Abstract
Background
Ontogenetic differences in response to ethanol challenge have been observed under a variety of circumstances, including varying reports of developmental differences in the expression of tolerance to ethanol. The purpose of the present experiment was to further explore potential differences in acute and chronic tolerance expression between adolescent and adult, male and female Sprague-Dawley rats, using the social interaction test.
Methods
Acute (AT) and chronic (CT) tolerance to the social suppressing effects of a moderate dose of ethanol was assessed in adolescent and adult rats following i.p. injections of 2.0 g/kg EtOH or saline daily for 10 days. At test, adults and adolescents were challenged with 1.0 or 1.25 g/kg EtOH, respectively, with AT and CT assessed at 5 and 25 min post-injection using ratios of impairment to brain ethanol levels (BrECs) at each time period (CT) and within-session declines in impairment relative to BrECs (AT).
Results
In adolescents, 10 days of ethanol pre-exposure resulted in evidence of chronic tolerance at 25 min post-injection, perhaps associated with an enhanced expression of acute tolerance. Among adults, signs of chronic tolerance were seen at 5 min post-injection in adults, and may reflect neuroadaptations unassociated with acute tolerance, as with evidence of tolerance emerging only in adult control animals repeatedly exposed to saline injection prior to ethanol challenge on test day. Sex differences in tolerance expression were not observed at either age.
Conclusions
Our results show ontogenetic differences between adolescents and adults in the short and long-term neuroadaptations that they express in response to repeated perturbations with ethanol. Together these findings add age of exposure and time of testing within the intoxication period as critical variables to be considered when exploring the complex relationship between acute and chronic tolerance.
Keywords: adolescent, ethanol, tolerance, social interaction test, sex differences
Introduction
Adolescence is a developmental period of transition from dependence on the mother to relative independence. During this period, organisms across a variety of mammalian species undergo hormonal, brain and behavioral changes (see Spear, 2000, for review). Some of the behavioral changes observed during this period include increases in interactions with peers and risk taking behaviors (see Spear, 2007 for references and review). Increased alcohol consumption is also seen during this developmental transition, in both animals (Brunell et al., 2005; Doremus et al., 2005; Lancaster et al., 1997; Vetter et al., 2007) and humans (Johnston et al., 2009; SAMHSA, 2008). Binge drinking (i.e. five or more drinks consumed in one sitting) has been reported in individuals as young as in the 8th grade (Johnston et al., 2009). According to the Monitoring the Future Study, by 2009 the percentage of 8th, 10th, and 12th graders who reported binge levels of drinking were 20%, 28%, and 42%, respectively (Johnston et al., 2009).
Increased levels of alcohol consumption observed in adolescents may be due to their relative insensitivity to some of the negative effects of EtOH that may help to curb intake. Rodent studies have shown adolescent animals to be less sensitive to the sedative (Silveri and Spear, 1998), motor impairing (White et al., 2002), anxiogenic and social inhibitory (Varlinskaya and Spear, 2002, 2006) effects of EtOH than adults. Social interactions play a critical role in both human (Brendt, 1982) and animal (see Vanderschuren et al., 1997, for review) development, and hence the impact of alcohol on social interactions is of particular importance and relevance. The social interaction test is sensitive to the impairing effects of moderate doses of EtOH and has been used for over 25 years to assess anxiolytic and anxiogenic effects of drugs, including EtOH (see File and Seth, 2003, for review), as well as tolerance to EtOH-induced social impairment in rodents (Varlinskaya and Spear, 2006; 2007).
Developmental differences in the expression of tolerance to EtOH may contribute to age differences in EtOH sensitivity. Chronic tolerance occurs after repeated exposures to EtOH and is indexed by a reduction in ethanol sensitivity relative to EtOH-naïve organisms. Acute tolerance on the other hand, represents a decline in EtOH sensitivity occurring within a single EtOH exposure. Literature on age differences in the development of acute and chronic tolerance is limited, although a number of studies have shown that adolescent animals may express acute tolerance under conditions where adults do not (Grieve and Littleton, 1979; Silveri and Spear, 1998; Varlinskaya and Spear, 2006). Available evidence regarding differences between adolescents and adults in the development of chronic tolerance to EtOH is more mixed (Pohorecky et al., 1986; Swartzwelder et al., 1998; Varlinskaya and Spear, 2007).
Some researchers argue that both acute and chronic tolerance represent the same underlying process as evidenced, for example, by a reduction or complete blockade in the development of both acute and chronic tolerance after lesions of the median raphe nucleus (Campanelli et al., 1988; Khanna et al., 1987). Kalant and colleagues postulated two potential explanations underlying the association between acute and chronic tolerance (Kalant et al., 1971). First, they proposed that the adaptive change due to acute tolerance could endure to the next drug exposure; according to this proposition, chronic tolerance may reflect a persisting change from the previous drug exposure. The second possibility proposed was that of a “carryover” effect, whereby subsequent drug exposures facilitate the rate of acute tolerance acquisition; here, chronic tolerance is viewed as potentially representing an increase in magnitude of acute tolerance. Other researchers, however, have argued that acute and chronic tolerance represent different underlying processes and are not related mechanistically (Pohorecky and Roberts, 1992; Tabakoff, 1982). A developmental approach could prove useful in further exploring the relationship between these processes.
The goal of the present experiment was to examine potential sex and age differences in the emergence of acute and chronic tolerance in adolescent and adult rats. Both sexes were included given that, despite evidence for sex differences in EtOH consumption (Blanchard et al., 1993; Doremus et al., 2005; Lancaster et al., 1996), relatively few studies have examined potential sex differences in the emergence of tolerance to EtOH, and among those that have, the findings are mixed (Linsenbardt et al., 2009; Webb et al., 2002, Varlinskaya and Spear, 2009; Burnett and Walker, 2002). Examining ontogenetic differences in the emergence of acute and chronic tolerance is important given the presumptive relationship of tolerance with alcoholism, with tolerance included as one of the criteria for alcohol dependence in the DSM-IV-TR. Furthermore, there is an abundance of evidence to suggest a relationship between high levels of EtOH consumption and the more rapid acquisition of acute and chronic tolerance (Lê and Kiianmaa, 1988; Li et al., 1993; Erwin et al., 1980; Tampier and Quintanilla, 2002; although see Khanna et al., 1991), findings suggesting that the greater alcohol intake seen during adolescence may contribute to the elevated levels of alcohol dependence (e.g. tolerance) reported among late adolescents (18–23 years old) relative to more mature individuals (Harford et al., 2005). Yet, despite evidence for age differences in a variety of EtOH-related measures of intoxication and consumption, relatively few researchers have examined the relationship between acute and chronic tolerance in adult male animals, let alone in females or in adolescent animals.
Materials and Methods
Subjects
Male and female Sprague-Dawley rats derived from our breeding facility were used in the current experiment. On postnatal day (P) 1, all litters were culled to 8–10 pups with a sex ratio of six males to four females kept whenever possible. Pups were weaned at P21 when they were pair housed with a same-sex littermate unless otherwise noted. Rats were given ad libitum access to food (Purina Lab chow, Lowell, MA) and water, and were maintained in a temperature-controlled vivarium with a 14:10 hr light-dark cycle (lights on at 0700 hr). At all times animals were treated in accordance with guidelines for animal care established by the National Institutes of Health under protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental Design
Both male and female rats received repeated exposure to either 2.0 g/kg ethanol (EtOH) or saline (Sal) during adolescence or adulthood. On test day, animals in all groups were challenged with either saline or ethanol, and tested in the social interaction test either 5 or 25 min thereafter. Thus, the experimental design for this study was a 2 age (adolescent, adult) x 2 sex x 2 pre-exposure (chronic Sal, chronic EtOH) x 2 injection-test interval (5, 25 min) x 2 acute challenge (Sal, EtOH) factorial, with eight animals placed in each of the 32 groups defined by this experimental design. On test day, the ethanol challenge dose used for adults was 1.0 g/kg EtOH whereas adolescents were challenged with a 1.25 g/kg EtOH dose due to their apparent insensitivity to the lower EtOH challenge dose of 1.0 g/kg revealed in initial test runs. Subsequent analysis confirmed the insensitivity of adolescents to the 1.0 g/kg EtOH dose, with adolescents given this challenge dose not differing from Sal controls in their overall social activity (49.6±3.97 and 53.3±3.27, respectively). Hence, a challenge dose of 1.25 g/kg EtOH was used for adolescents in all the assessments reported here.
Chronic Exposure
On P21 or P66, rats were re-housed with a same-sex, same-age non-littermate assigned to the same chronic exposure condition, with no more than one animal from a given litter placed into an experimental group, thus reducing the possibility of litter effects (Holson and Pearce, 1992). Beginning four days thereafter (i.e. from P24–33 or P69–78), animals received daily injections of EtOH (2.0 g/kg as a 12.6% v/v solution in 0.9% saline) or Sal for 10 days. Immediately after injection, animals were returned to their home cage with their similarly injected cage mate. On the last day of the chronic exposure period, and about 2 hr prior to the daily injection, all experimental animals were placed individually into the test context to be used for the social interaction (SI) test for a 30-min habituation period and then returned to their home cage.
Social Interaction testing
On test day (i.e. P34 or P79), animals were tested in the SI test between the hours of 0900 and 1300, under dim lighting (3 lux). Each experimental animal was weighed, marked with a vertical black line across the back (in order to differentiate each experimental animal from its partner during the SI test), and isolated in a 47 × 25.4 × 20.3 cm holding cage for a total of 30 min prior to testing in order to facilitate social interactions (File and Seth, 2003). Rats in the 5 min injection-test interval group were placed in the holding cage for 25 min, injected with either EtOH or Sal and returned to the holding cage for an additional 5 min before testing, whereas rats in the 25 min group were placed in the holding cage for 5 min, injected with either EtOH or Sal and returned to the holding cage for an additional 25 min prior to testing. At the onset of the 5-min test session, each animal was simultaneously placed into the SI apparatus with a non-manipulated partner of the same sex and age that was unfamiliar with both the SI apparatus and the experimental animal. Weight differences between experimental animals and their partners were minimized to the extent possible, with no more than a 20 g difference between each pair tested.
Each social interaction apparatus was made of clear Plexiglas (Binghamton Plate Glass, Binghamton, NY) and size-scaled for each test age: 30 × 20 × 20 cm for adolescents and 45 × 30 × 20 cm for adults. The apparatus was divided on the long axis into two equal sized compartments by a wall containing an aperture measuring 7 × 5 cm for adolescents and 9 × 7 cm for adults, thereby allowing animals to move between compartments. Before placing an animal into the SI apparatus, the walls/floors were wiped clean with a 3% hydrogen peroxide solution, dried, and fresh pine shavings were added.
All sessions in the SI apparatus were conducted without an experimenter present in the room. A video camera (Supercircuits model PC-23C, Austin, TX) located above the apparatus recorded each session for later scoring. Behaviors of the experimental animal during the 5-min test session were scored from the videotape by two experimenters blind to the experimental condition of each animal, with at least 90% inter-experimenter agreement. Overall social activity was determined by summing the occurrence of the following behaviors: investigation (sniffing any body part of the partner), contact (crawling over or under the partner and social grooming) and play (pouncing or playful nape attack, chasing and pinning). A social preference/avoidance coefficient [Coefficient (%) = (crossovers to the partner − crossovers away from the partner)/(crossovers to the partner + crossovers away from the partner) × 100] was used as an index of social motivation toward its partner, with positive scores indicating a preference and negative scores indicating relative avoidance of the partner. An index of motor movement in the test apparatus was calculated on both the habituation and test days by summing the number of crosses between compartments Although recognizing that number of crosses into each compartment may be influenced by exploratory behavior (File and Seth, 2005), compartment entries is a common way of indexing motor activity (e.g. the use of total number of arm entries in the elevated plus maze to index locomotor activity), and was used as such in this social context.
Blood and Brain Ethanol Determination
Immediately following testing, experimental animals were decapitated. Trunk blood and brains were collected, placed, and maintained at −80°C until analysis of blood and brain ethanol concentration (BEC and BrEC, respectively). Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (GC) (Wilmington, DE). Blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the GC. Ethanol concentrations in each sample were determined using HP Chemstation software which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions. Brain samples were thawed at time of analysis and 2 ml of cold distilled water added per gram of brain tissue before the sample was homogenized at 5 °C and analyzed using the same procedure used for analysis of BEC.
Data Analysis
Unless otherwise noted, data were analyzed by factorial or repeated measures analyses of variance (ANOVA), with significant effects and interactions explored further using Fisher’s LSD post-hoc tests.
Test day analyses focused first on behavior of animals receiving Sal challenge on test day to assess potential withdrawal-related alterations. Overall social activity of each EtOH challenged animal was then converted to an impairment score reflecting its impairment relative to the corresponding Sal-challenged control group, to control for any age or sex-related differences seen among these Sal challenged animals prior to assessment of EtOH effects. The formula used for calculating impairment scores was: [impairment score = 100 − ([each animal’s social behavior/mean of Sal group] × 100)], resulting in scores from 0 (no impairment) to 100 (maximum impairment). Any EtOH-challenged animal who exhibited greater social activity at test compared to its control group was automatically given an impairment value of zero (9 adults and 12 adolescents met this criteria).
Chronic tolerance was assessed by comparing the impairment scores following EtOH challenge in animals with a history of EtOH relative to EtOH-naïve (chronic Sal) animals. Because interpretation of the response to EtOH challenge should presumably reflect, in part, the amount of EtOH in the brain, data were expressed as social impairment/BrEC ratios (impairment ratio = impairment score/BrEC). A significantly smaller impairment ratio in EtOH-exposed relative to EtOH-naïve animals at a given injection-test interval was used as evidence for CT. Although most assessments of CT focus only on a single injection-test interval, analysis of CT in the present experiment was conducted at both the 5- and 25- min test interval due to evidence demonstrating that CT expression may vary with time following the ethanol challenge dose (Pohorecky and Roberts, 1992; Kalant, 1971).
Acute tolerance can be indexed a number of different ways, most of which involve assessing the relationship between BECs and EtOH-induced impairment of a particular function (e.g. sedation, motor impairment). Radlow (1994) argues for the use of an output function (that relates change in BEC to change in EtOH induced impairment) over time. Using this method, AT is defined as being present when animals display less impairment relative to their brain EtOH levels over time post-EtOH. To directly compare BrEC with EtOH-induced impairment using the Radlow method, the first step is to convert the social impairment score for each EtOH challenged animal into a % maximum impairment score [% maximum impairment = (impairment of each animal/mean maximum impairment for that experimental group) × 100]; therefore with this transformation, maximum impairment is expressed as 100%. The next step is to likewise convert each animals BrECs to % of the average maximum BrEC within each age/sex/pre-exposure condition [% maximum BrEC = (BrEC of each animal/maximum mean BrECs) × 100]. Finally, an output function is formed by subtracting the percent maximum social impairment from the percent maximum BrEC (% max BrEC − % max social impairment) for each animal, with each of these data points plotted against time. With this output function, higher scores reflect less impairment relative to BrECs. Acute tolerance is then assessed via regression analysis of each output function over time to determine if the slope differs significantly from zero (see Radlow, 1994). Using this approach, acute tolerance is defined as greater output values over time, with EtOH-induced social impairment declining more rapidly than BrECs, as indexed by a positive slope of the linear regression of this output function with time.
The advantage of the Radlow method is that it converts data across groups to the same scale (% of maximum impairment) prior to assessing AT in each group. The disadvantage of this approach is the many transformations needed in order to obtain each output function, with the final analysis far removed from the raw data. Due to this concern, acute tolerance was also assessed more directly using social impairment/BrEC ratios at each age and exposure condition. When assessing AT using these ratios, AT is indexed by significant declines in the ratios over time—i.e., significantly smaller ratios at the 25 min than 5 min injection-test interval (see Pohorecky and Roberts, 1992).
Results
BrECs and overall social activity data were checked for outliers and any values greater than 2 standard deviations from the mean were excluded from analysis. As a result, data from a total of four adolescents and five adults were removed prior to analysis, with no more than one animal removed from any experimental condition.
Means (±SEM) are provided for all behavioral measures in adolescent (table 3a) and adult (table 3b) male and female rats.
Table 3a.
Mean (± SEM) of the social interaction test data among adolescent male and female rats
| Saline Challenge | Ethanol Challenge | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-exposure | Saline | Ethanol | Pre-exposure | Saline | Ethanol | ||||||
| Males | Females | Males | Females | Males | Females | Males | Females | ||||
| Measure | Interval | Measure | Interval | ||||||||
| Overall Social Activity | 5 | 66.12±9.85 | 44.57±3.06 | 51.00±4.76 | 49.86±2.39 | Overall Social Activity | 5 | 32.87±4.01 | 35.37±6.51 | 37.50±5.39 | 35.75±2.57 |
| 25 | 42.12±6.44 | 57.62±8.20 | 45.87±5.50 | 43.00±3.27 | 25 | 35.50±5.22 | 29.62±4.41 | 39.57±1.80 | 48.62±5.42 | ||
| Total # of crossovers | 5 | 29.00±2.66 | 25.00±1.31 | 25.37±1.90 | 25.14±2.20 | Total # of crossovers | 5 | 27.00±3.32 | 29.75±4.05 | 34.87±3.90 | 28.37±2.96 |
| 25 | 19.62±2.05 | 28.12±2.56 | 19.87±1.78 | 22.00±1.29 | 25 | 25.00±2.66 | 21.50±3.2 | 29.14±2.04 | 27.25±4.30 | ||
| Social Motivation | 5 | 33.50±6.38 | 20.33±11.82 | 20.49±8.47 | 32.95±9.40 | Social Motivation | 5 | 5.22±8.40 | 5.62±8.85 | 16.63±9.38 | 31.06±9.42 |
| 25 | 37.34±5.70 | 24.83±6.35 | 24.34±6.60 | 35.57±8.05 | 25 | 13.20±9.01 | 20.13±13.22 | 11.09±1.92 | 30.93±5.42 | ||
| Social Impairment ratio (for CT & AT) | 5 | --------- | --------- | --------- | --------- | Social Impairment ratio (for CT & AT) | 5 | 0.52±0.06 | 0.24±0.12 | 0.27±0.09 | 0.27±0.05 |
| 25 | --------- | --------- | --------- | --------- | 25 | 0.27±0.09 | 0.55±0.08 | 0.15±0.04 | 0.08±0.04 | ||
Table 3b.
Mean (± SEM) of the social interaction test data among adult male and female rats
| Saline Challenge | Ethanol Challenge | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-exposure | Saline | Ethanol | Pre-exposure | Saline | Ethanol | ||||||
| Males | Females | Males | Females | Males | Females | Males | Females | ||||
| Measure | Interval | Measure | Interval | ||||||||
| Overall Social Activity | 5 | 48.00±4.79 | 54.37±8.07 | 41.37±6.86 | 55.00±7.24 | Overall Social Activity | 5 | 12.71±2.08 | 22.86±1.97 | 25.87±3.86 | 33.43±3.37 |
| 25 | 49.62±5.11 | 47.28±4.66 | 51.00±6.15 | 45.86±4.73 | 25 | 31.25±6.47 | 47.50±8.72 | 38.87±6.02 | 41.67±3.83 | ||
| Total # of crossovers | 5 | 22.12±1.52 | 22.62±2.54 | 18.87±0.97 | 20.12±1.52 | Total # of crossovers | 5 | 11.86±1.59 | 16.00±1.40 | 19.50±1.91 | 19.86±2.38 |
| 25 | 21.37±1.05 | 21.43±0.92 | 17.87±2.07 | 19.57±1.34 | 25 | 22.12±3.97 | 24.50±1.84 | 17.75±1.67 | 22.33±3.37 | ||
| Social Motivation | 5 | 26.66±9.10 | 22.20±6.10 | 11.01±8.74 | 29.50±6.47 | Social Motivation | 5 | −14.72±10.42 | 1.89±9.80 | 13.78±7.94 | 15.78±9.54 |
| 25 | 20.12±8.34 | 27.66±6.21 | 17.31±10.30 | 26.89±9.29 | 25 | 0.54±13.04 | 25.75±8.93 | 2.07±7.48 | 26.99±12.00 | ||
| Impairment ratio (for CT & AT) | 5 | --------- | --------- | --------- | --------- | Impairment ratio (for CT & AT) | 5 | 0.71±0.04 | 0.64±0.06 | 0.41±0.09 | 0.44±0.06 |
| 25 | --------- | --------- | --------- | --------- | 25 | 0.45±0.13 | 0.24±0.12 | 0.34±0.10 | 0.19±0.06 | ||
Body Weight Gain
To determine whether chronic EtOH exposure altered weight gain during the 14 days of the experiment, a 2 (age) X 2 (sex) X 2 (pre-exposure) factorial ANOVA was performed. This analysis revealed main effects of age [F (1,237) = 2243.83, p<.001], sex [F (1,237) = 236.40, p<.001] and pre-exposure [F (1,237) = 94.34, p<.001], as well as interactions of sex with pre-exposure [F (1,237) = 9.62, p<.01] and age [F (1,237) = 17.05, p<.001], and a 3-way interaction of these variables [F (1,237) = 5.84, p<.001] (table 1). Fisher’s post hoc tests did not reveal meaningful differences underlying these interactions, although adolescents were found to gain significantly more weight than adults, rats exposed to EtOH for 10 days to gain significantly less weight than their Sal-exposed counterparts, and males at each age and exposure condition to gain significantly greater weight gains than females.
Table 1.
Absolute body weight gain (in grams) across the 14 day experimental procedure in adolescent and adult male and female rats exposed to 2.0 g/kg EtOH or an equivalent volume of saline daily for 10 days
| Chronic saline | Chronic ethanol | |||
|---|---|---|---|---|
|
|
||||
| Adolescent | Male | Female | Male | Female |
| 97.8 ± 1.64 | 78.0 ± 1.20 ** | 90.3 ± 1.66* | 72.0 ± 1.49 | |
|
|
||||
| Chronic saline | Chronic ethanol | |||
|
|
||||
| Adult | Male | Female | Male | Female |
| 47.7 ± 2.26 | 22.6 ± 1.59 ** | 24.2 ± 1.97* | 12.4 ± 1.74** | |
EtOH-exposed animals gained significantly less weight than Sal animals at both ages
Females gained significantly less weight than males at both ages and exposure conditions
Total number of crosses on the habituation day
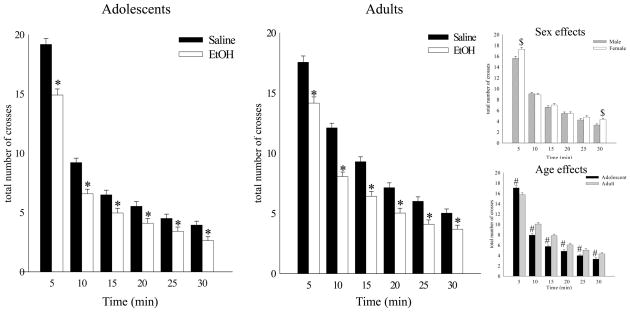
A 2 (age) X 2 (sex) X 2 (pre-exposure) X 6 (5-min bins) repeated measures ANOVA of motor activity (number of crosses) during the 30-min habituation period revealed significant main effects of sex [F (1,235) = 4.37, p<.05], age [F (1,235) = 15.36, p<.001], pre-exposure [F (1,235) = 69.58, p<.001] and time bin [F (5,1175) = 859.37, p<.001], as well as interactions of bin with sex [F (5,1175) = 4.60, p<.001], age [F (5,1175) = 14.66, p<.001] and pre-exposure [F (5,1175) = 10.42, p<.001]. Females generally were more active than males, a sex difference that reached significance during the first and last 5 min of habituation (see top insert Fig. 1). Adolescents were significantly more active than adults during the first 5 min, although their activity dropped quickly; reaching levels significantly lower than that of adults at each time bin thereafter (see bottom insert Fig. 1). Regardless of age, EtOH pre-exposed animals had significantly less crosses than Sal pre-exposed animals during all time bins, although this difference tended to diminish as activity in both groups diminished over time (Fig. 1). Since habituation took place 22 hours following the previous day’s injection, the lower motor activity of EtOH pre-exposed animals at both ages may be due, in part, to withdrawal or “hangover” effects (Macey et al., 1996).
Figure 1. Total number of crosses during habituation.
Locomotor activity (total number of crossovers) (± SEM) during the 30 min habituation session in adolescent (left) and adult (right) rats (collapsed across sex). Top insert: * denotes a significant sex effect. Bottom insert: * denotes a significant age effect.
Baseline levels of Motor Activity, Overall Social Activity, and Social Motivation on test day
In order to further explore possible withdrawal effects, behaviors of Sal-challenged animals on test day were examined during the 5-min SI test, using a 2 (age) X 2 (sex) X 2 (pre-exposure) factorial ANOVA for each behavior: motor activity (chamber crosses); overall social activity; social motivation. Analysis of the number of crosses between chambers during the 5-min test revealed only a main effect of age [F (1,54) = 14.55, p<.05], with adolescents showing more chamber crosses in the social context than adults. A similar analysis of overall social activity (sum of social investigation, contact behavior, and play) revealed only a significant sex X age interaction [F (1,54) = 5.32, p<.05], with adolescent males being more socially active than adult males and no other group differences. The analysis of social motivation, as indexed by social preference/avoidance coefficients, revealed a significant sex X pre-exposure interaction [F (1,54) = 4.21, p<.05], although post-hoc analyses of this interaction on data collapsed across age revealed no significant differences. None of these behavioral assessments of animals challenged with saline on test day revealed evidence of pre-exposure effects, thus there was no indication that chronic EtOH exposed animals were displaying signs of withdrawal in this test at either age.
Brain Ethanol Concentration (BrEC)
Correlations conducted relating BEC and BrEC levels at each age revealed positive correlations for adults as well as adolescents, r=0.76 and 0.84, respectively. Therefore, analyses focused on BrECs (see table 2 for BrEC and BEC values).
Table 2.
Mean ± SEM for blood and brain EtOH concentration in adolescent (top) and adult (bottom) male and female rats
| Pre-exposure | Saline | Ethanol | |||
|---|---|---|---|---|---|
| Interval | 5 | 25 | 5 | 25 | |
| Males | BEC | 96.49±3.82 | 86.04±2.45 | 102.82±3.49 | 91.23±2.55 |
| BrEC | 96.16±5.67 | 91.73±2.35 | 110.59±5.48 | 92.63±2.69 | |
| Females | BEC | 92.20±2.63 | 85.45±2.86 | 99.32±4.44 | 85.19±3.12 |
| BrEC | 96.36±3.93 | 88.76±2.51 | 104.54±4.54 | 88.55±3.01 | |
| Pre-exposure | Saline | Ethanol | |||
|---|---|---|---|---|---|
| Interval | 5 | 25 | 5 | 25 | |
| Males | BEC | 98.44±1.78 | 85.49±3.18 | 92.31±4.22 | 81.01±3.96 |
| BrEC | 103.03±3.40 | 87.40±3.20 | 91.69±4.54 | 81.41±2.37 | |
| Females | BEC | 90.76±2.79 | 71.14±2.59 | 89.48±4.99 | 77.45±2.95 |
| BrEC | 91.66±2.84 | 78.64±4.84 | 87.77±3.73 | 74.77±1.81 | |
Analyses of BrECs were conducted separately at each age due to the different EtOH challenge doses administered to animals at each age. The 2 (sex) X 2 (pre-exposure) X 2 (injection-test interval) ANOVA of BrECs at each age revealed a significant main effect of injection-test interval in adolescents [F (1,55) = 16.36, p<.001], as well as in adults [F (1,51) = 25.80, p<.001], with significantly higher BrECs at 5 min post-injection than 25 min at both ages. A main effect of pre-exposure in adolescents [F (1,55) = 4.19, p<.05)] revealed that EtOH pre-exposed adolescents had higher BrECs on test day than chronic Sal pre-exposed adolescents, an effect seemingly driven by the 5 min data (table 2), although the pre-exposure X time interaction did not reach significance. In contrast, the main effect of pre-exposure [F (1,51) = 6.02, p<.01] in adults revealed that adults with a history of chronic EtOH had significantly lower BrECs than those of chronic Sal adults (table 2). A main effect of sex was only evident in adult animals [F (1,51) = 9.01, p<.01], with adult females exhibiting overall lower BrECs (83.35 ± 2.18) than males (90.49 ± 2.17).
Chronic Tolerance (Impairment/BrEC Ratios)
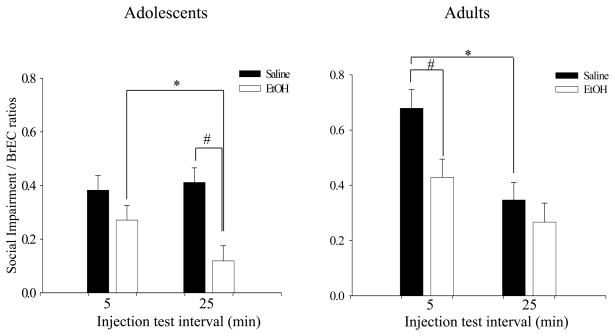
The 2 (age) X 2 (sex) X 2 (pre-exposure) X 2 (injection-test interval) ANOVA of the social impairment/BrEC ratios revealed main effects of age [F (1,106) = 9.79, p<.01], pre-exposure [F (1,106) = 18.23, p<.001], and injection-test interval [F (1,106) = 12.92, p<.001]. Additionally, interactions of age X injection-test interval [F (1,106) = 4.67, p<.05], age X sex X injection-test interval [F (1,106) = 5.51, p<.05], as well as age X pre-exposure X injection-test interval [F (1,106) = 4.17, p<.05] emerged. Due to these interactions, to further explore pre-exposure (CT) effects, separate ANOVAs were run for each age and time interval. The 2 (sex) X 2 (pre-exposure) X 2 (injection-test interval) ANOVA of the social impairment/BrEC ratios of adolescents at 5 min post-EtOH administration, revealed no evidence of CT in adolescents, whereas evidence of CT was seen among adolescents at the 25-min injection-test interval [main effect of pre-exposure: F (1,27) = 17.71, p<.001] (see # in left side of Fig. 2). Conversely, among adults, evidence for CT was seen only at the 5-min injection-test interval (main effect of pre-exposure: [F (1,25) = 12.89, p<.01]) (see # in the right side of Fig. 2).
Figure 2. Social Impairment/Brain EtOH concentration ratios.
Social impairment/BrEC ratios at 5 and 25 min post-injection in adolescent (left) and adult (right) rats with data collapsed across sex. * denotes a significant injection-test interval effect (acute tolerance); # denotes a significant pre-exposure effect (chronic tolerance).
When chronic tolerance to the motor impairing effects of EtOH on test day was similarly analyzed, no evidence for the expression of CT to the motor impairing effects was evident among adolescents at either injection-test interval, whereas evidence for CT again emerged in adults only at the 5-min injection-test interval (see table 4).
Table 4.
Mean (± SEM) for locomotor impairment/BrEC ratios 5 and 25 min post-injection in adolescent and adult rats
| Pre-exposure | 5 min post-EtOH | 25 min post-EtOH | |
|---|---|---|---|
|
|
|||
| Adolescent | Saline | 0.13 ± 0.04 | 0.18 ± 0.06 |
| EtOH | 0.06 ± 0.02 | 0.08 ± 0.07 | |
|
|
|||
| Pre-exposure | 5 min post-EtOH | 25 min post-EtOH | |
|
|
|||
| Adult | Saline | 0.38 ± 0.05 | 0.18 ± 0.06 |
| EtOH | 0.11 ± 0.04* | 0.14 ± 0.05 | |
Significantly different from corresponding saline control
Acute Tolerance
Radlow’s approach of assessing AT as an output function over time revealed that adolescents chronically exposed to EtOH expressed AT, whereas AT was evident only among adults with a history of chronic Sal injections (see table 5).
Table 5.
Acute tolerance assessed via Radlow’s approach
| Pre-Exposure | Slope | p-value | |
|---|---|---|---|
| Adolescents | Saline | −0.25 ± 0.98 | 0.80 |
| EtOH | 2.40 ± 1.09* | 0.04 | |
| Adults | Saline | 2.10 ± 0.64* | 0.00 |
| EtOH | 1.55 ± 0.99 | 0.14 |
Slope significantly different from zero
When acute tolerance was assessed using social impairment/BrEC ratios via a 2 (age) X 2 (sex) X 2 (pre-exposure) X 2 (injection-test interval) ANOVA, the same main effects and interactions as in the assessment of CT emerged; therefore, ANOVAs were run separately at each age yielding significant main effects of pre-exposure at both ages (adolescents: [F (1,55) = 13.47, p<.001]; adults: [F (1,51) = 6.13, p<.05]). When these effects were explored in adolescents using separate 2 (sex) X 2 (injection-test interval) factorial ANOVAs for each pre-exposure condition, only adolescents with a history of chronic EtOH exposure were found to demonstrate AT as evidenced by a significant main effect of injection-test interval [F (1, 27) = 6.51, p< .05], reflecting significantly smaller impairment ratios 25 min than 5 min post-injection (see * on left side of Fig. 2). Adult data demonstrated an opposite pattern to that seen among adolescents, with evidence for AT emerging only in Sal pre-exposed adults, but not adults chronically exposed to EtOH [F (1,26) = 10.99, p<.01] (denoted by * on right side of Fig. 2).
Discussion
The results of the present study demonstrate that adaptations to chronic ethanol exposure are different in adolescents and adults. In adults, males and females expressed CT to the social and motor impairing effects of EtOH, with ethanol-exposed adults being less impaired than saline-exposed adults when tested shortly following administration on test day. Adolescents chronically exposed to EtOH, on the other hand, demonstrated less ethanol-induced social impairment relative to chronically saline-exposed adolescents only at the 25 min injection-test interval, suggesting that chronic tolerance at this age could be related, at least in part, to the enhancement of acute, within-session tolerance. Indeed, AT was evident in adolescents with a history of chronic EtOH, whereas adolescents with a history of chronic Sal showed no evidence of AT. In contrast, only Sal-exposed, but not EtOH-exposed adults displayed AT to the social impairing effects of EtOH. Despite these age differences in the impact of chronic EtOH exposure on later sensitivity to EtOH, at both ages daily treatment with EtOH for 10 days was sufficient to suppress body weight gain in males and females, and to reduce baseline locomotor activity during the habituation session, hypoactivity that may be indicative of ethanol withdrawal.
Chronic tolerance, in the classical definition (Lê and Mayer, 1996), essentially eliminates post-EtOH administration-to-test interval as a factor. However, research has shown initial sensitivity to be positively correlated with the development of chronic functional tolerance (Crabbe et al., 1982; Kalant et al., 1971; Khanna et al., 1985; San-Marina et al., 1989; Tabakoff et al., 1980). In the present study, initial sensitivity to EtOH-induced social suppression, at least when indexed via the response to EtOH seen at the 5 min injection-test interval, differed notably as a function of age, to the extent that adolescents required a higher (1.25 g/kg) dose to display significant social suppression that was seen at 1.0 g/kg in adults. Similar results have previously been demonstrated in our lab, with adults showing greater EtOH-induced impairment than adolescents at the same dose (Varlinskaya and Spear, 2009). To the extent that initial sensitivity to EtOH is related to development of CT, it should perhaps not be surprising that adults, being initially more sensitive to ethanol-induced social impairment, showed CT when tested 5 min following EtOH administration, whereas adolescents did not at this early period post-administration.
BrECs were also lower following repeated EtOH in adults, suggesting they had developed some degree of metabolic tolerance, consistent with other research findings in the literature (Khanna et al., 1982; Varlinskaya and Spear, 2007). Although the emergence of metabolic tolerance may contribute to a reduced sensitivity to EtOH challenge in this group, this alone cannot explain the decrease in their impairment, given that CT was assessed via relating impairments to BrECs using impairment/BrEC ratios. The results using these impairment ratios are consistent with other common findings for the emergence of chronic functional tolerance after repeated exposure to EtOH in adult rodents (LeBlanc et al., 1969; Pohorecky et al., 1986). Reminiscent of other research from our lab (Varlinskaya and Spear, 2006; 2007), no sex differences emerged at either age in the expression of acute or chronic tolerance to EtOH in the present study. This lack of sex differences in alcohol adaptations were seen despite the adult females having lower BrECs following EtOH challenge than adult males, regardless of pre-exposure condition. The lack of sex differences in acute or chronic tolerance development in adults in the present study despite sex differences in their BrECs may be related, at least in part, to the use of impairment ratios indexing EtOH sensitivity relative to corresponding BrECs.
In contrast to the expression of CT shortly after EtOH challenge on test day among adults, expression of CT was seen among adolescents at only the 25-min injection-to-test interval. These results lend support to the fact that adolescents, too, can express chronic tolerance to EtOH, and is a finding reminiscent of the human survey data where human adolescents often report development of tolerance to alcohol (see Chung et al., 2001, for references and review). And, although few studies have examined CT in developing animals and available results are complex, there are reports that adolescents and even younger animals (preweanlings) as well can express CT to EtOH under some conditions (Hunt et al., 1993; Varlinskaya and Spear, 2007). In the present study, CT was evident only at the later injection-test interval among adolescents, a time when significant AT was evident in these animals. Hence, it is possible that this CT expression may reflect enhancement of AT in these adolescents, with expression of AT building over repeated drug exposures (Kalant et al., 1971).
Acute tolerance to EtOH in the present study was expressed in both adolescent and adult rats, but was dependent on pre-exposure condition. Adolescents with a history of chronic EtOH expressed AT, whereas only saline pre-exposed adults did so. Research supports that AT develops early in life and often declines during ontogeny, with adults frequently expressing little to no AT under conditions supporting robust AT in adolescents and even younger rats (Draski et al., 2001; Grive and Littleton, 1979; Silveri and Spear, 1998; 2001; Varlinskaya and Spear, 2006). Yet, there is ample evidence that under some test conditions adult rats can demonstrate AT to EtOH (see Lê and Mayer, 1996, for references and review; LeBlanc et al., 1974; Silveri and Spear, 2004). Although a previous study from our lab did not see AT to the social impairing effects of EtOH in adult rats (Varlinskaya and Spear, 2006), a major difference between that study and the present one was that animals in the earlier study were not manipulated prior to pre-test habituation to the test apparatus, whereas rats in the present study had been injected daily for 10 days. Pre-test perturbation has been suggested to enhance expression of AT in adults (Silveri and Spear, 2004). A potentially even more important difference is the drug challenge dose, with adult and adolescent rats in our experiment receiving different challenge doses on test day (1.0 and 1.25 g/kg EtOH, respectively), in contrast to use of the same challenge dose in the Varlinskaya and Spear (2006) study. Previous work from our lab has shown that when dose was varied with age to equate across-age levels of motor impairment in a swim task, AT was expressed in both adolescents and adults, with adults perhaps expressing even stronger AT than adolescents (Silveri and Spear, 2001). Additionally, age differences in rapid and chronic tolerance were not present. By adjusting the challenge dose to induce similar levels of social impairment on test day at each age, it is possible that we may have minimized possible age-related differences in the emergence of AT. In contrast, because of age-related differences in EtOH sensitivities (e.g. Varlinskaya and Spear, 2002), by administering the same absolute EtOH dose across age during the pre-exposure period, it is possible that adolescents and adults may have developed different neuroadaptations to the repeated exposures. Indeed there is evidence to suggest that the same amount of chronic EtOH exposure can produce age-dependent neuroadaptations (Pian et al., 2010). For example, Pian and colleagues (2010) found that following 2 weeks of EtOH vapor, adolescents showed a reduction in NR2A subunits in the hippocampus, with the opposite finding observed in adults. The results of these studies demonstrate the importance of considering whether to maintain equal EtOH doses across age or equate for impairment (e.g. by varying dose) when designing ontogenetic experiments.
The question of whether or not acute and chronic tolerance represent the same or different mechanisms remains unanswered. The results of the present study partially lend support for Kalant and colleagues’ notion that development of acute and chronic tolerance are functionally related (Kalant et al., 1971), given that adolescents chronically exposed to EtOH developed both acute and chronic tolerance (with the latter expressed at the later injection interval). Work relating history of chronic EtOH to facilitation of AT expression has been reported in adult humans (Bennett et al., 1993) and rodents (Hiltunen and Jarbe, 1992; Kalant et al., 1978), with some evidence in both species for similar relationships developmentally (Beirness and Vogel-Sprott, 1984; García-Moreno et al., 2004). This suggests that, at least early in life, expression of CT may be related to an enhancement of AT, as Kalant and colleagues have postulated (Kalant et al., 1971).
This does not appear to be the case with adults in the current study, given that only Sal-exposed adults showed AT. Therefore, CT in these adult rats does not seem to reflect accelerated development of AT due to chronic EtOH exposure but rather changes in EtOH sensitivity based on almost immediate adaptation of neurons. These adult data are more consistent with the idea that acute and chronic tolerance may be mediated by different processes (Khanna et al., 1992; Pohorecky and Roberts, 1992). Indeed, it is important to consider the dynamics of expression of CT within an alcohol exposure session, and how that may bidirectionally relate to expression of AT (Pohorecky and Roberts, 1992). These results provide additional support for the necessity of carefully selecting post-EtOH administration interval testing. CT should be assessed at different time points in order to separate CT based on changes in constitutive sensitivity of the CNS to ethanol (perhaps driven almost immediate adaptations of neurons) from that related to the enhancement of AT that builds over time during the EtOH intoxication period.
Although genetic factors were not tested in the present experiment, there is evidence to suggest that genetics can influence the development of tolerance to EtOH. Research has shown that inbred strains of mice and lines of rats demonstrate differences in the expression of alcohol tolerance that are line and strain dependent (Crabbe et al., 1982; Reed et al., 2001; Waller et al., 1983; Tampier and Quintanilla, 2002; Kurtz et al., 1996). For example, C57BL/6 mice develop greater tolerance to EtOH while DBA mice do not (Grieve and Littleton, 1979; Tabakoff and Ritzmann, 1979), differences that are likely related at least in part to strain differences in neural systems critical for expression of EtOH tolerance. Included among critical EtOH-sensitive neural systems undergoing tolerance-related changes are NMDAR systems, with rats given EtOH in a 2 week liquid diet showing tolerance to EtOH-induced sedation and having NMDARs that were resistant to inhibition following an acute EtOH challenge when compared to EtOH-naïve rats (Wu et al., 2010). Ontogenetic studies have also been conducted to examine the effects of NMDA system blockade on the expression of tolerance (Silveri and Spear, 2004; Ramirez et al., in press). Thus, although we found age, EtOH-exposure, and injection-to test interval to be important factors in mediating the expression of tolerance to EtOH, research examining genetic factors and neural systems clearly has revealed the importance of these levels of analysis as well as for understanding contributors to ethanol tolerance.
Regardless of age or sex, animals in the present study that were chronically exposed to EtOH exhibited significantly lower levels of motor activity than animals exposed chronically to Sal, when motor activity was indexed via crosses during the 30-min habituation session. Since animals in this study were given this habituation session 22 hr after their last injection, this motor suppression likely reflects withdrawal-induced hypomotility, which has been observed in rats up to 48 hr since their last EtOH administration (Macey et al., 1996). Decreases in motor activity join other measures, including convulsions, hypothermia, increased anxiety, hyperactivity, and rebound hyperthermia as being indicative of the withdrawal that occurs when chronic exposure to EtOH is terminated (Finn and Crabbe, 1997; Sinclair and Taira, 1988; Rasmussen et al., 2001; see Kliethermes, for review, 2005). There is ample evidence that adult rats show notable withdrawal after chronic EtOH exposure (File, 1991; 1993; 1994); however, withdrawal following chronic EtOH exposure during adolescence has been little investigated. Slawecki and Roth (2004) reported age differences in hypoactivity following chronic EtOH, with adolescents showing enhanced withdrawal hypoactivity when assessed via activity chamber monitors, but not when examined in the open field. These results demonstrate that the expression of hypoactivity observed during withdrawal may express itself differently developmentally depending on the task.
Whereas adults in the present study demonstrated evidence for metabolic tolerance, a common finding in the literature, (e.g. see Miceli and Le Magnen, 1979), an opposite pattern was seen in adolescent animals, with adolescent male and female rats with a history of EtOH having significantly higher BrECs than Sal pre-exposed adolescents. Although this was an unexpected finding, some researchers have found higher BrECs in rats after chronic EtOH exposure compared with Sal pre-exposed rats (Silveri and Spear, 2001). Given that repeated stress has been shown to result in higher BECs after an EtOH challenge (Parker et al., 2008), the higher BrECs seen in these adolescents could potentially be due to differential stress effects across age. This possibility seems unlikely, though, given that weight gain was similarly suppressed at both ages by chronic EtOH, as has been commonly reported in the literature (Slawecki et al., 2005). The emergence of EtOH-induced liver damage following chronic adolescent EtOH exposure is also a possibility, since the liver contains the largest proportion of enzymes used to metabolize EtOH (Pawan, 1972). There is evidence for human adolescents who alcohol dependent to have higher serum liver enzyme levels, which are indicative of liver damage than adolescents who have not consumed alcohol (Clark et al., 2001; Zeigler et al., 2005).
Taken together, the results of this study continue to demonstrate the complexity in the relationship between acute and chronic tolerance to EtOH, as reported by other laboratories (Pohorecky and Roberts, 1992; for review, Lê and Mayer, 1996). Our results show that tolerance is dependent on injection-test interval, pre-exposure history, and importantly, age. It appears that in adolescence, repeated EtOH exposure resulted in the expression of CT to ethanol-induced social impairment that appears based, in part, on the enhancement of AT. In adults, CT was presumably based on neuroadaptations separable from the expression of AT, as AT was only expressed following the repeated Sal exposure period in adults. These results demonstrate possible ontogenetic differences in the underlying neuroadaptations evoked in response to acute and chronic EtOH, and illustrate the utility of ontogenetic assessments when further exploring the dynamic relationships between these two adaptations and their underlying neural substrates (Kalant, 1993).
Acknowledgments
The research presented in this paper was supported by NIAAA grants R37 AA12525 and R01 AA018026 to Linda P. Spear and AA012453 to Elena I. Varlinskaya.
References
- Beirness D, Vogel-Sprott M. The development of alcohol tolerance: acute recovery as a predictor. Psychopharmacology. 1984;84:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Bennett RH, Cherek DR, Spiga R. Acute and Chronic Alcohol Tolerance in Humans: Effects of Dose and Consecutive Days of Exposure. Alcohol Clin Exp Res. 1993;17:740–745. doi: 10.1111/j.1530-0277.1993.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex Differences in Ethanol-induced Dopamine Release in Nucleus Accumbens and in Ethanol Consumption in Rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of Stress on the Voluntary Intake of a Sweetened Ethanol Solution in Pair-Housed Adolescent and Adult Rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Campanelli C, Dzung A, Khanna J, Kalant H. Effect of raphe lesions on the development of acute tolerance to ethanol and pentobarbital. Psychopharmacology. 1988;96:454–457. doi: 10.1007/BF02180023. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS, Winters KC, Langenbucher JW. Assessment of alcohol tolerance in adolescents. J Stud Alcohol. 2001;62:687–695. doi: 10.15288/jsa.2001.62.687. [DOI] [PubMed] [Google Scholar]
- Clark DB, Lynch KG, Donovan JE, Block GD. Health Problems in Adolescents With Alcohol Use Disorders: Self-Report, Liver Injury, and Physical Examination Findings and Correlates. Alcohol Clin Exp Res. 2001;25:1350–1359. [PubMed] [Google Scholar]
- Crabbe JC, Janowsky JS, Young ER, Kosobud A, Stack J, Rigter H. Tolerance to Ethanol Hypothermia in Inbred Mice: Genotypic Correlations with Behavioral Responses. Alcohol Clin Exp Res. 1982;6:446–458. doi: 10.1111/j.1530-0277.1982.tb05007.x. [DOI] [PubMed] [Google Scholar]
- Crawford S, Ryder D. A study of sex differences in cognitive impairment in alcoholics using traditional and computer-based tests. Drug Alcohol Depend. 1986;18:369–375. doi: 10.1016/0376-8716(86)90101-8. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors Influencing Elevated Ethanol Consumption in Adolescent Relative to Adult Rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70:387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Erwin VG, McClearn GE, Kuse AR. Interrelationships of alcohol consumption, actions of alcohol, and biochemical traits. Pharmacol Biochem Behav. 1980;13(Suppl 1):297–302. doi: 10.1016/s0091-3057(80)80045-1. [DOI] [PubMed] [Google Scholar]
- File S. Chronic exposure to noise modifies the anxiogenic response, but not the hypoactivity, detected on withdrawal from chronic ethanol treatment. Psychopharmacology. 1994;116:369–372. doi: 10.1007/BF02245342. [DOI] [PubMed] [Google Scholar]
- File SE, Andrews N, al-Farhan M, Wu PY. The role of 5-HT in the anxiogenic effects of acute ethanol withdrawal and in the long-lasting cognitive effects. Alcohol Alcohol Suppl. 1993;2:495–499. [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Gulati K. Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology. 1991;30:183–190. doi: 10.1016/0028-3908(91)90202-m. [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol Health Res World. 1997;21:149–156. [PMC free article] [PubMed] [Google Scholar]
- García-Moreno LM, Capilla A, García-Sánchez O, Luque J, Senderek K, Conejo NM, Arias JL. Alcohol tolerance in rats submitted to different periods of chronic and acute ethanol intake. Psicothema 2004 [Google Scholar]
- Grieve SJ, Littleton JM. Age and strain differences in the rat in development of functional tolerance by mice. J Pharm Pharmacol. 1979;31:696–700. doi: 10.1111/j.2042-7158.1979.tb13631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi Hy, Chen CM. Patterns of DSM-IV Alcohol Abuse and Dependence Criteria Among Adolescents and Adults: Results From the 2001 National Household Survey on Drug Abuse. Alcohol Clin Exp Res. 2005;29:810–828. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- Hiltunen A, Järbe T. Acute and chronic ethanol tolerance: operant behaviour in naive and ethanol tolerant rats. Psychopharmacology. 1992;107:511–516. doi: 10.1007/BF02245264. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Rajachandran L, Spear LP, Spear NE. Chronic administration of alcohol in the developing rat: Expression of functional tolerance and alcohol olfactory aversions. Behav Neural Biol. 1993;59:87–99. doi: 10.1016/0163-1047(93)90795-j. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 09-7401. 2009. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008; pp. 1–79. [Google Scholar]
- Kalant H. Problems in the search for mechanisms of tolerance. Alcohol Alcohol Suppl. 1993;2:1–8. [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–191. [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ, Wilson A. Accelerated development of tolerance during repeated cycles of ethanol exposure. Psychopharmacology. 1978;60:59–65. doi: 10.1007/BF00429180. [DOI] [PubMed] [Google Scholar]
- Khanna J, Kalant H, Sharma H, Chau A. Initial sensitivity, acute tolerance and alcohol consumption in fischer 344 and long evans rats. Psychopharmacology. 1991;105:175–180. doi: 10.1007/BF02244305. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Campanelli C, Lê AD, Kalant H. Effect of raphe lesions on the development of chronic tolerance to pentobarbital and cross-tolerance to ethanol. Psychopharmacology. 1987;91:473–478. doi: 10.1007/BF00216013. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Israel Y, Kalant H, Mayer JM. Metabolic tolerance as related to initial rates of ethanol metabolism. Biochem Pharmacol. 1982;31:3140–3141. doi: 10.1016/0006-2952(82)90098-3. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Weiner J, Chau A, Shah G. Ketamine retards chronic but not acute tolerance to ethanol. Pharmacol Biochem Behav. 1992;42:347–350. doi: 10.1016/0091-3057(92)90538-q. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Lê AD, LeBlanc AE, Shah G. Initial sensitivity versus acquired tolerance to ethanol in rats selectively bred for ethanol sensitivity. Psychopharmacology. 1985;86:302–306. doi: 10.1007/BF00432218. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li T-K, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex Differences in Alcohol Preference and Drinking Patterns Emerge during the Early Postpubertal Period in Sprague-Dawley Rats. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Kiianmaa K. Characteristics of ethanol tolerance in alcohol drinking (AA) and alcohol avoiding (ANA) rats. Psychopharmacology. 1988;94:479–483. doi: 10.1007/BF00212841. [DOI] [PubMed] [Google Scholar]
- Lê AD, Mayer JM. Aspects of alcohol tolerance in humans and experimental animals. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; Boca Raton, FL: 1996. pp. 251–268. [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ. Acute tolerance to ethanol in the rat. Psychopharmacology. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ, Berman ND. Acquisition and loss of tolerance to ethanol by the rat. J Pharmacol Exp Ther. 1969;168:244–250. [PubMed] [Google Scholar]
- Li T-K, Lumeng L, Doolittle D. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., II Sensitivity and Tolerance to the Hypnotic and Ataxic Effects of Ethanol in Adolescent and Adult C57BL/6J and DBA/2J Mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: Effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Miceli D, Le Magnen J. Relations between metabolic and nervous tolerance toward ethanol in naive and chronically intoxicated rats. Pharmacol Biochem Behav. 1979;10:329–334. doi: 10.1016/0091-3057(79)90192-8. [DOI] [PubMed] [Google Scholar]
- Parker CC, Ponicsan H, Spencer RL, Holmes A, Johnson TE. Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol. 2008;42:477–485. doi: 10.1016/j.alcohol.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawan GLS. Metabolism of alcohol (ethanol) in man. Proc Nutr Soc. 1972;31:83–89. [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J, Carpenter JA. Assessment of the Development of Tolerance to Ethanol Using Multiple Measures. Alcohol Clin Exp Res. 1986;10:616–622. doi: 10.1111/j.1530-0277.1986.tb05155.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Roberts P. Daily dose of ethanol and the development and decay of acute and chronic tolerance and physical dependence in rats. Pharmacol Biochem Behav. 1992;42:831–842. doi: 10.1016/0091-3057(92)90037-g. [DOI] [PubMed] [Google Scholar]
- Radlow R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology. 1994;114:1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- Ramirez LR, Varlinskaya EI, Spear LP. in press. [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Reed CL, Hood KE, Cortes DA, Jones BC. Genetic-environment analysis of sensitivity and acute tolerance to ethanol in mice. Pharmacol Biochem Behav. 2001;69:461–467. doi: 10.1016/s0091-3057(01)00520-2. [DOI] [PubMed] [Google Scholar]
- San-Marina A, Khanna JM, Kalant H. Relationship between initial sensitivity, acute tolerance and chronic tolerance to ethanol in a heterogeneous population of Swiss mice. Psychopharmacology. 1989;99:450–457. doi: 10.1007/BF00589891. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased Sensitivity to the Hypnotic Effects of Ethanol Early in Ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, Rapid, and Chronic Tolerance During Ontogeny: Observations When Equating Ethanol Perturbation Across Age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The Effects of NMDA and GABAA Pharmacological Manipulations on Acute and Rapid Tolerance to Ethanol During Ontogeny. Alcohol Clin Exp Res. 2004;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Taira T. Hangover hypothermia in rats: relation to tolerance and external stimuli. Psychopharmacology. 1988;94:161–166. doi: 10.1007/BF00176838. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Jiménez-Vasquez P, Mathé AA, Ehlers CL. Effect of ethanol on brain neuropeptides in adolescent and adult rats. J Stud Alcohol. 2005;66:46–52. doi: 10.15288/jsa.2005.66.46. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the Onset of Hypoactivity and Anxiety-Like Behavior During Alcohol Withdrawal in Adolescent and Adult Rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: Biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl. 2002;(14):71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: Rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2008. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental Differences in the Acquisition of Tolerance to Ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Melchior CL, Hoffman PL. Commentary on ethanol tolerance. Alcohol Clin Exp Res. 1982;6:252–259. doi: 10.1111/j.1530-0277.1982.tb04971.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF. Acute tolerance in inbred and selected lines of mice. Drug Alcohol Depend. 1979;4:87–90. doi: 10.1016/0376-8716(79)90043-7. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF, Raju TS, Deitrich RA. Characterization of acute and chronic tolerance in mice selected for inherent differences in sensitivity to ethanol. Alcohol Clin Exp Res. 1980;4:70–73. doi: 10.1111/j.1530-0277.1980.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME. Effect of a dose of ethanol on acute tolerance and ethanol consumption in alcohol drinker (UChB) and non-drinker (UChA) rats. Addiction Biology. 2002;7:279–284. doi: 10.1080/13556210220139488. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Pee JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute Effects of Ethanol on Social Behavior of Adolescent and Adult Rats: Role of Familiarity of the Test Situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute Ethanol Withdrawal (Hangover) and Social Behavior in Adolescent and Adult Male and Female Sprague-Dawley Rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in Sensitivity to Ethanol-Induced Social Facilitation and Social Inhibition from Early to Late Adolescence. Ann N Y Acad Sci. 2004;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: Social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Are the sobering effects of stress associated with increases in acute tolerance?. Poster presented at the annual meeting of the Research Society on Alcoholism; San Diego, CA. 2009. [Google Scholar]
- Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time Course of Elevated Ethanol Intake in Adolescent Relative to Adult Rats Under Continuous, Voluntary-Access Conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex Differences in Ethanol-Induced Hypnosis and Hypothermia in Young Long-Evans Rats. Alcohol Clin Exp Res. 2002;26:695–704. [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wu PH, Coultrap S, Browning MD, Proctor WR. Correlated changes in NMDA receptor phosphorylation, functional activity, and sedation by chronic ethanol consumption. Journal of Neurochemistry. 2010;115:1112–1122. doi: 10.1111/j.1471-4159.2010.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]