Abstract
A number of studies have found a disjunction between women’s attention to, and memory for, handsome men. Although women pay initial attention to handsome men, they do not remember those men later. The present study examines how ovulation might differentially affect these attentional and memory processes. We found that women near ovulation increased their visual attention to attractive men. However, this increased visual attention did not translate into better memory. Discussion focuses on possible explanations, in the context of an emerging body of findings on disjunctions between attention to, and memory for, other people.
Keywords: Attention, Memory, Ovulation, Fertility, Menstrual cycle, Evolutionary psychology
Introduction
On entering a crowded room, to whom do we pay attention? Who do we later remember? A number of studies suggest that simple social cognitive processes are often biased in functionally sensible ways (e.g., Ackerman et al., 2009; Becker, Kenrick, Neuberg, Blackwell, & Smith, 2007; Maner et al., 2005). Some of this research suggests sex differences in such processing. For instance, whereas men pay attention to, selectively encode, and selectively remember physically attractive women, women attend to, but do not later remember, handsome men (Becker, Kenrick, Guerin, & Maner, 2005; Maner et al., 2003).
These findings make sense in terms of typical male and female mating strategies: whereas men are interested in, and relatively nonselective about, possible relationships with female strangers, women have generally higher standards for casual relationships and are less inclined to have such relationships with male strangers (e.g., Clark & Hatfield, 1989; Greitemeyer, 2005; Kenrick, Sadalla, Groth, & Trost, 1990; Li & Kenrick, 2006; Wiederman & Hurd, 1999). For women, the relative costs of casual relationships are higher than they are for men. In particular, a short-term relationship could result in pregnancy, which brings necessarily high costs for women, but not necessarily for men. Although there is some value in noticing physically attractive male strangers, it might not generally be a good use of cognitive resources for women to extensively process such men in the absence of indicators of other desirable characteristics (Kenrick, Delton, Robertson, Becker, & Neuberg, 2007).
There might, however, be an exception to the above generalizations. Women adopt different mating strategies under different circumstances (Buss & Schmitt, 1993; Gangestad & Simpson, 2000; Haselton & Miller, 2006), and an emerging literature reveals that hormonal fluctuations near ovulation alter women’s mating preferences and behaviors in important ways. Compared to other points in their menstrual cycle, women near ovulation dress more attractively and provocatively (Haselton, Mortezaie, Pillsworth, Bleske-Recheck, & Frederick, 2007). Ovulating women are more attracted to men showing high levels of masculinity (e.g.,Penton-Voak et al., 2003) and signs of creativity (Haselton & Miller, 2006). They also prefer the scent of symmetrical men (Gangestad & Thornhill, 1998). Most critically, women in the most fertile part of their cycle are more interested in extra-pair sexual relations, particularly with men more attractive than their long-term partners (Pillsworth & Haselton, 2006).
Why would women, even those with partners, be especially interested in attractive men during ovulation? Symmetry, high masculinity, and creative displays, much like colorful and symmetrical displays in peacocks, appear to reflect the possession of genetic traits well-suited to survival (Haselton & Miller, 2006). When choosing a mate, then, females may face trade-offs between mates who will stay around and provide resources versus those who, because of their attractiveness, may have more opportunities to stray. A casual liaison with an attractive man is thus a double-edged sword: although it is a means of acquiring beneficial genes for off-spring, it risks the loss of a (less attractive but more committed) partner willing to provide resources. One strategy for balancing this trade-off is to engage in temporally limited and concealed extra-pair liaisons with highly attractive males during the period of maximal fertility (Gangestad, Thornhill, & Garver-Apgar, 2005; Haselton & Miller, 2006). Of course, none of this is presumed to be consciously mediated, and cyclic effects are not found for women on hormonal birth control (which changes normal hormonal patterns).
Despite evidence of ovulatory shifts in overt behavior and expressed preferences, few researchers have explored how ovulation affects early-stage cognitive processing. During ovulation, it might be expected that women pay increased attention to handsome men. Given that highly fertile women are more attracted to, and more interested in mating with, highly attractive men, we predicted that women near ovulation would spend more time attending to attractive men than those in less fertile periods.
Will this extra attention for attractive men translate into better memory? The default assumption would be that increased visual attention to any target will increase memory for that target. Given that highly fertile women are more interested in short-term sexual encounters with men of high genetic quality, they may be expected to spend extra cognitive resources subsequently processing the faces of attractive men to more carefully evaluate cues linked to genetic quality (e.g., facial masculinity, symmetry). In a similar vein, they may expend extra cognitive resources assessing the faces of attractive men for cues suggestive of additional desirable characteristics (e.g., resources, dependability, trustworthiness)—characteristics suggesting that the man also possesses long-term relationship potential. In both cases, such enhanced processing should lead to enhanced memory.
On the other hand, it is not always the case that increased visual attention translates into increased memory. For example Rock and Gutman (1981) had participants look at sets of two overlapping figures while searching for a particular feature. While the task required that participants look at both figures, they later showed poor memory for the figure that lacked the target feature. In another study, participants playing a computerized version of the matching game Concentration were asked to match pairs of faces in a matrix after having the opportunity to look at all faces before they were masked. Female participants gave evidence of having initially attended to the handsome faces, as judged by performance on the first trial. However, unlike performance for attractive female faces, which remained efficient throughout the game, efficiency for matching attractive male faces decreased over trials (Becker et al., 2005).
In the case of ovulating women, there are several reasons why enhanced looking might not translate into enhanced memory. Because of the costs associated with liaisons with unfamiliar men, for example, there could be some suppression of deeper subsequent cognitive processing. Alternatively, increased visual attention to attractive men could serve functions that are not fundamentally about cognitive processing and thus would not translate into increased memory. For example, visual attention is a tactic used by women to communicate interest and encourage men to approach (Moore, 1985). If increased looking serves a non-cognitive function, we would not expect it to contribute to increased memory. In the present study, we measured effects of fertility on visual attention to faces varying on attractiveness and gender using an eye tracking device, and also tested women’s memory for those faces.
Method
One hundred twelve females in an Introductory Psychology course participated in exchange for partial fulfillment of course requirements. Prescreening questionnaires excluded those using hormonal birth control or indicating highly irregular cycle length. Equipment malfunctions and calibration difficulties rendered eye tracking data from 22 participants unusable, leaving a final sample of 90 participants. These participants were classified as high fertility (N = 24) or low fertility (N = 66) based on information they provided about their menstrual cycle (see below).
To minimize the possibility that participants would consciously try to control eye movements, they were told the study investigated visual and auditory perception using a portable electroencephalograph; the apparent electroencephalograph was actually a headband containing magnetic sensors that allowed the Applied Science Laboratories Series 5000 eye tracker to reduce eye-capture loss. After calibrating the eye tracking software, participants viewed a slide show consisting of four slides. Each slide contained eight faces (two exemplars each of the factorial combination of male/female and attractive/average) in a roughly circular array. These faces were neutrally-expressive, White young adults, and were pre-rated for physical attractiveness for an earlier study. More detailed information can be found in Maner et al. (2003). Each slide appeared for 10 s with a 2 s break between slides.
Participants next completed the memory test. The memory test consisted of the 32 faces from the slide show and 32 distracter faces also varying on gender and attractiveness. Participants indicated whether they had seen each face on a six-point scale ranging from “Definitely did not see” to “Definitely did see.”
At the end of the study, participants provided information about their menstrual cycle length and regularity and were asked to email researchers the date of their next menses onset. To determine fertility phase, we employed the reverse-cycle day method (cf. Haselton & Miller, 2006). The 5 days prior to ovulation and the day of ovulation itself (i.e., reverse count days 15–20) are considered high fertility days, while the remaining days are considered low fertility. We based our fertility calculation on the most recent cycle information available. For 50 participants, this information came from the requested follow-up email. The other 40 participants did not contact us at the start of their next menses, so we used the information they provided during the experimental session. Although the reverse-cycle day method is less precise than hormonal methods of measuring fertility, women in the high fertility group will be, on average, more fertile than those in the low fertility group. Any errors in categorization worked against our hypotheses.
Results
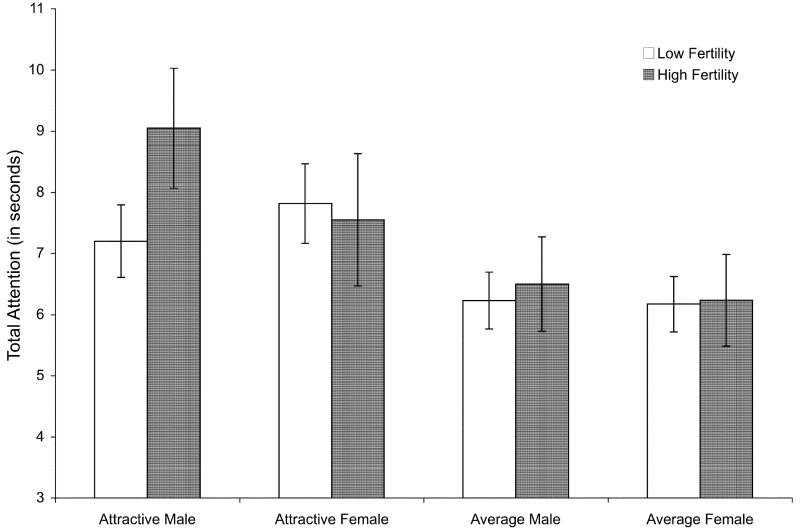
To test the effects of fertility on attention to faces, we conducted a mixed ANOVA on the total attention to each face type with fertility as a between-subjects factor and target gender and attractiveness as within-subjects factors. Overall, there was a main effect of target attractiveness, F(1, 88) = 44.21, p < .001, = .33, such that participants paid more attention to attractive targets (M = 7.90 seconds, SD = 2.05) than average targets (M = 6.28 seconds, SD = 1.92).
This attractiveness main effect was qualified, however, by a three-way interaction of target gender, target attractiveness, and fertility, F(1, 88) = 4.98, p = .028, ; see Fig. 1. The two-way interaction between fertility and target gender was significant within attractive targets, F(1, 88) = 6.15, p = .015, , but not within average targets (F < .3). As expected, high fertility women paid more attention to attractive male targets than did low fertility women, F(1, 88) = 10.28, p = .002, ; fertility had no effect on attention to other face types (all Fs < .40, ps > .56). High fertility women paid more attention to men than women, F(1, 88) = 4.15, p = .045, ; this effect was not found in low fertility women. Both high and low fertility women paid more attention to attractive than average men; this effect was larger for high fertility women, t(33.81) = 2.367, p = .024. Additionally, high fertility women paid more attention to attractive males than attractive females, F(1, 88) = 4.22, p = .043, .
Fig. 1.
Mean time spent looking at each face type. Error bars represent 95% CI. The apparent main effect of fertility status is not statistically significant.
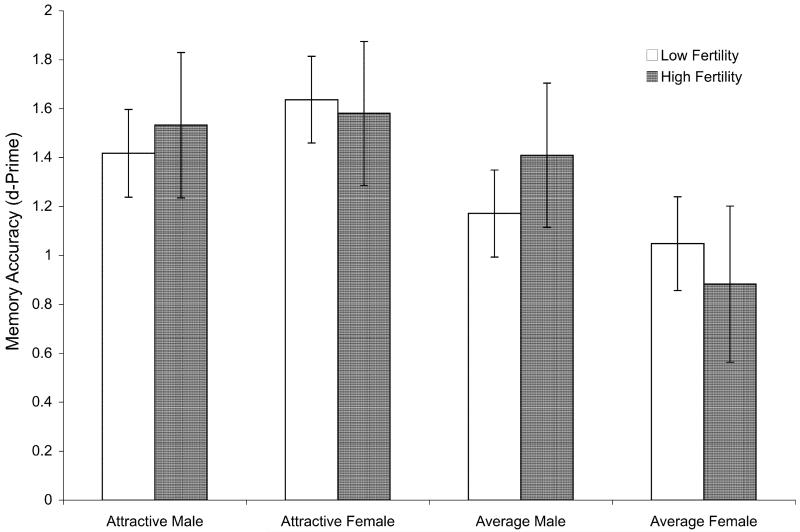
To test the effects of fertility on memory, we first dichotomized participant responses into either “Did not see” or “Did see.” Using these scores, we calculated d-prime (a measure of recognition sensitivity that controls for false alarms) for each face type (e.g., for all attractive male faces). We then conducted a mixed ANOVA on the d-prime scores with fertility as a between-subjects factor and target gender and attractiveness as within-subjects factors. Overall, attractive faces were remembered better than average, F(1, 88) = 26.95, p < .001, ; see Fig. 2.
Fig. 2.
Memory accuracy for each face type. Error bars represent 95% CI.
The significant three-way interaction between target gender, target attractiveness, and fertility status found in the attention data was not replicated in the memory data, F(1, 88) = .80, p = .373. However, the two-way interaction between target gender and target attractiveness was significant, F(1, 88) = 12.369, p = .001, : Attractive women were remembered significantly better than average women, F(1, 88) = 44.65, p < .001, but attractive men were remembered only marginally better than average men, F(1, 88) = 2.80, p = .095. Fertility status did not significantly affect memory within any target type (ps > .17).
Discussion
Using an eye tracking device, we found that ovulating women paid relatively more attention to the attractive male targets in arrays of varying faces. However, fertility status had no effect on attention to other face types, and did not produce an analogous effect on memory.
What function is served by fertility-enhanced attention to attractive men? Recall that ovulating women, in particular, perceive such men to be relatively more desirable (e.g., Haselton & Miller, 2006; Penton-Voak et al., 2003). This fertility-enhanced visual attention may thus reflect a more thorough cognitive assessment of these men. However, fertility status did not enhance subsequent recognition memory for these handsome men, mitigating against this cognitive processing explanation. As we mentioned earlier, women face costs as well as benefits from liaisons with unfamiliar attractive men, and those costs may be enhanced during ovulation. Although it may be difficult to monitor initial visual attention to such men, there may be mechanisms in place for suppressing additional processing, especially when such men are strangers (Kenrick et al., 2007). Alternatively, because eye contact serves to nonverbally signal romantic interest (Moore, 1985), perhaps the increased visual attention by highly fertile women reflected not extended cognitive processing but rather a strategic (albeit nonconscious) inclination to communicate romantic interest to desirable men. That ovulating women exhibited especially enhanced looking at, but not especially enhanced memory for, handsome men, is consistent with that possibility. Future research might profitably explore these alternatives in more detail.1 Research measuring both implicit and explicit memory, for example, may provide evidence for or against a suppression explanation of the decoupling of attention and memory. Moreover, if the extra attention truly serves a flirtation function, we might expect this attention to be focused on the targets’ eyes, whereas other functions would be supported by a broader visual scanning of the faces. Alternatively, if these effects are linked to suppression effects, we might expect to see them more strongly in women involved in highly committed relationships. Stronger effects for single (or less committed) individuals, in contrast, would support a flirtation explanation.
This finding links to a broader set of findings indicating adaptively tuned discrepancies between visual attention and memory (Ackerman et al., 2009; Becker et al., 2010). For example, in contrast to desirable targets such as attractive faces, which are looked at but not remembered, people look away from physically threatening targets, such as outgroup males, but nonetheless remember them well later (Becker et al., 2010).
The results for attractive females contrast with those for attractive males. Replicating earlier findings (e.g., Maner et al., 2003), women, whether ovulating or not, showed initial attention to, as well as good memory for, attractive female targets. This increased attention to beautiful women makes sense to the extent that attractive women can be viewed as threatening others’ existing relationships, given men’s interest and attention to even unfamiliar attractive women (Li & Kenrick, 2006; Maner et al., 2003). Unlike the possible costs associated with downstream cognitive processing of handsome men, then, no such costs are associated with processing other women.
More broadly, these findings lend further support to the growing appreciation that perceptual and cognitive biases of various kinds often serve functionally sensible aims (e.g., Kenrick, Neuberg, Griskevicius, Becker, & Schaller, 2010). Finally, it is useful to note that ovulation status lies outside the theoretical architecture of traditional social psychological theories of relationships. As such, these data combine with other findings demonstrating important effects of various hormones (e.g., Durante & Li, 2009; Miller & Maner, 2010; Roney & Simmons, 2008) to illustrate the value of generating integrative, biosocial models of social cognition.
Acknowledgment
The contributions of Douglas Kenrick and Steven Neuberg were supported by a grant from the National Institute of Mental Health (R01MH064734).
Footnotes
One might wonder whether the extra 1.8 s of attention given to attractive males (compared to average) by ovulating women, divided among the eight attractive male exemplars, is insufficient to increase memory. Other data from this study suggests, however, that this concern is unfounded. Ignoring ovulation condition, attractive females received only 1.48 s more attention than average females, yet they were still remembered better, suggesting that relatively small increases in attention can have significant effects on memory—but not when ovulating women are attending to attractive men.
References
- Ackerman JM, Becker DV, Mortensen CR, Sasaki T, Neuberg SL, Kenrick DT. A pox on the mind: Disjunction of attention and memory in the processing of physical disfigurement. Journal of Experimental Social Psychology. 2009;45:478–485. doi: 10.1016/j.jesp.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DV, Anderson US, Neuberg SL, Maner JK, Shapiro JR, Ackerman JM, et al. More memory bang for the attentional buck: Self-protection goals enhance encoding efficiency for potentially threatening males. Social Psychological and Personality Science. 2010;1:182–189. doi: 10.1177/1948550609359202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DV, Kenrick DT, Guerin S, Maner JK. Concentrating on beauty: Sexual selection and sociospatial memory. Personality & Social Psychology Bulletin. 2005;31:1643–1652. doi: 10.1177/0146167205279583. [DOI] [PubMed] [Google Scholar]
- Becker DV, Kenrick DT, Neuberg SL, Blackwell KC, Smith DM. The confounded nature of angry men and happy women. Journal of Personality and Social Psychology. 2007;92:179–190. doi: 10.1037/0022-3514.92.2.179. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: A contextual evolutionary analysis of human mating. Psychological Review. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Clark RD, Hatfield E. Gender differences in receptivity to sexual offers. Journal of Psychology and Human Sexuality. 1989;2:39–55. [Google Scholar]
- Durante KM, Li NP. Oestradiol and opportunistic mating in women. Biology Letters. 2009;5:179–182. doi: 10.1098/rsbl.2008.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences. 2000;23:573–644. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R. Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society of London B. 1998;265:727–733. doi: 10.1098/rspb.1998.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R, Garver-Apgar CE. Adaptations to ovulation implications for sexual and social behavior. Current Directions in Psychological Science. 2005;14:312–316. [Google Scholar]
- Greitemeyer T. Receptivity to sexual offers as function of sex, socioeconomic status, physical attractiveness, and intimacy of the offer. Personal Relationships. 2005;12:373–386. [Google Scholar]
- Haselton MG, Miller GF. Women’s fertility across the cycle increases the short-term attractiveness of creative intelligence. Human Nature. 2006;17:50–73. doi: 10.1007/s12110-006-1020-0. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Recheck AE, Frederick DA. Ovulation and human female ornamentation: Near ovulation, women dress to impress. Hormones and Behavior. 2007;51:41–45. doi: 10.1016/j.yhbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Kenrick DT, Delton AW, Robertson T, Becker DV, Neuberg SL. How the mind warps: A social evolutionary perspective on cognitive processing disjunctions. In: Forgas JP, Haselton MG, Von Hippel W, editors. The evolution of the social mind: Evolution and social cognition. Psychology Press; New York: 2007. pp. 49–68. [Google Scholar]
- Kenrick DT, Neuberg SL, Griskevicius V, Becker DV, Schaller M. Goal-driven cognition and functional behavior: The fundamental motives framework. Current Directions in Psychological Science. 2010;19:63–67. doi: 10.1177/0963721409359281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick DT, Sadalla EK, Groth G, Trost MR. Evolution, traits, and the stages of human courtship: Qualifying the parental investment model. Journal of Personality. 1990;58:97–117. doi: 10.1111/j.1467-6494.1990.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Li NP, Kenrick DT. Sex similarities and differences in preferences for short-term mates: What, whether, and why. Journal of Personality and Social Psychology. 2006;90:468–489. doi: 10.1037/0022-3514.90.3.468. [DOI] [PubMed] [Google Scholar]
- Maner J, Kenrick DT, Becker D, Delton A, Hofer B, Wilbur C, et al. Sexually selective cognition: Beauty captures the mind of the beholder. Journal of Personality and Social Psychology. 2003;85:1107–1120. doi: 10.1037/0022-3514.85.6.1107. [DOI] [PubMed] [Google Scholar]
- Maner JK, Kenrick DT, Becker DV, Robertson TE, Hofer B, Neuberg SL, et al. Functional projection: How fundamental social motives can bias interpersonal perception. Journal of Personality and Social Psychology. 2005;88:63–78. doi: 10.1037/0022-3514.88.1.63. [DOI] [PubMed] [Google Scholar]
- Miller SL, Maner JK. Scent of a woman: Men’s testosterone responses to olfactory ovulation cues. Psychological Science. 2010;21:276–283. doi: 10.1177/0956797609357733. [DOI] [PubMed] [Google Scholar]
- Moore MM. Nonverbal courtship patterns in women: Context and consequences. Ethology and Sociobiology. 1985;6:237–247. [Google Scholar]
- Penton-Voak IS, Little AC, Jones BC, Burt DM, Tiddeman BP, Perrett DJ. Female condition influences preferences for sexual dimorphism in faces of male humans (Homo sapiens) Journal of Comparative Psychology. 2003;117:264–271. doi: 10.1037/0735-7036.117.3.264. [DOI] [PubMed] [Google Scholar]
- Pillsworth EG, Haselton MG. Male sexual attractiveness predicts differential ovulatory shifts in female extra-pair attraction and male mate retention. Evolution and Human Behavior. 2006;27:247–258. [Google Scholar]
- Rock I, Gutman D. The effect of inattention on form perception. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:275–285. doi: 10.1037//0096-1523.7.2.275. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Women’s oestradiol predicts preference for facial cues of men’s testosterone. Hormones and Behavior. 2008;53:14–19. doi: 10.1016/j.yhbeh.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Wiederman MW, Hurd C. Extradyadic involvement during dating. Journal of Social and Personal Relationships. 1999;16:265–274. [Google Scholar]