=family history of CRC, Group=randomized worksite study group
p < .05;
p < .01;
p < .001
Hypothesis Testing
Models with Intention as the Outcome Variable
In the perceived benefits model predicting changes in intention between baseline and year 2 follow-up, perceived susceptibility was not a significant moderator of the change in perceived benefits and was not associated with year 1 benefits even after the non-significant interaction term was dropped from the model (Table 3). The direct effect of perceived susceptibility on the change in CRC screening intention also was not significant.
Table 3.
Independent Models Predicting Year 2 Screening Intention
| Standardized | Unstandardized | Std Error | p-value | |
|---|---|---|---|---|
| Model: Benefits Y1 benefits R2=0.38, p<.001; Y2 Intention R2=0.41, p<.001 | ||||
| BL PS -> Y2 intent (path 4) | 0.051 | 0.069 | 0.037 | 0.061 |
| BL PS -> Y1 benefits (path1) | −0.001 | <−0.001 | 0.031 | 0.978 |
| BL benefits -> Y1 benefits | 0.496 | 0.473 | 0.052 | <0.001 |
| Y1 benefits -> Y2 intent (path2) | 0.280 | 0.411 | 0.053 | <0.001 |
| BL intent -> Y1 benefits | 0.179 | 0.122 | 0.037 | 0.001 |
| BL intent -> Y2 intent | 0.441 | 0.441 | 0.043 | <0.001 |
| Study group ->Y1 benefits | −0.047 | −0.032 | 0.042 | 0.447 |
| Study group -> Y2 intent | 0.003 | 0.003 | 0.049 | 0.959 |
| BL Age -> Y1 benefits | −0.003 | −0.002 | 0.002 | 0.366 |
| BL Age -> Y2 intent | −0.004 | −0.004 | 0.002 | 0.079 |
| BL FamHx -> Y1 benefits | 0.037 | 0.025 | 0.054 | 0.637 |
| BL FamHx -> Y2 intent | 0.077 | 0.077 | 0.068 | 0.260 |
| Model: Barriers Y1 barriers R2=0.28, p<.001; Y2 Intention R2=0.37, p<.001 | ||||
| BL PS -> Y2 intent (path4) | 0.071 | 0.097 | 0.037 | 0.009 |
| BL PS -> Y1 barriers (path1) | 0.037 | 0.050 | 0.046 | 0.281 |
| BL barriers -> Y1 barriers | 0.471 | 0.469 | 0.037 | <.001 |
| Y1 barriers -> Y2 intent (path2) | −0.139 | −0.142 | 0.024 | <.001 |
| BL PS*barriers -> Y1 barriers (path3) | 0.085 | 0.116 | 0.042 | 0.006 |
| BL intent -> Y1 barriers | −0.124 | −0.122 | 0.042 | 0.004 |
| BL intent -> Y2 intent | 0.570 | 0.570 | 0.039 | <0.001 |
| Study group ->Y1 barriers | 0.124 | 0.122 | 0.071 | 0.085 |
| Study group -> Y2 intent | 0.006 | 0.006 | 0.048 | 0.896 |
| BL Age -> Y1 barriers | −0.007 | −0.007 | 0.003 | 0.035 |
| BL Age -> Y2 intent | −0.007 | −0.007 | 0.003 | 0.008 |
| BL FamHx -> Y1 barriers | −0.045 | −0.044 | 0.083 | 0.597 |
| BL FamHx -> Y2 intent | 0.073 | 0.073 | 0.060 | 0.220 |
| Model: Self-efficacy (SE) Y1 SE R2=0.20, p<.001; Y2 Intention R2=0.39, p<.001 | ||||
| BL PS -> Y2 intent (path4) | 0.072 | 0.098 | 0.043 | 0.023 |
| BL PS -> Y1 SE (path1) | −0.019 | −0.025 | 0.060 | 0.681 |
| BL SE -> Y1 SE | 0.365 | 0.371 | 0.032 | <.001 |
| Y1 SE -> Y2 intent (path2) | 0.194 | 0.198 | 0.028 | <.001 |
| BL PS*SE -> Y1 SE (path3) | 0.077 | 0.106 | 0.051 | 0.038 |
| BL intent -> Y1 SE | 0.170 | 0.167 | 0.035 | <0.001 |
| BL intent -> Y2 intent | 0.535 | 0.535 | 0.039 | <0.001 |
| Study group ->Y1 SE | −0.008 | −0.007 | 0.077 | 0.923 |
| Study group -> Y2 intent | 0.007 | 0.007 | 0.040 | 0.870 |
| BL Age -> Y1 SE | 0.001 | 0.001 | 0.002 | 0.591 |
| BL Age -> Y2 intent | −0.006 | −0.006 | 0.002 | 0.008 |
| BL FamHx -> Y1 SE | −0.002 | −0.002 | 0.071 | 0.981 |
| BL FamHx -> Y2 intent | 0.091 | 0.091 | 0.066 | 0.172 |
| Model: Family Influence (Fam) Fam R2=0.30, p<.001; Y2 Intention R2=0.40, p<.001 | ||||
| BL PS -> Y2 intent (path4) | 0.035 | 0.047 | 0.038 | 0.221 |
| BL PS -> Y1 Fam (path1) | 0.102 | 0.626 | 0.225 | 0.005 |
| BL Fam -> Y1 Fam | 0.322 | 0.325 | 0.048 | <0.001 |
| Y1 Fam -> Y2 intent (path2) | 0.244 | 0.054 | 0.008 | <0.001 |
| BL intent -> Y1 Fam | 0.294 | 1.325 | 0.269 | <0.001 |
| BL intent -> Y2 intent | 0.484 | 0.484 | 0.045 | <0.001 |
| Study group ->Y1 Fam | −0.123 | −0.556 | 0.264 | 0.035 |
| Study group -> Y2 intent | 0.034 | 0.034 | 0.048 | 0.480 |
| BL Age -> Y1 Fam | 0.001 | 0.004 | 0.011 | 0.697 |
| BL Age -> Y2 intent | −0.006 | −0.006 | 0.003 | 0.019 |
| BL FamHx -> Y1 Fam | −0.075 | −0.338 | 0.377 | 0.370 |
| BL FamHx -> Y2 intent | 0.112 | 0.112 | 0.062 | 0.069 |
Significant paths of interest are bolded.
PS=perceived susceptibility, BL=baseline, Y1=year 1 follow-up, Y2=year 2 follow-up, SE=self-efficacy, Fam=family influence, FamHx=family history of colorectal cancer
In the perceived barriers model, perceived susceptibility and barriers at baseline significantly interacted to influence year 1 perceived barriers of screening, thus supporting the moderator hypothesis (Table 3). Men with high perceived susceptibility at baseline had a greater increase in perceived barriers from baseline to year 1 compared to men with low perceived susceptibility at baseline. Perceived susceptibility also maintained a significant direct effect on the change in CRC screening intention from baseline to year 2 follow-up.
Like the barriers model, perceived susceptibility was a significant moderator of the changes in self-efficacy for CRC screening over time and the direct effect of perceived susceptibility on the change in intention was significant (Table 3). The interaction effect supported the moderation hypothesis and suggested that men with high perceived susceptibility at baseline had a greater increase in self-efficacy from baseline to year 1 compared to men with low perceived susceptibility at baseline.
In the full model with family influence, neither the mediation nor moderation hypothesis was supported. When the non-significant interaction term was dropped from the model, however, clear support for family influence as a mediator of the effect of perceived susceptibility on the change in CRC screening intention was found; (z = 2.36, p = .018). The direct effect of perceived susceptibility on year 2 intention was no longer significant, suggesting significant mediation (Table 3).
Models with CRC screening as the Outcome Variable
Like the perceived benefits model predicting intention, neither the mediation or moderation hypothesis was supported. However, the direct effect of perceived susceptibility on the change in CRC screening between baseline and year 2 follow-up was significant (Table 4). Similar to the model predicting changes in intention, baseline perceived susceptibility significantly moderated the change in perceived barriers over time when predicting changes in CRC screening behavior (Table 4). For the self-efficacy model predicting changes in behavior, only the direct effect of perceived susceptibility was significant even after removing the non-significant interaction (Table 4). Consistent with the models predicting changes in intention, when the non-significant interaction was removed from the model, the change in family influence was a significant mediator of the effect of perceived susceptibility on the change in CRC screening behavior (z = 2.13, p = .021). The direct effect of perceived susceptibility on the change in behavior was no longer significant, suggesting significant mediation in the family influence model (Table 4).
Table 4.
Independent Models Predicting Year 2 Colorectal Cancer Screening (CRCS)
| Standardized | Unstandardized | SE | p-value | |
|---|---|---|---|---|
| Model: Benefits Y1 benefits R2=0.22, p<.001; Y2 CRCS R2=0.11, p<.001 | ||||
| BL PS -> Y2 CRCS (path4) | 0.188 | 0.256 | 0.113 | 0.024 |
| BL PS -> Y1 benefits (path1) | 0.001 | 0.001 | 0.034 | 0.983 |
| BL benefits -> Y1 benefits | 0.527 | 0.503 | 0.038 | <0.001 |
| Y1 benefits -> Y2 CRCS (path2) | 0.304 | 0.446 | 0.077 | <0.001 |
| BL CRCS -> Y1 benefits | 0.323 | 0.220 | 0.037 | <0.001 |
| BL CRCS -> Y2 CRCS | 1.351 | 1.351 | 0.206 | <0.001 |
| Study group -> Y1 benefits | −0.050 | −0.034 | 0.042 | 0.416 |
| Study group -> Y2 CRCS | −0.178 | −0.178 | 0.160 | 0.265 |
| BL Age -> Y1 benefits | −0.004 | −0.003 | 0.002 | 0.239 |
| BL Age -> Y2 CRCS | 0.015 | 0.015 | 0.007 | 0.045 |
| BL FamHx -> Y1 benefits | 0.036 | 0.024 | 0.046 | 0.597 |
| BL FamHx -> Y2 CRCS | −0.002 | −0.002 | 0.155 | 0.992 |
| Model: Barriers Y1 barriers R2=0.16, p<.001; Y2 CRCS R2=0.10, p<.001 | ||||
| BL PS -> Y2 CRCS (path4) | 0.222 | 0.302 | 0.109 | 0.006 |
| BL PS -> Y1 barriers (path1) | 0.022 | 0.029 | 0.047 | 0.538 |
| BL barriers -> Y1 barriers | 0.488 | 0.486 | 0.035 | <0.001 |
| Y1 barriers -> Y2 CRCS (path2) | −0.155 | −0.158 | 0.068 | 0.019 |
| BL PS*barriers -> Y1 barriers (path3) | 0.079 | 0.108 | 0.041 | 0.009 |
| BL CRCS -> Y1 barriers | −0.138 | −0.136 | 0.069 | 0.049 |
| BL CRCS -> Y2 CRCS | 1.491 | 1.491 | 0.210 | <0.001 |
| Study group -> Y1 barriers | 0.121 | 0.118 | 0.074 | 0.111 |
| Study group -> Y2 CRCS | −0.170 | −0.170 | 0.154 | 0.269 |
| BL Age -> Y1 barriers | −0.006 | −0.006 | 0.003 | 0.065 |
| BL Age -> Y2 CRCS | 0.011 | 0.011 | 0.007 | 0.121 |
| BL FamHx -> Y1 barriers | −0.052 | −0.051 | 0.081 | 0.524 |
| BL FamHx -> Y2 CRCS | 0.012 | 0.012 | 0.159 | 0.942 |
| Model: Self-efficacy (SE) Y1 SE R2=0.10, p<.001; Y2 CRCS R2=0.13, p<.001 | ||||
| BL PS -> Y2 CRCS (path4) | 0.207 | 0.281 | 0.111 | 0.011 |
| BL PS -> Y1 SE (path1) | 0.009 | 0.012 | 0.054 | 0.831 |
| BL SE -> Y1 SE | 0.362 | 0.368 | 0.031 | <0.001 |
| Y1 SE -> Y2 CRCS (path2) | 0.392 | 0.400 | 0.078 | <0.001 |
| BL CRCS -> Y1 SE | 0.356 | 0.348 | 0.062 | <0.001 |
| BL CRCS -> Y2 CRCS | 1.355 | 1.355 | 0.209 | <0.001 |
| Study group -> Y1 SE | −0.013 | −0.013 | 0.075 | 0.867 |
| Study group -> Y2 CRCS | −0.175 | −0.175 | 0.157 | 0.266 |
| BL Age -> Y1 SE | 0.001 | 0.001 | 0.002 | 0.801 |
| BL Age -> Y2 CRCS | 0.012 | 0.012 | 0.008 | 0.113 |
| BL FamHx -> Y1 SE | −0.016 | −0.016 | 0.072 | 0.867 |
| BL FamHx -> Y2 CRCS | 0.021 | 0.021 | 0.165 | 0.900 |
| Model: Family Influence (Fam) Y1 Fam R2=0.15, p<.001; Y2 CRCS R2=0.11, p<.001 | ||||
| BL PS -> Y2 CRCS (path4) | 0.165 | 0.225 | 0.117 | 0.055 |
| BL PS -> Y1 Fam (path1) | 0.126 | 0.772 | 0.202 | 0.001 |
| BL Fam -> Y1 Fam | 0.376 | 0.379 | 0.038 | <0.001 |
| Y1 Fam -> Y2 CRCS (path2) | 0.268 | 0.059 | 0.018 | 0.001 |
| BL CRCS -> Y1 Fam | 0.453 | 2.041 | 0.227 | <0.001 |
| BL CRCS -> Y2 CRCS | 1.376 | 1.376 | 0.203 | <0.001 |
| Study group -> Y1 Fam | −0.122 | −0.550 | 0.248 | 0.027 |
| Study group -> Y2 CRCS | −0.148 | −0.148 | 0.160 | 0.356 |
| BL Age -> Y1 Fam | −0.002 | −0.007 | 0.012 | 0.553 |
| BL Age -> Y2 CRCS | 0.013 | 0.013 | 0.008 | 0.087 |
| BL FamHx -> Y1 Fam | −0.063 | −0.285 | 0.329 | 0.386 |
| BL FamHx -> Y2 CRCS | 0.037 | 0.037 | 0.159 | 0.815 |
Significant paths of interest are bolded.
PS=perceived susceptibility, BL=baseline, Y1=year 1 follow-up, Y2=year 2 follow-up, SE=self-efficacy, Fam=family influence, FamHx=family history of colorectal cancer, CRCS=colorectal cancer screening
Discussion
Our findings suggest that perceived susceptibility interacted differently with four psychosocial constructs that influence CRC screening intention or behavior. Perceived susceptibility was independent of perceived benefits, moderated the change in perceived barriers and self-efficacy, and was mediated by the change in family influence. Our results are consistent with theoretical models that posit perceived susceptibility as a more distal predictor of intention and behavior; however, our models often included a small, but significant residual direct effect of perceived susceptibility on changes in CRC screening intention and behavior. The residual effect is consistent with the argument that perceived susceptibility is a necessary, but not sufficient, significant predictor of intention and behavior and may be due to the omission of other important mediating variables. Inadequate measurement of psychosocial constructs also may reduce the power to detect significant mediation.
Only the change in family influence was a significant mediator of the effect of perceived susceptibility on CRC screening intention and behavior. Acknowledgement of personal susceptibility may heighten one’s perceived family support for getting screened. Family support may become a more proximal predictor of the decision (or action) to get screened and become a more salient influence for the individual. Our results were not consistent with previous studies that found perceived benefits (15, 16, 18) or self-efficacy (43) to be a mediator of perceived susceptibility’s influence on other health behaviors (e.g., flossing teeth, breast self-exams, sun protection). Discrepant findings may in part be attributable to previous studies modeling cross-sectional data, whereas the current study modeled longitudinal data, and we adjusted for the effects of baseline values of mediator and outcome variables and other covariates. More research is needed to examine the potential indirect effects of perceived susceptibility on intention and behavior, whether there are different mediators for different behaviors, and under what conditions significant mediation occurs.
Our findings provide some support for the hypothesis that perceived susceptibility moderates changes in psychosocial variables such as perceived barriers of CRC screening and self-efficacy for CRC screening over time. The moderation hypothesis posited that people who acknowledged their personal risk of developing CRC would attend more to risk information and show greater change in health beliefs. Contrary to expectations, an initial heightened perceived susceptibility may also make barriers to screening (e.g., screening uncomfortable) more salient. Future studies should assess whether the changes in these beliefs reflect a more realistic assessment of the “work” involved in making behavior changes or whether a selection effect occurred because we focused on individuals who did not take action during the first year after intervention.
Our examination of separate models predicting CRC screening intention or behavior produced different findings for models including self-efficacy. One explanation for the difference may be that people can perform behaviors for which they have strong self-efficacy regardless of strong perceived susceptibility, whereas intending to make a behavior change may require an increase in self-efficacy over time which may be dependent on some level of perceived susceptibility.
There is no consensus on the best way to measure perceived susceptibility (44). Although some researchers have combined measures of perceived susceptibility (45), other studies have reported different results for absolute and comparative measures (46–48). Two studies attempted to examine mediators of individuals’ comparative perceived susceptibility with cross-sectional data and reported different findings from ours and others that measured absolute perceived susceptibility (49, 50). Future research should investigate absolute and comparative perceived susceptibility in the same longitudinal model to better understand their direct and indirect influences on intention and behavior through the same or different mediating variables.
Implications for intervention
Previous work has found inconsistent associations between perceived susceptibility on health behaviors, but knowing the statistical significance or direction of association does not explain how it affects behavior. Our study was the first to critically examine different hypotheses of the role of perceived susceptibility of CRC on CRC screening intention and behavior. Although more research is needed to replicate the effects reported here, it is clear that the role of perceived susceptibility is not limited to a weak, direct effect on behavior, but rather may involve mediating and moderating pathways of influence. Understanding the underlying mechanisms that link predictors to behavior will allow researchers and practitioners to make more thoughtful decisions about how and when to intervene in the behavior change process. Additionally, hypothesizing and testing models involving mediation and moderation effects is necessary for evaluating behavioral interventions. Beyond the behavior differences by study group, it is important to know whether intervention targets successfully influence intermediate outcomes (and for whom) and how those outcomes affected behavior -- either directly or indirectly. Whether or not an intervention showed significant changes in behavior, only a thorough evaluation of the mediating and moderating pathways of influence on behavioral outcomes can inform us about how the intervention did or did not work as intended, for whom, and under what conditions (51).
Intervention researchers are tasked with selecting clear targets for intervention that will produce expected changes in mediating variables and/or outcomes. For example, interventions that attempt to change individuals’ perceptions of risk without influencing more proximal predictors of behavior may not successfully change desired behavioral outcomes (52). Once personal risk is acknowledged, external reinforcements such as social influence may be a more salient motivator for CRC screening. Despite our support for perceived susceptibility as a moderator, it is unclear whether health messages regarding the importance of CRC screening should first address individuals’ susceptibility to CRC before providing messages addressing CRC screening benefits, barriers, and self-efficacy or whether order matters (13, 14, 53). Tailoring approaches may be useful if people with higher perceived susceptibility require different messages than people with lower perceived susceptibility for CRC.
Limitations
Our study population was limited to non-Hispanic white male automotive workers who were at increased risk for CRC and were offered CRC screening through a worksite screening program. Future research will need to confirm these results in more diverse samples. Our decision to exclude workers who completed CRC screening by year 1 follow-up was useful for reducing complexity in the longitudinal conceptual models, but results may not generalize to individuals who respond more quickly to CRC screening recommendations. Some psychosocial mediators were measured with single items, which may indicate less than adequate representation of constructs and underestimation of true effects. For example, barriers in this study were limited to perceptions of the test being uncomfortable; future studies should consider using validated, multi-item measures (54–57). Possible ceiling effects on measured variables also may have attenuated the ability to detect significant associations. Measurement intervals less than one year apart may be better suited to examining the intermediate effect of perceived susceptibility on other psychosocial variables; however, longer intervals may be appropriate for examining intermittent behaviors like cancer screening. Our longitudinal analyses were able to control for baseline measures of dependent variables, which may reduce the amount of variance to be explained and the likelihood of finding significant mediation. However, inclusion of these covariates is important for obtaining estimates of the true indirect effect of perceived susceptibility on screening intention and behavior.
Although the mean levels of the psychosocial constructs (i.e., self-efficacy) may be higher in more recent studies due to greater public awareness about CRC screening (58), the psychosocial constructs measured in this study have remained significant predictors of CRC screening over time in different samples (11). Additionally, our focus for this study was the theoretical relationships between constructs, which we expect will be similar across time and samples. Our findings can inform future tests of competing theories of the role of perceived susceptibility. With the exception of digital rectal exams, the screening tests recommended during this study are still included in current screening recommendations (1). Interventions to increase CRC screening uptake thus far have had modest success and future interventions should address more environmental (e.g., physician- and system-level) determinants of behavior in addition to individual psychosocial factors (10).
Conclusions
We extended previous research by using longitudinal data to test different hypotheses about the role of perceived susceptibility in longitudinal path models predicting CRC screening intention and behavior. Future interventions are needed that can experimentally test hypothesized mechanisms of influence and help refine our current models of health behavior change and inform the design of future interventions. Identification of moderators is also important for targeting populations of interest and for appropriately tailoring intervention materials.
Acknowledgements
The Next Step Trial was supported by a National Cancer Institute (NCI) grant, Colorectal Cancer Screening and Nutrition Intervention (5R01CA052605-04; PI: Barbara C. Tilley). Secondary analyses were supported by a supplement grant from the NCI (5R01CA113828-04; PI: Gregory J. Norman). Dr. McQueen was supported by an American Cancer Society Mentored Research Scholar Grant (CPPB-113766) and a NCI Cancer Prevention and Control training grant (R25CA57712-11). Dr. Vernon was supported by a NCI grant, Tailored Interactive Intervention to Increase CRC Screening (R01CA097263).
Footnotes
Preliminary findings from this report were presented at the 30th annual meeting of the Society of Behavioral Medicine, April 23, 2009, in Montreal, Canada.
References
- 1.American Cancer Society. Cancer facts and figures 2009. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Task Force recommendation. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 5.Meissner HI, Breen NL, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the US. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 6.Swan J, Breen NL, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: Results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 7.Cokkinides VE, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among U.S. adults, age 50 years and older. Prev Med. 2003;36:85–91. doi: 10.1006/pmed.2002.1127. [DOI] [PubMed] [Google Scholar]
- 8.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult US population: results from the 2000 National Health Interview Survey. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: A review. Prev Med. 2004;38:536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Vernon SW, McQueen A. Colorectal cancer screening. In: Holland JC, Breitbart WS, Jacobson PB, et al., editors. Psychooncology. New York, NY: Oxford University Press; 2010. pp. 71–83. [Google Scholar]
- 11.McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automotive workers. Cancer Epidemiol Biomarkers Prev. 2007;16:500–509. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- 12.Janz NK, Becker MH. The health belief model: A decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein ND, Sandman PM. The precaution adoption process model. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education. San Francisco, CA: Jossey-Bass; 2002. pp. 121–143. [Google Scholar]
- 14.Witte K. Putting the fear back into fear appeals: The extended parallel processing model. Communication Monographs. 1992;59:330–349. [Google Scholar]
- 15.Aiken LS, West SG, Woodward CK, Reno RR, Reynolds KD. Increasing screening mammography in asymptomatic women: Evaluation of a second-generation, theory-based program. Health Psychol. 1994;13:526–538. doi: 10.1037//0278-6133.13.6.526. [DOI] [PubMed] [Google Scholar]
- 16.Ronis DL. Conditional health threats: Health beliefs, decisions, and behaviors among adults. Health Psychol. 1992;11:127–134. doi: 10.1037//0278-6133.11.2.127. [DOI] [PubMed] [Google Scholar]
- 17.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- 18.Ronis DL, Kaiser MK. Correlates of breast self-examination in a sample of college women: Analyses of linear structural relations. J Appl Soc Psychol. 1989;19:1068–1084. [Google Scholar]
- 19.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein ND, Lyon JE, Sandman PM, Cuite CL. Experimental evidence for stages of health behavior change: The precaution adoption process model applied to home radon testing. Health Psychol. 1998;17:445–453. doi: 10.1037//0278-6133.17.5.445. [DOI] [PubMed] [Google Scholar]
- 21.Polacsek M, Celentano DD, O'Campo P, Santelli J. Correlates of condom use stage of change: Implications for intervention. AIDS Educ Prev. 1999;11:38–52. [PubMed] [Google Scholar]
- 22.Schnoll RA, Rothman RL, Newman H, et al. Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psychooncology. 2004;13:346–358. doi: 10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- 23.Rothman AJ. "Is there nothing more practical than a good theory?": Why innovations and advances in health behavior change will arise if interventions are used to test and refine theory. Int J Behav Nutr Phys Act. 2004;1:1–7. doi: 10.1186/1479-5868-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilley BC, Vernon SW, Glanz K, et al. Worksite cancer screening and nutrition intervention for high-risk auto workers: Design and baseline findings of the Next Step Trial. Prev Med. 1997;26:227–235. doi: 10.1006/pmed.1996.0132. [DOI] [PubMed] [Google Scholar]
- 25.Tilley BC, Vernon SW, Myers R, et al. The Next Step Trial: Impact of a worksite colorectal cancer screening promotion program. Prev Med. 1999;28:276–283. doi: 10.1006/pmed.1998.0427. [DOI] [PubMed] [Google Scholar]
- 26.Robinson CR, Waxweiler RJ, McCammon CS. Pattern and model makers, proportional mortality 1972–1978. Am J Ind Med. 1980;1:159–165. doi: 10.1002/ajim.4700010206. [DOI] [PubMed] [Google Scholar]
- 27.Swanson GM, Belle SH, Burrows RW. Colon cancer incidence among modelmakers and patternmakers in the automobile manufacturing industry: A continuing dilemma. J Occup Med. 1985;27:567–569. doi: 10.1097/00043764-198508000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Tilley BC, Glanz K, Kristal AR, et al. Nutrition intervention for high-risk auto workers: Results of the Next Step Trial. Prev Med. 1999;28:284–292. doi: 10.1006/pmed.1998.0439. [DOI] [PubMed] [Google Scholar]
- 29.Myers RE, Vernon SW, Tilley BC, Lu M, Watts BG. Intention to screen for colorectal cancer among white male employees. Prev Med. 1998;27:279–287. doi: 10.1006/pmed.1998.0264. [DOI] [PubMed] [Google Scholar]
- 30.Eddy D. ACS report on the cancer-related health checkup. CA Cancer J Clin. 1980;30:194–240. [PubMed] [Google Scholar]
- 31.Levin B, Murphy GP. Revision in American Cancer Society recommendations for the early detection of colorectal cancer. CA A Cancer Journal for Clinicians. 1992;42:296–299. doi: 10.3322/canjclin.42.5.296. [DOI] [PubMed] [Google Scholar]
- 32.Bandura A. Social foundations of thought and action: a social cognitive theory. NJ: Prentice Hall; 1986. [Google Scholar]
- 33.Fishbein M, Ajzen I. Belief, attitude, intention and behavior: An introduction to theory and research. Reading, MA: Addison-Wesley; 1975. [Google Scholar]
- 34.Antonovsky A. Health Stress and Coping. San Francisco, CA: Jossey-Bass; 1979. [Google Scholar]
- 35.Myers RE, Ross E, Jepson C, et al. Modeling adherence to colorectal cancer screening. Prev Med. 1994;23:142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- 36.Myers RE, Chodak GW, Wolf TA, et al. Adherence by African American men to prostate cancer education and early detection. Cancer. 1999;86:88–104. doi: 10.1002/(sici)1097-0142(19990701)86:1<88::aid-cncr14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Myers RE, Wolf TA, McKee L, et al. Factors associated with intention to undergo annual prostate cancer screening among African American men in Philadelphia. Cancer. 1996;78:471–479. doi: 10.1002/(SICI)1097-0142(19960801)78:3<471::AID-CNCR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 38.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–832. [PubMed] [Google Scholar]
- 39.Ajzen I, Fishbein F. Scaling and testing multiplicative combinations in the expectancy-value model of attitudes. J Appl Soc Psychol. 2008;38:2222–2247. [Google Scholar]
- 40.Sobel ME, Leinhardt S. Sociological methodology. San Francisco: Jossey-Bass; 1982. Asymptotic confidence intervals for indirect effects in structural equation models; pp. 290–312. [Google Scholar]
- 41.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 42.Hu L, Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural Equation Modeling. Thousand Oaks,CA: Sage Publications; 1995. pp. 76–99. [Google Scholar]
- 43.Jackson KM, Aiken LS. A psychosocial model of sun protection and sunbathing in young women: The impact of health beliefs, attitudes, norms, and self-efficacy for sun protection. Health Psychol. 2000;19:469–578. doi: 10.1037//0278-6133.19.5.469. [DOI] [PubMed] [Google Scholar]
- 44.Vernon SW. Risk perception and risk communication for cancer screening behaviors: A review. J Natl Cancer Inst Monogr. 1999:101–119. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- 45.Gerend MA, Aiken LS, West SG, Erchull MJ. Beyond medical risk: Investigating the psychological factors underlying women's perceptions of susceptibility to breast cancer, heart disease, and osteoporosis. Health Psychol. 2004;23:247–258. doi: 10.1037/0278-6133.23.3.247. [DOI] [PubMed] [Google Scholar]
- 46.Blalock SJ, DeVellis BM, Afifi RA, Sandler RS. Risk perceptions and participation in colorectal cancer screening. Health Psychol. 1990;9:792–806. doi: 10.1037//0278-6133.9.6.792. [DOI] [PubMed] [Google Scholar]
- 47.Lipkus IM, Klein WMP. Effects of communicating social comparison information on risk perceptions for colorectal cancer. J Health Commun. 2006;11:391–407. doi: 10.1080/10810730600671870. [DOI] [PubMed] [Google Scholar]
- 48.Lipkus IM, Klein WMP, Skinner CS, Rimer BK. Breast cancer risk perceptions and breast cancer worry: What predicts what? J Risk Res. 2005;8:439–452. [Google Scholar]
- 49.Hay JL, Ford JS, Klein D, et al. Adherence to colorectal cancer screening in mammography-adherent older women. J Behav Med. 2003;26:553–576. doi: 10.1023/a:1026253802962. [DOI] [PubMed] [Google Scholar]
- 50.Manne S, Markowitz A, Winawer SJ, et al. Understanding intention to undergo colonoscopy among intermediate risk siblings of colorectal cancer patients: a test of a mediational model. Prev Med. 2003;36:71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- 51.Norman GJ. Answering the "What works?" question in health behavior change. Am J Prev Med. 2008;34:449–450. doi: 10.1016/j.amepre.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipkus IM, Skinner CS, Dement J, et al. Increasing colorectal cancer screening among individuals in the carpentry trade: Test of risk communication interventions. Prev Med. 2005;40:489–501. doi: 10.1016/j.ypmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Keller PA. Converting the unconverted: The effect of inclination and opportunity to discount health-related fear appeals. J Appl Psychol. 1999;84:403–415. doi: 10.1037/0021-9010.84.3.403. [DOI] [PubMed] [Google Scholar]
- 54.McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiol Biomarkers Prev. 2008;17:2231–2237. doi: 10.1158/1055-9965.EPI-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rawl SR, Champion VL, Menon U, Loehrer PJ, Vance GH, Skinner CS. Validation of scales to measure benefits and barriers to colorectal cancer screening. J Psychosoc Oncol. 2001;19:47–63. [Google Scholar]
- 56.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14:2855–2861. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- 57.Ritvo P, Myers R, Del Giudice ML, et al. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening in Ontario, Canada--a replication study. Cancer Epidemiol Biomarkers Prev. 2008;17:3279–3283. doi: 10.1158/1055-9965.EPI-08-0241. [DOI] [PubMed] [Google Scholar]
- 58.Cram P, Fendrick AM, Inadomi JM, Cowen ME, Carpenter D, Vijan S. The impact of a celebrity promotional campaign on the use of colon cancer screening. Arch Intern Med. 2003;163:1601–1605. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]