Abstract
One dimension of understanding the functions of the prefrontal cortex is knowledge of cortical connectivity. We have surveyed three aspects of prefrontal cortical connections: local projections (within the frontal lobe), the termination patterns of long association (post-Rolandic) projections, and the trajectories of major fiber pathways. The local connections appear to be organized in relation to dorsal (hippocampal origin) and ventral (paleocortical origin) architectonic trends. According to the proposal of a dual origin of the cerebral cortex, cortical areas can be traced as originating from archicortex (hippocampus) on the one hand, and paleocortex, on the other hand, in a stepwise manner (e.g., Sanides, 1969; Pandya and Yeterian, 1985). Prefrontal areas within each trend are connected with less architectonically differentiated areas, and, on the other hand, with more differentiated areas. Such organization may allow for the systematic exchange of information within each architectonic trend. The long connections of the prefrontal cortex with post-Rolandic regions seem to be organized preferentially in relation to dorsal and ventral prefrontal architectonic trends. Prefrontal areas are connected with post-Rolandic auditory, visual and somatosensory association areas, and with multimodal and paralimbic regions. This long connectivity likely works in conjunction with local connections to serve prefrontal cortical functions. The afferent and efferent connections of the prefrontal cortex with post-Rolandic regions are conveyed by specific long association pathways. These pathways as well appear to be organized in relation to dorsal and ventral prefrontal architectonic trends. Finally, although prefrontal areas have preferential connections in relation to dual architectonic trends, it is clear that there are interconnections between and among areas in each trend, which may provide a substrate for the overall integrative function of the prefrontal cortex. Prefrontal corticocortical connectivity may help to elucidate both region-specific and integrative perspectives on the functions of the prefrontal cortex.
Keywords: prefrontal cortex, local connections, long association connections, fiber pathways, architectonic trends
The frontal lobe in primates comprises three main sectors: motor cortex, premotor cortex, and the prefrontal region. Whereas the premotor and motor cortical regions are closely related to motor programming and movement production per se, the prefrontal region is neither purely motor nor sensory but rather underlies higher-order control processes of cognition and emotion (e.g., Fuster, 2008; Stuss and Knight, 2002). The role of the prefrontal cortex in executive processing is of course effected by means of the interaction of the various prefrontal cortical areas with post-Rolandic association cortical areas. The present review will focus on the connectional anatomy of the prefrontal cortex in macaque monkeys, which underlies the diverse functions of this cortical region. Recent investigations in humans have demonstrated the relevance of the connectional anatomy of the prefrontal cortex in nonhuman primates to the organization of the human brain (e.g., Kelly et al., 2010). Some of these findings will be considered in the final portion of this review.
The prefrontal cortex has been the focus of intensive investigation for well over a century. In the nineteenth century researchers such as Harlow (1848, 1868), Broca (1865), Ferrier (1876), and Bianchi (1895) conducted clinical as well as experimental studies of the role of the frontal cortex in behavior, cognition and emotion. During the nineteenth and twentieth century, the terms “frontal lobe syndrome” and “frontal lobe personality” came to include a constellation of behavioral changes, including disinhibition, impaired judgment, unpredictability, altered emotionality, apathy, loss of motivation, and abnormal executive function (e.g., Luria, 1973; Meyer, 1974). By the mid-twentieth century, numerous investigators had attempted to relate particular functions to specific portions of the prefrontal cortex in both human and nonhuman primates (e.g., Benton, 1991; Luria, 1973; Meyer, 1974; Warren and Akert, 1964). In the late twentieth century, greater insight regarding the specificity of prefrontal functions was provided by the development of new brain imaging techniques and increasingly sophisticated neuropsychological methodologies (see, e.g., Fuster, 2008 for review). These approaches have added significantly to our knowledge of the workings of the prefrontal cortex, yet much remains to be learned.
At the same time, the prefrontal cortex has been examined from a morphological perspective, i.e., in terms of cortical architecture and connections. Some investigations have focused on the anatomical demarcation of distinct prefrontal cortical areas (e.g., Barbas and Pandya, 1989; Bonin and Bailey, 1947; Brodmann, 1909; Carmichael and Price, 1994; Economo and Koskinas, 1925; Mai et al., 2008; Petrides and Pandya, 1994; Sanides, 1969; Sarkissov et al., 1955; Vogt and Vogt, 1919; Walker, 1940). Others have detailed the connectional relationships of prefrontal areas (e.g., Barbas and Pandya, 1989; Carmichael and Price, 1995a, 1995b; Cavada and Goldman-Rakic, 1989; Jones and Powell, 1970; Kuypers et al., 1965; Nauta, 1964; Pandya and Kuypers, 1969; Pandya et al., 1971; Petrides and Pandya, 1999, 2002a, 2002b, 2006, 2007, 2009; Schall et al., 1995; Stanton et al., 1995). Many of these connectional studies have concentrated on a single area, or a limited number of areas, within the context of a specific architectonic parcellation map of the prefrontal cortex. A smaller number of studies have examined prefrontal cortical connectivity from the perspective of an overall architectonic-connectional framework (e.g., Barbas and Pandya, 1989; Pandya and Yeterian, 1996).
It has been well established that the prefrontal cortex consists of a large number of distinct areas with varied architecture and connections. The question arises whether there is an overall architectonic-connectional theme that underpins the organization of the prefrontal cortex. A detailed comparison of human and macaque monkey prefrontal cortical cytoarchitecture has recently been provided by Petrides and Pandya (1994, 1999, 2002a). This comparison facilitates the translation of macaque monkey connectional findings to the human prefrontal cortex (see Petrides et al., 2011 in this issue).
The issue of the extent to which architecture is comparable between the monkey and the human frontal cortex is dealt in the companion paper by Petrides et al. (this issue) that examines comparative architecture and also considers the problem of drawing inferences between the human and the macaque monkey brain using different methodologies: diffusion tensor imaging and resting functional connectivity in the human and direct anatomical tracing using anterograde and retrograde tracers in the monkey. Many of the details of macaque monkey neuroanatomy remain to be validated in the human brain and this is a complex issue as discussed by Petrides et al. in this issue. Nevertheless, the monkey neuroanatomy provides important hypotheses to be tested and validated in the human brain using the available imaging methodologies.
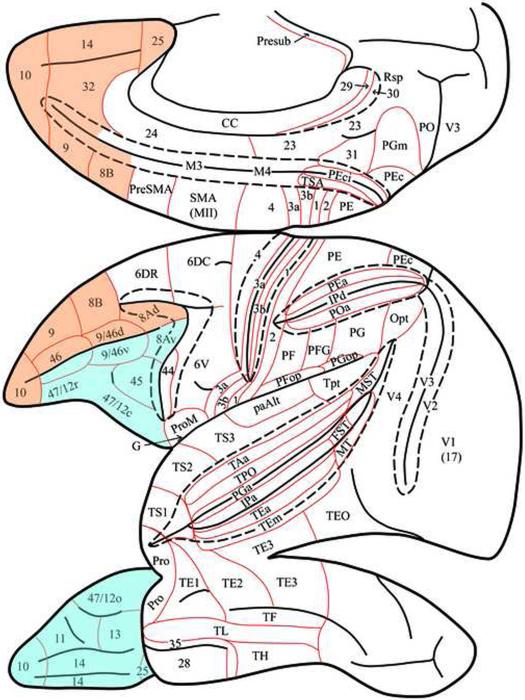
In the present review, we focus on the afferent and efferent cortical connectivity of the prefrontal cortex. We have drawn primarily from experimental studies in our laboratory while at the same time synthesizing data from many other investigations of prefrontal cortical connectivity. Collectively, these studies have employed both anterograde (e.g., autoradiography and biotinylated dextran amines) and retrograde (e.g., horseradish peroxidase and fluorescent dyes) tracing methods. In describing the connectivity of the prefrontal cortex we have used the parcellation and nomenclature of Petrides and Pandya (1994, 2007) for specific prefrontal areas (Fig. 1). For the remainder of the cerebral hemisphere, we have defined the cytoarchitectonic areas of the posterior parietal cortex according to Pandya and Seltzer (1982), the superior temporal gyrus according to Pandya and Sanides (1973), the inferotemporal cortex and the cortex of the superior temporal sulcus according to Seltzer and Pandya (1978), the posterior parahippocampal gyrus according to Rosene and Pandya (1983), and the cingulate cortex and retrosplenial cortex according to Vogt et al. (1987).
Figure 1.
Schematic representations of the medial, lateral, and ventral surfaces of the cerebral cortex of the rhesus monkey (Macaca mulatta) according to Petrides and Pandya (1994, 2007). The architectonic areas depicted in these diagrams are based on the findings of several investigators: occipital (Paxinos et al., 2000), parietal (Pandya and Seltzer, 1982), inferotemporal and superior temporal sulcal (Seltzer and Pandya, 1978; Ungerleider and Desimone, 1986), superior temporal gyrus and supratemporal plane (Pandya and Sanides, 1973; Krubitzer and Kaas, 1990); insular, parietotemporal opercular and frontotemporal opercular (Jones and Burton, 1976; Mesulam and Mufson, 1982; Krubitzer and Kaas, 1990; Krubitzer et al., 1995), premotor (Barbas and Pandya, 1987), prefrontal (Petrides and Pandya, 1994), cingulate (Vogt et al., 1987), and parahippocampal (Blatt et al., 2003) regions. Areas of the dorsal prefrontal (archicortical) architectonic trend are shown in orange, and those of the ventral prefrontal (paleocortical) architectonic trend are shown in blue.
Before considering the cortical connectivity of the prefrontal cortex, it is important to note that what follows represents a collation of data from numerous studies in monkeys involving diverse methodologies with relative advantages and limitations, and differing architectonic schemas. Thus, while offering an overview and perspective on prefrontal corticocortical relationships, the present discussion is inherently limited by the differences in the data from which it is drawn. Moreover, we do realize that the functions of the prefrontal cortex depend on subcortical connectivity as well as on corticocortical relationships. although a consideration of prefrontal-subcortical relationships is beyond the scope of the present review. In this regard, the importance of integrating subcortical and cortical connectivity in attempting to understand the functions of the cerebral cortex has been addressed in detail recently by Schmahmann and Pandya (2008) and Parvizi (2009).
Local Connectivity of the Prefrontal Cortex
There are several architectonically distinct areas in the prefrontal region. These areas have specific laminar characteristics, ranging from those with a relatively rudimentary type of cell distribution and layering (e.g., areas 25 and 13) to those with six highly differentiated cortical layers (e.g., areas 9/46 and 8A). Moreover, it has been suggested that within the prefrontal cortex there are two specific architectonic trends, within which four major subdivisions have been identified. The most primitive type of cortical subdivision, with three cortical layers, is termed allocortex. The cortex immediately surrounding the allocortex is designated as periallocortex, with relatively undifferentiated neuronal distribution among its layers. Adjoining the periallocortex is a ring of cortices which show an incipient laminar pattern and are termed proisocortex. From the proisocortex can be traced the so-called iso- or neocortex, with a well defined, six-layered pattern of lamination. In the frontal lobe, a dorsal trend originates from the medial prefrontal allocortex and encompasses the medial and dorsolateral prefrontal regions, and a ventral trend originates from the caudal orbital allocortex and involves the ventral and ventrolateral prefrontal regions. Prefrontal areas within each architectonic trend appear to be connected to neighboring cortical areas within the frontal lobe. More specifically, each area is connected, on the one hand, to one or more increasingly architectonically differentiated cortical regions, and, on the other hand, to one or more less differentiated regions (e.g., Barbas and Pandya, 1989; Pandya and Yeterian, 1985).
The local connections of the medial and dorsolateral prefrontal regions
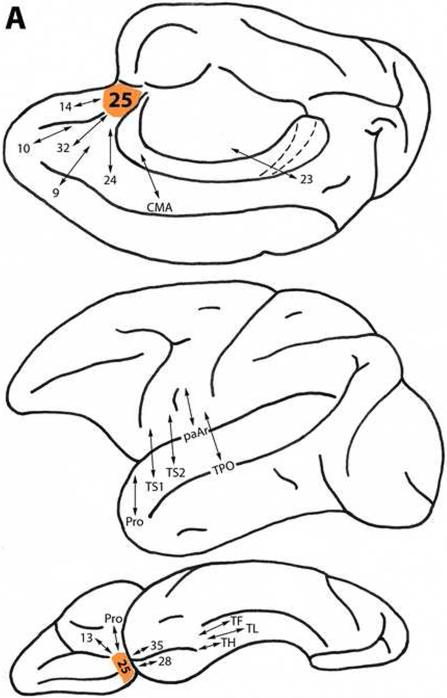
Area 25
This area is located in the ventromedial prefrontal region, below the genu of the corpus callosum (Fig. 1). It is connected with more differentiated dorsal trend prefrontal areas 32, 14, 10 and 9, and with area 24 and the cingulate motor area (CMA) primarily on the medial surface of the frontal lobe. Area 25 also is connected with the ventral trend paralimbic prefrontal areas, proisocortex (Pro) and area 13 on the orbital frontal surface (Fig. 2A) (Arikuni et al., 1994; Barbas and Pandya, 1989; Carmichael and Price, 1996a; Morecraft and Van Hoesen, 1998; Pandya et al., 1981; Petrides and Pandya, 2007; Vogt and Pandya, 1987).
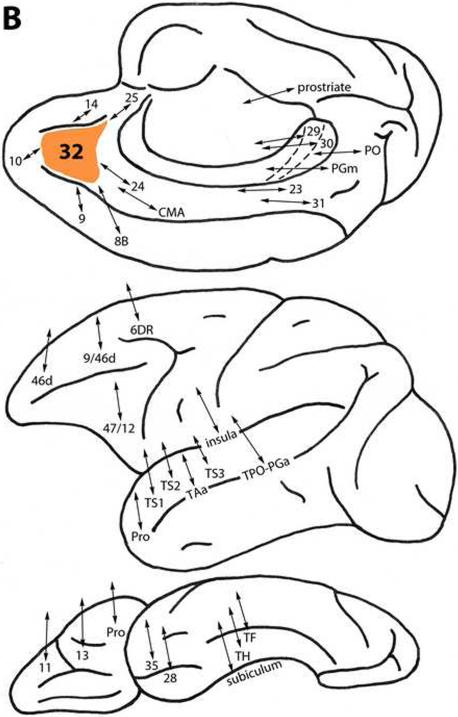
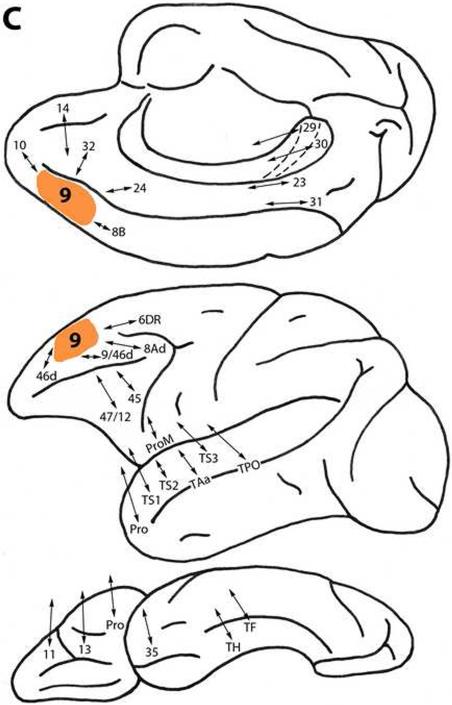
Figure 2.
A. Diagrammatic representations of the medial, lateral and ventral surfaces of the macaque monkey cerebral hemisphere showing bidirectional local (within the frontal lobe) and long (post-Rolandic) association connections of areas (shown in orange) in the dorsal prefrontal architectonic trend. A: Area 25. B: Area 32. C: Area 9. D: Area 8B. E: Area 10. F: Area 46d. G: Area 9/46d. H: Area 8Ad. Note that in these diagrams and in those in Figure 3, the arrows indicate the connectivity of the different prefrontal areas of interest and not the specific trajectory of those connections.
Area 32
This area is located in the medial prefrontal region, dorsal to area 25 and rostral to area 24 (Fig.1). Like area 25, it is connected with more differentiated dorsal trend areas 14, 10 and 9 medially, and 46d and 9/46d on the lateral surface. Area 32 also is connected with less differentiated areas 24 and 25, and with the cingulate motor area (CMA), area 8B and the dorsal premotor cortex (area 6DR). Additionally, area 32 is related to ventral trend area 47/12, and orbital areas Pro, 13 and 11 (Fig. 2B) (Arikuni et al., 1994; Barbas, 1988; Barbas and Pandya, 1989; Barbas et al., 1999; Carmichael and Price, 1995, 1996a; Morecraft and Van Hoesen, 1998; Pandya et al., 1981; Petrides and Pandya, 2007; Saleem et al., 2008; Vogt and Pandya, 1987).
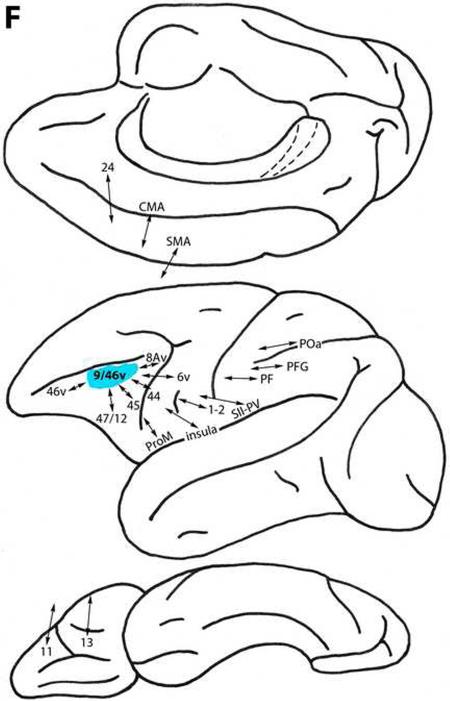
Area 9
This area is located dorsomedially above the cingulate sulcus and extends onto the dorsolateral surface (Fig. 1). Area 9 is connected with less differentiated dorsal trend areas 32 and 14, as well as with area 24 on the medial surface. It is related to more differentiated dorsal trend areas 8B, 10, 46d, 9/46d, 8Ad and 6DR. Area 9 is also connected with ventral trend areas 45, 47/12 and ProM, ventrolaterally, and areas Pro, 13, and 11 on the orbital frontal surface (Fig. 2C) (Arikuni et al., 1994; Barbas and Pandya, 1989; Barbas et al., 1999; Bates and Goldman-Rakic, 1993; Carmichael and Price, 1996; Gerbella et al., 2010; Huerta and Kaas, 1990; Pandya et al., 1981; Petrides and Pandya, 1999, 2002a, 2007; Vogt and Pandya, 1987; Vogt et al., 1979).
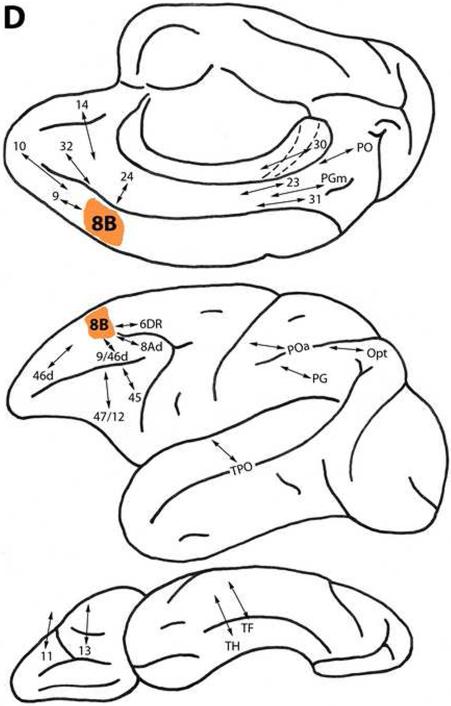
Area 8B
This area is located dorsomedially above the cingulate gyrus and caudal to area 9, and extends onto the lateral surface (Fig. 1). It is connected with less differentiated dorsal trend areas 14 and 32. Area 8B is also connected with areas 10 and 9, as well as with area 24 on the medial surface and area 6DR dorsolaterally. Like area 9, it is connected with more differentiated dorsal trend areas 46d, 9/46d and 8Ad. Area 8B is related to ventral trend areas 47/12 and 45 ventrolaterally, and 11 and 13 on the orbital surface (Fig. 2D) (Arikuni et al., 1988, 1994; Barbas and Mesulam, 1981, 1985; Barbas and Pandya, 1987, 1989; Gerbella et al., 2010; Jacobson and Trojanowski, 1977; Luppino et al., 2003; Petrides and Pandya, 1999, 2002a, 2006, 2007).
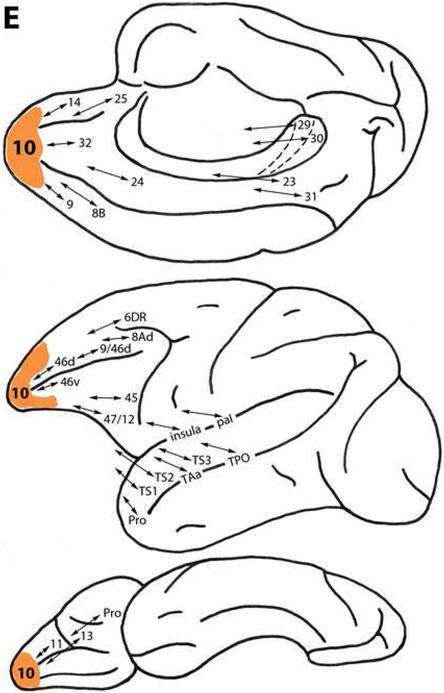
Area 10
This area, which is located at the rostral tip of the frontal lobe and extends on its medial, lateral and ventral surfaces (Fig. 1), is connected with less differentiated dorsal trend areas 25, 32, 14, 9, 8B and 24. Like areas 9 and 8B, it is connected with more differentiated dorsal trend areas 46d, 9/46d and 8Ad. Area 10 also is related to ventral trend areas 46v, 45 and 47/12, ventrolaterally, and Pro, 13 and 11 on the orbital frontal surface (Fig. 2E) (Arikuni et al., 1994; Barbas and Pandya, 1989; Barbas et al, 1999; Barbas and Mesulam, 1985; Carmichael and Price, 1996; Goldman-Rakic et al., 1984; Hackett et al., 1999; Jacobson and Trojanowski, 1977; Petrides and Pandya, 1999, 2007; Vogt and Pandya, 1987).
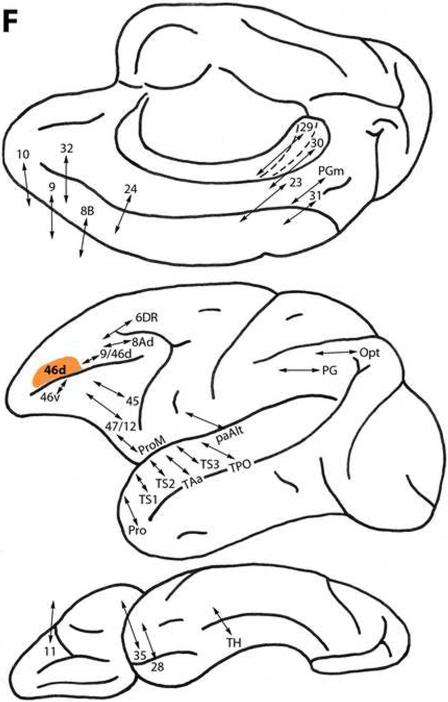
Area 46d
This area, located caudal to area 10 and above and within the principal sulcus (Fig. 1), is connected with less differentiated dorsal trend areas 32, 10, 9, 8B and 24 on the medial surface, and with area 6DR and more differentiated areas 9/46d and 8Ad, dorsolaterally. It is also related to ventral trend area 46v below the principal sulcus, and areas 45, 47/12 and ProM, ventrolaterally. Area 46d has limited connectivity with the orbital surface, with area 11 (Fig. 2F) (Arikuni et al., 1994; Barbas, 1988; Barbas and Mesulam, 1981; Barbas and Pandya, 1989; Barbas et al., 1999; Gerbella et al., 2010; Goldman-Rakic et al. 1984; Hackett et al., 1999; Jacobson and Trojanowski, 1977; Morecraft and Van Hoesen, 1993; Pandya et al., 1981; Petrides and Pandya, 1984, 1999, 2002a,2006, 2007; Vogt and Pandya, 1987).
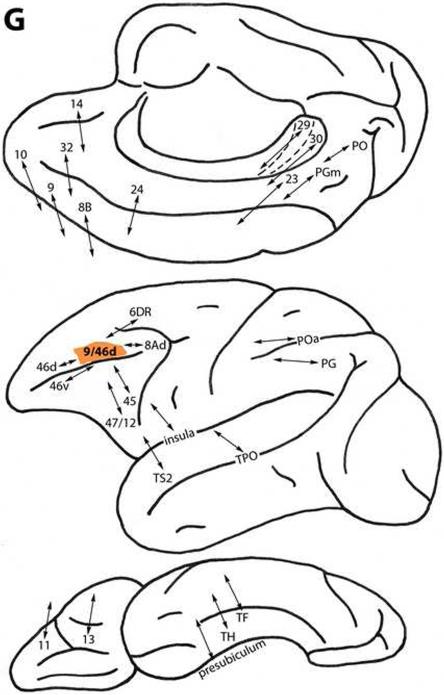
Area 9/46d
Located caudal to area 46d and above the principal sulcus (Fig. 1), this area is connected with less differentiated dorsal trend areas 32, 14, 9, 8B and 10, medially and dorsally. Area 9/46d is also related to area 24, and to dorsolateral area 6DR. On the lateral surface, it is connected with dorsal trend area 46d and with more differentiated area 8Ad. Area 9/46d is related to ventral trend areas 46v, 45 and 47/12, ventrolaterally, as well as to areas 11 and 13 on the orbital surface (Fig. 2G) (Barbas, 1988; Barbas and Mesulam, 1981, 1985; Barbas and Pandya, 1987, 1989; Bates and Goldman-Rakic, 1993; Ghosh and Gattera, 1995; Goldman-Rakic et al., 1984; Hackett et al., 1999; Huerta et al. 1987; Jacobson and Trojanowski, 1977; Luppino et al., 2003; Morecraft and Van Hoesen, 1993; Pandya et al., 1981; Petrides and Pandya, 1999, 2006; Vogt et al., 1979; Vogt and Pandya, 1987; Wang et al., 2002).
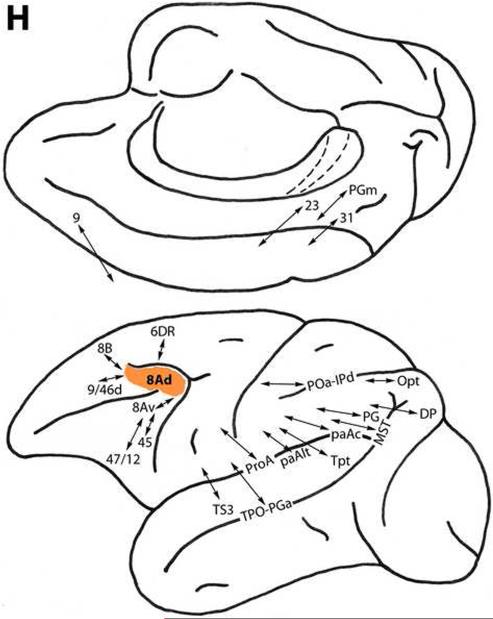
Area 8Ad
This area represents the most highly differentiated region in the dorsal prefrontal architectonic trend. Located in the dorsal portion of the concavity of the arcuate sulcus (Fig. 1), area 8Ad is connected with less differentiated dorsal trend area 9, dorsomedially. It is related to areas 8B, 6DR and 9/46d in the dorsolateral frontal region. Area 8Ad also is connected with ventral trend areas 8Av, 45 and 47/12 ventrolaterally (Fig. 2H) (Arikuni et al., 1998; Barbas, 1988; Barbas and Mesulam, 1981, 1985; Barbas and Pandya, 1987, 1989; Bates and Goldman-Rakic, 1993; Caminiti et al., 1999; Gerbella et al., 2010; Ghosh and Gattera, 1995; Goldman-Rakic et al., 1984; Hackett et al., 1999; Huerta et al., 1987; Jacobson and Trojanowski, 1977; Luppino et al., 1990, 2003; Luppino and Rizzolatti, 2000; Morán et al., 1987; Morecraft et al. 1993; Petrides and Pandya, 1999, 2006, 2007; Stanton et al., 1993; Wang et al., 2002).
Overall, it appears that the medial and dorsolateral prefrontal regions are connected strongly with other medial and dorsolateral prefrontal regions (dorsal trend areas). These regions also have some connectivity with ventrolateral and orbital prefrontal areas (ventral trend areas). Thus, each area has a distinctive and relatively complex set of connections within the frontal lobe, both within its respective trend and with the opposite trend.
The local connections of the orbital and ventrolateral prefrontal regions
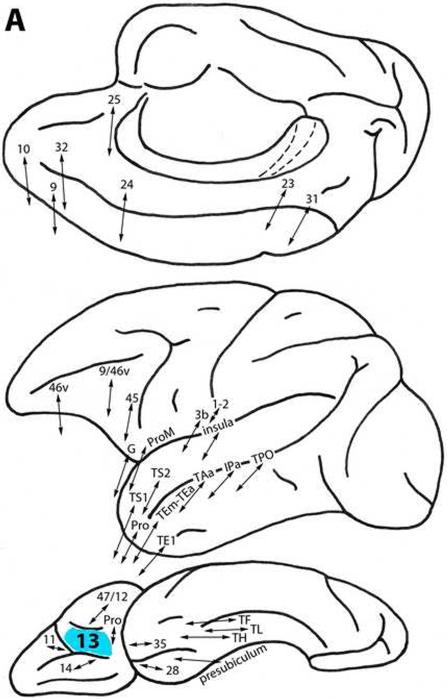
Area 13
This area lies rostral to the proisocortex on the orbital surface of the frontal lobe (Fig. 1). It is connected with the surrounding areas on the orbital surface, i.e., with less differentiated ventral trend area Pro and with more differentiated areas 14, 11, and 47/12. It is related to more differentiated ventral trend areas 45, 9/46v, and 46v on the ventrolateral surface, and with area ProM and the gustatory area in the frontal operculum. Area 13 has significant connections also with dorsal trend areas 25, 32 and 24 medially, and with areas 10 and 9 (Fig. 3A) (Arikuni et al., 1994; Barbas, 1988, 1993; Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Barbas et al., 1999; Carmichael and Price, 1995a, 1995b, 1996; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Morecraft et al., 1992, 1993; Morecraft and Van Hoesen, 1998; Pandya et al., 1981; Petrides and Pandya, 1999, 2002a, 2006, 2007; Saleem et al., 2008; Vogt and Pandya, 1987).
Figure 3.
A. Diagrammatic representations of the medial, lateral and ventral surfaces of the macaque monkey cerebral hemisphere showing bidirectional local (within the frontal lobe) and long (Post-Rolandic) association connections of areas (shown in blue) in the ventral prefrontal architectonic trend. A: Area 13. B: Area 14. C: Area 11. D: Area 47/12. E: Area 46v. F: Area 9/46v. G: Area 45. H: Area 8Av.
Area 14
This area is located medial to areas 13 and 11 and ventral to area 32, occupying the ventromedial prefrontal region (Fig. 1). (It should be noted that the medial part of area 14 is located within the territory of the dorsal trend and could be regarded as belonging to that trend.) It is connected with less differentiated ventral trend areas Pro and 13 on the orbital surface, and with more differentiated areas 11, 47/12, 45, and 46v ventrally and laterally. Like area 13, area 14 is related to dorsal trend areas 25, 32 and 24 medially, to areas 10 and 9, and to area 46d dorsolaterally (Fig. 3B) (Barbas, 1988; Barbas and Pandya, 1989; Barbas et al., 1999; Carmichael and Price, 1995a, 1996; Morecraft et al., 1992; Morecraft and Van Hoesen, 1993; Petrides and Pandya, 1999, 2002a, 2007; Vogt and Pandya, 1987).
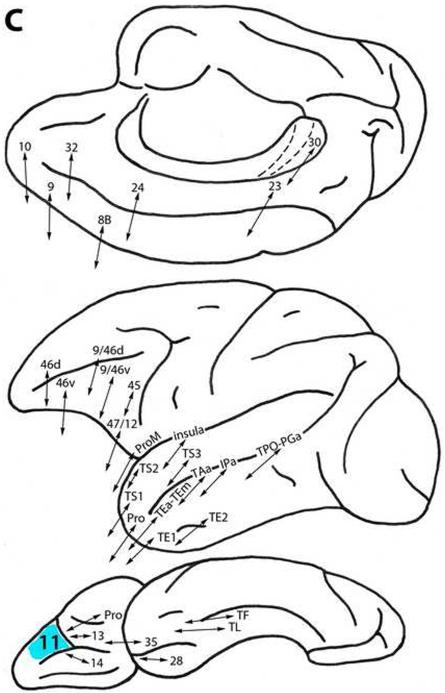
Area 11
Located rostral to area 13 and lateral to area 14 on the orbital surface (Fig. 1), this area is connected with less differentiated ventral trend areas Pro, 13 and 14 on the orbital surface, as well as with area ProM, ventrolaterally. It is related to more differentiated ventral trend areas 47/12, 45, 46v and 9/46v on the lateral surface. Area 11 also is connected with dorsal trend areas 32 and 24 on the medial surface, with areas 10, 9 and 8B, and with areas 46d and 9/46d dorsolaterally (Fig. 3C) (Barbas, 1988, 1993; Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Barbas et al., 1999; Bates and Goldman-Rakic, 1993; Carmichael and Price, 1995a, 1995b, 1996; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Jacobson and Trojanowski, 1977; Kondo et al., 2005; Morecraft et al., 1992; Pandya et al., 1981; Petrides and Pandya, 1999, 2002a, 2006, 2007; Saleem et al., 2008; Shiwa, 1987; Vogt and Pandya, 1987).
Area 47/12
Located on the ventrolateral convexity of the frontal lobe (Fig. 1), this area is connected with less differentiated ventral trend areas Pro, 13 and 11 on the orbital surface. It is related to more differentiated ventral trend areas 46v, 9/46v, 8Av, 45, 44 and 6v on the lateral surface, as well as to the gustatory area and area ProM in the frontal opercular region. In addition, area 47/12 is connected with dorsal trend areas 32 and 24 medially, and with areas 10 and 9 (Fig. 3D) (Arikuni et al., 1994; Barbas, 1988, 1993; Barbas and Mesulam, 1985; Barbas and Pandya, 1987, 1989; Barbas et al., 1999; Bates and Goldman-Rakic, 1993; Carmichael and Price, 1995a, 1995b, 1996; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Jacobson and Trojanowski, 1977; Morecraft et al., 1992; Morecraft and Van Hoesen, 1993, 1998; Pandya et al., 1981; Petrides and Pandya, 1999, 2002a, 2006, 2007; Saleem et al., 2008; Shiwa, 1987; Stanton et al., 1993; Vogt and Pandya, 1987).
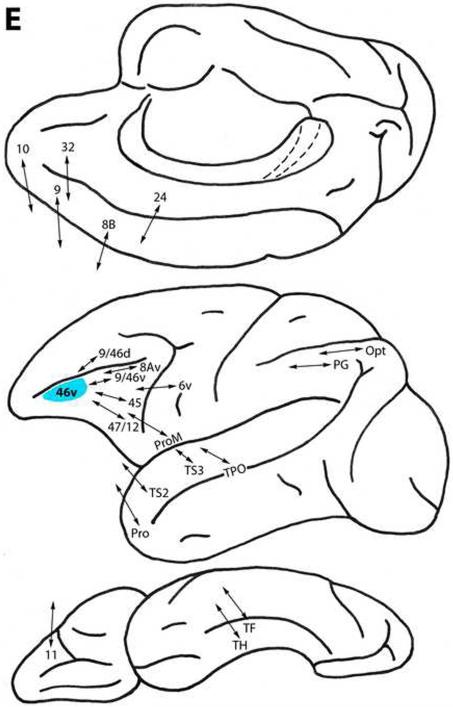
Area 46v
This area, located caudal to area 10 and below and within the principal sulcus (Fig. 1), is connected with less differentiated ventral trend areas 47/12 and ProM ventrolaterally, with area 6v, and with area 11 on the orbital surface. Area 46v is related to more differentiated ventral trend areas 9/46v, 45 and 8Av ventrolaterally. In addition, area 46v has connections with dorsal trend areas 32 and 24 medially, with areas 10, 9 and 8B, and with 9/46d dorsolaterally (Fig. 3E) (Barbas, 1988; Barbas and Mesulam, 1985; Barbas and Pandya, 1987, 1989; Barbas et al., 1999; Goldman-Rakic et al., 1984; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Jacobson and Trojanowski, 1977; Morecraft and Van Hoesen, 1993; Petrides and Pandya, 1999, 2002a, 2006, 2007; Preuss and Goldman-Rakic, 1989; Vogt and Pandya, 1987).
Area 9/46v
Located caudal to area 46v and below the principal sulcus (Fig. 1), this area has connections with less differentiated ventral trend areas 46v, 47/12 and ProM on the ventrolateral surface, and orbital frontal areas 11 and 13. It is related to more differentiated ventral trend areas 45, 44 and 8Av, and with area 6v ventrolaterally. Area 9/46v also is related to dorsal trend area 24, the CMA, and the supplementary motor area (SMA) dorsomedially (Fig. 3F) (Barbas, 1988; Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Bates and Goldman-Rakic, 1993; Carmichael and Price, 1996; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Matelli et al., 1986; Morecraft et al. 1992; Pandya et al., 1981; Petrides and Pandya, 1999, 2002a, 2006, 2007; Stanton et al., 1993).
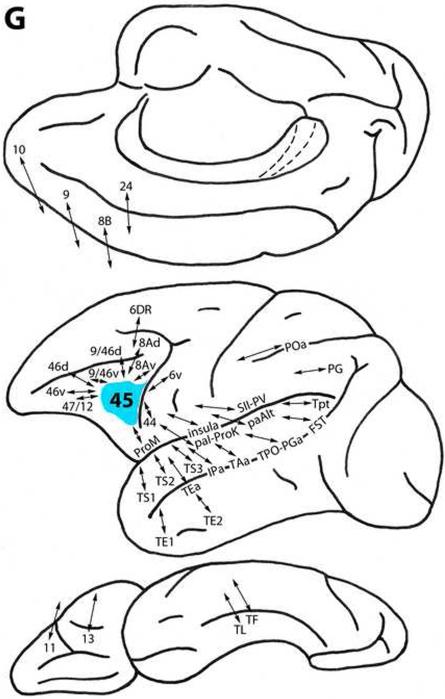
Area 45
We have modified the extent of area 45 in accordance with the observations of Petrides and Pandya (1994) (see Petrides et al., 2011 in this issue). This area is located in front of the lower limb of the arcuate sulcus and extends from the arcuate sulcus to the fronto-orbital sulcus. Area 45 is connected with less differentiated ventral trend areas 46v, 9/46v, 47/12, 44 and ProM ventrolaterally, and with areas 11 and 13 on the orbital surface. It is related to more differentiated ventral trend area 8Av in the arcuate concavity. In addition, it is connected with dorsal trend area 24 medially, with areas 10, 9 and 8B dorsomedially, and with areas 46d, 9/46d, 8Ad and 6DR dorsolaterally (Fig. 3G) (Arikuni et al., 1988, 1994; Barbas, 1988; Barbas and Mesulam, 1985; Barbas and Pandya, 1987, 1989; Carmichael and Price, 1996; Gerbella et al., 2010; Godschalk et al., 1984; Goldman-Rakic et al., 1984; Jacobson and Trojanowski, 1977; Luppino et al., 1990, 2003; Morecraft et al., 1992, 1993; Morecraft and Van Hoesen, 1993; Petrides and Pandya, 1999, 2002a, 2006, 2007; Shiwa, 1987; Stanton et al., 1993).
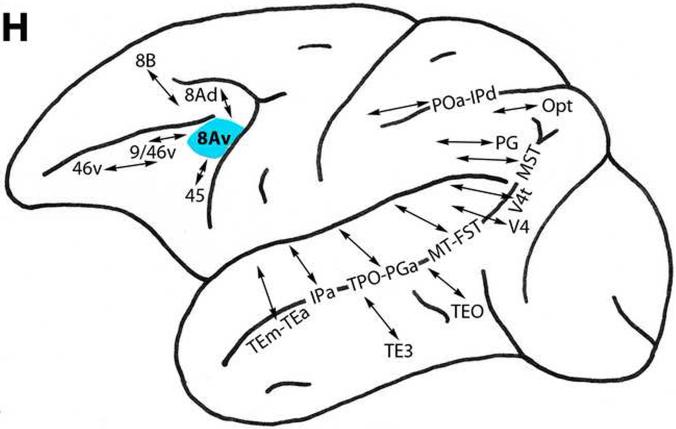
Area 8Av
This area represents the most highly differentiated region in the ventral prefrontal architectonic trend (Fig. 1). Located in the ventral arcuate concavity dorsal to area 45, area 8Av is connected with less differentiated ventral trend areas 46v, 9/46v, and 45 on the ventrolateral surface. It is also related to dorsal trend areas 8B and 8Ad (Fig. 3H) (Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Goldman-Rakic et al., 1984; Gerbella et al., 2010; Petrides and Pandya, 1999, 2002a, 2006; Stanton et al., 1993).
Thus, it seems that orbital and ventrolateral prefrontal areas have strong ties with orbital and ventrolateral prefrontal areas (ventral trend areas). These areas are also connected with medial and dorsomedial prefrontal regions (dorsal trend areas). Barring some minor differences, in both the dorsal and the ventral trend, the afferent and efferent local connections appear to be reciprocal.
The Connectivity of the Prefrontal Cortex with Post-Rolandic Cortical Regions
The prefrontal cortical areas are connected with post-Rolandic sensory association, multimodal, and paralimbic areas. These connections are largely bidirectional and are conveyed by specific fiber pathways.
The long, post-Rolandic connections of the medial and dorsolateral prefrontal regions
Area 25
This area is connected with auditory-related cortical areas TS1, TS2 and paAr of the rostral superior temporal region, and with multimodal area TPO of the superior temporal sulcus (Fig. 2A). It is also related to the paralimbic temporal polar proisocortex (Pro), the perirhinal (area 35) and entorhinal (area 28) areas of the ventromedial temporal region, the parahippocampal gyrus (areas, TF, TL and TH), and area 23 of the cingulate gyrus (Bachevalier et al., 1997; Barbas et al., 1999; Blatt et al., 2003; Kondo et al., 2005, 2007; Lavenex et al., 2002; Mohedano-Moriano et al., 2007; Morán et al., 1987; Morecraft et al., 2004; Pandya, 1995; Parvizi et al., 2006; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Vogt and Pandya, 1987).
Area 32
This area is connected with auditory-related areas TS1, TS2, TS3, and TAa of the rostral superior temporal gyrus, with somatosensory-related area 31 of the medial parietal region, and with visually-related area PO of the medial preoccipital region (Fig. 2B). Area 32 is related to multimodal areas TPO and PGa of the superior temporal sulcus, and area PGm of the medial parietal region. This area has connections with paralimbic regions: the temporal polar proisocortex, the insula, the perirhinal and entorhinal regions, parahippocampal areas TH and TF, the subiculum, and areas 23, 29 and 30 and the prostriate area in the caudal cingulate region (Bachevalier et al., 1997; Barbas, 1988; Barbas et al., 1999; Carmichael and Price, 1995a; Insausti et al., 1987; Kobayashi and Amaral, 2003; Kondo et al., 2005, 2007; Lavenex et al., 2002; Pandya, 1995; Pandya et al., 1981; Petrides and Pandya, 2007; Parvizi et al., 2006; Romanski et al., 1999a; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Vogt et al., 1979; Vogt and Pandya, 1987).
Area 9
This area is connected with rostral auditory-related areas TS1, TS2, TS3, and TAa of the superior temporal gyrus, and with somatosensory-related area 31 of the medial parietal region (Fig. 2C). Area 9 is related to multimodal area TPO of the superior temporal sulcus. In addition, this area is connected with the paralimbic temporal polar proisocortex, the perirhinal region, parahippocampal areas TH and TF, and caudal cingulateretrosplenial areas 23, 29 and 30 (Baleydier and Mauguière, 1980; Barbas et al., 1999; Bates and Goldman-Rakic, 1993; Cavada and Goldman-Rakic, 1989; Kobayashi et al., 2003; Lavenex et al., 2002; Morecraft et al., 2004; Pandya, 1995; Pandya et al., 1981; Parvizi et al., 2006; Petrides and Pandya, 1999, 2007, 2009; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Vogt and Pandya, 1987; Yeterian and Pandya, 2010).
Area 8B
This area is connected with visually-related area PO, and with area 31 of the medial parietal region (Fig. 2D). It is related to multimodal areas TPO of the superior temporal sulcus, POa, PG, and Opt in the inferior parietal lobule, and PGm on the medial parietal surface. Area 8B is connected with paralimbic areas TF and TH of the parahippocampal gyrus, and areas 23 and 30 of the caudal cingulate-retrosplenial region (Andersen et al., 1990; Bullier et al., 1996; Cavada and Goldman-Rakic, 1989; Maioli et al., 1998; Mesulam et al., 1977; Morecraft et al., 2004; Pandya et al., 1981; Parvizi et al., 2006; Petrides and Pandya, 1984, 1999, 2006; Rozzi et al., 2006; Schall et al., 1995; Schwartz and Goldman-Rakic, 1984; Seltzer and Pandya, 1989; Yeterian and Pandya, 2010).
Area 10
This area is connected with auditory-related association areas TS1, TS2, TS3, TAa, and paI of the rostral superior temporal gyrus, and with somatosensory-related area 31 of the medial parietal region (Fig. 2E). It is related to multimodal area TPO of the superior temporal sulcus. Additionally, it is connected with the paralimbic temporal polar proisocortex, the insula, and areas 23, 29 and 30 of the caudal cingulate-retrosplenial region (Bachevalier et al., 1997; Baleydier and Mauguière, 1980; Barbas and Mesulam, 1985; Barbas et al., 1999; Carmichael and Price, 1995a; Goldman-Rakic et al., 1984; Hackett et al., 1999; Jacobson and Trojanowski, 1977; Kobayashi et al., 2003; Kondo et al., 2007; Morán et al., 1987; Morecraft et al., 2004; Pandya et al., 1981; Parvizi et al., 2006; Petrides and Pandya, 2007, 2009; Romanski et al., 1999a, 1999b; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Vogt et al., 1979).
Area 46d
This area is connected with auditory-related association areas TS1, TS2, TS3, TAa and paAlt of the superior temporal gyrus, and with area 31 of the medial parietal region (Fig. 2F). Area 46d is connected with multimodal areas TPO of the superior temporal sulcus, and PG, Opt and PGm of the parietal lobe. This area also is related to the paralimbic temporal polar proisocortex, the perirhinal and entorhinal regions, area TH of the parahippocampal gyrus, and areas 23, 29 and 30 of the caudal cingulate-retrosplenial region (Andersen et al., 1990; Baleydier and Mauguière, 1980; Ban et al., 1991; Cavada and Goldman-Rakic, 1989; Hackett et al., 1999; Jacobson and Trojanowski, 1977; Kobayashi and Amaral, 2003; Morecraft et al., 2004; Morris et al., 1999; Pandya, 1995; Pandya et al., 1981; Parvizi et al., 2006; Petrides and Pandya, 1984, 1999, 2009; Romanski et al., 1999b; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Vogt and Pandya, 1987).
Area 9/46d
This area has limited connections with the superior temporal gyrus, primarily with area TS2. It is related to areas PG and POa of the inferior parietal lobule and area PGm of the medial parietal lobe. In addition, it has connections with visually-related area PO of the medial preoccipital region (Fig. 2G). Area 9/46d is related to multimodal area TPO of the superior temporal sulcus. It is also connected with paralimbic regions: the insula, areas TH and TF of the parahippocampal gyrus, the presubiculum, and areas 23, 29 and 30 of the caudal cingulateretrosplenial cortex (Andersen et al., 1990; Baleydier and Mauguière, 1980; Ban et al., 1991; Barbas and Mesulam, 1985; Bates and Golman-Rakic, 1993; Blatt et al., 1990; Bullier et al., 1991; Cavada and Goldman-Rakic, 1989; Goldman-Rakic and Schwartz, 1982; Goldman-Rakic et al., 1984; Hackett et al., 1999; Kobayashi and Amaral, 2003; Lavenex et al., 2002; Lewis and Van Essen, 2000; Maioli et al., 1998; Mesulam et al., 1977; Mesulam and Mufson, 1982; Morecraft et al., 2004; Neal et al., 1990; Parvizi et al., 2006; Petrides and Pandya, 1999, 2006; Rozzi et al. 2008; Schall et al., 1995; Seltzer and Pandya, 1989; Stepniewska et al., 2005; Suzuki and Amaral, 1994; Vogt and Pandya, 1987; Yeterian and Pandya, 2010).
Area 8Ad
This area is connected with auditory-related areas TS3, ProA, paAlt, paAc and Tpt of the caudal superior temporal gyrus, with area 31 of the medial parietal region, and with preoccipital visually-related areas DP dorsomedially, and MST in the superior temporal sulcus (Fig. 2H). Area 8Ad is related to multimodal areas TPO and PGa of the superior temporal sulcus, and POa, IPd, PG, Opt and PGm of the parietal region. It is also connected with paralimbic area 23 of the caudal cingulate-retrosplenial region (Andersen et al., 1985, 1990; Baleydier and Mauguière, 1980; Ban et al., 1991; Barbas, 1988; Barbas and Mesulam, 1981, 1985; Bates and Goldman-Rakic, 1993; Blatt et al., 1990; Bullier et al., 1996; Caminiti et al., 1999; Cavada and Goldman-Rakic, 1989; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Hackett et al., 1999; Huerta et al., 1987; Jacobson and Trojanowski, 1977; Kobayashi and Amaral, 2003; Leichnetz, 1989; Lewis and Van Essen, 2000; Maioli et al., 1998; Morecraft et al., 1993, 2004; Neal et al., 1990; Pandya, 1995; Parvizi et al., 2006; Petrides and Pandya, 1984, 1999, 2006, 2009; Rozzi et al., 2006; Romanski et al., 1999a; Saleem et al., 2008; Schall et al., 1995; Schwartz and Goldman-Rakic, 1984; Seltzer and Pandya, 1989; Seltzer et al., 1996; Stanton et al., 1995; Stepniewska et al., 2005; Yeterian and Pandya, 2010).
The long, post-Rolandic connections of the orbital and ventrolateral prefrontal regions
Area 13
This area is connected with auditory-related association areas TS1, TS2 and TAa of the rostral superior temporal region, with visually-related areas TE1, TEm and TEa of the rostral inferior temporal region, and with somatosensory-related area 31 of the medial parietal region (Fig. 3A). Area 13 is related to multimodal areas TPO and IPa of the superior temporal sulcus. In addition, it is connected with paralimbic regions including the insula, the temporal polar proisocortex, the perirhinal (area 35) and entorhinal (area 28) regions, parahippocampal areas TF, TL and TH, and caudal cingulate area 23 (Barbas, 1993; Blatt et al., 2003; Carmichael and Price, 1995a, 1995b; Hackett et al., 1999; Kobayashi et al., 2003; Kondo et al., 2005, 2007; Lavenex et al., 2002; Martin-Elkins and Horel, 1992; Mesulam and Mufson, 1982; Morán et al., 1987; Morecraft et al. 1992, 1998, 2004; Mufson and Mesulam, 1982; Pandya, 1995; Petrides and Pandya, 2009; Romanski et al., 1999a; Saleem et al., 2008; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Van Hoesen et al., 1975; Webster et al., 1994).
Area 14
This area is connected with auditory-related association areas TS1, TS2, TS3 and TAa of the rostral superior temporal gyrus, and auditory area paI of the supratemporal plane (Fig. 3B). It also is connected with visually-related areas TE1, TE2, TEa and TEm of the rostral inferior temporal region. Area 14 is related to multimodal areas TPO and PGa of the superior temporal sulcus. Additionally, this area is related to paralimbic regions: the insula, the temporal polar proisocortex, the perirhinal and entorhinal regions, areas TF and TH of the parahippocampal region, and caudal cingulate-retrosplenial areas 29 and 30 (Bachevalier et al., 2007; Ban et al., 1991; Barbas et al., 1999; Carmichael and Price, 1995a; Hackett et al., 1999; Insausti and Amaral, 2008; Insausti et al., 1987; Kobayashi and Amaral, 2003; Kondo et al., 2005, 2007; Lavenex et al., 2002; Martin-Elkins and Horel, 1992; Morecraft et al., 1992; Petrides and Pandya, 2009; Saleem et al., 2008; Shiwa, 1987; Suzuki and Amaral, 1994; Vogt and Pandya, 1987).
Area 11
This area is related to auditory association areas TS1, TS2, TS3 and TAa of the rostral superior temporal gyrus, and to visually-related association areas TE1, TE2, TEa and TEm of the rostral inferior temporal region (Fig. 3C). Area 11 is related to multimodal areas TPO, PGa and IPa of the superior temporal sulcus. Area 11 has paralimbic connections with the insula, the temporal polar proisocortex, the perirhinal and entorhinal regions, areas TF and TL of the parahippocampal gyrus, and caudal cingulate-retrosplenial areas 23, 29, and 30 (Baleydier and Mauguière, 1980; Ban et al., 1991; Barbas, 1988, 1993; Bates and Goldman-Rakic, 1993; Blatt et al., 2003; Carmichael and Price, 1995a, 1995b; Insausti et al., 1987; Kobayashi et al., 2003; Kondo et al., 2005, 2007; Lavenex et al., 2002; Martin-Elkins and Horel, 1992; Mesulam and Mufson, 1982; Morán et al., 1987; Morecraft et al., 1998, 2004; Mufson and Mesulam, 1982; Parvizi et al., 2006; Potter and Nauta, 1979; Rempel-Clower and Barbas, 2000; Saleem et al., 2008; Pandya et al., 1981; Petrides and Pandya, 2007, 2009; Romanski et al., 1999b; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Vogt et al., 1979; Vogt and Pandya, 1987; Webster et al., 1994).
Area 47/12
This area has connections with visual association areas TE1, TE2, TE3 and TEa of the inferior temporal region (Fig. 3D). Area 47/12 is connected with somatosensory-related areas: the second somatosensory and parietal ventral areas (SII-PV) in the parietal operculum, and areas PF and PFG of the inferior parietal region. In addition, it is connected with paralimbic areas including the insula, the temporal polar proisocortex, the perirhinal cortex, and area TL of the parahippocampal gyrus (Ban et al., 1991; Barbas, 1988, 1993; Borra et al., 2010; Bullier et al., 1996; Carmichael and Price, 1995a, 1995b; Cavada and Goldman-Rakic, 1989; Gerbella et al., 2010; Kondo et al., 2005, 2007; Lewis and Van Essen, 2000; Martin-Elkins and Horel, 1992; Mesulam and Mufson, 1982; Morán et al., 1987; Morecraft et al., 1992; Mufson and Mesulam, 1982; Petrides and Pandya, 2002a; Rempel-Clower and Barbas, 2000; Romanski et al., 1999a; Saleem et al., 2008; Schall et al., 1995; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Webster et al., 1994).
Area 46v
This area is connected with auditory-related association areas TS2 and TS3 of the rostral superior temporal gyrus (Fig. 3E). Area 46v is related to multimodal areas TPO of the superior temporal sulcus and PG and Opt of the inferior parietal lobule. It has paralimbic connections with the temporal polar proisocortex, and areas TF and TH of the parahippocampal gyrus (Cavada and Goldman-Rakic, 1989; Blatt et al., 2003; Lavenex et al., 2002; Mesulam et al., 1977; Pandya, 1995; Petrides and Pandya, 1984; Preuss and Goldman-Rakic, 1989; Rozzi et al., 2006; Saleem et al., 2008; Shiwa, 1987; Suzuki and Amaral, 1994).
Area 9/46v
This area is connected with ventrolateral somatosensory-related areas 1–2 and SII-PV in the fronto-parietal operculum, and with areas PF, PFG and rostral POa of the inferior parietal lobule (Fig. 3F). It has paralimbic connections with the insula (Barbas, 1988; Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Blatt et al., 1990; Borra et al., 2008; Cavada and Goldman-Rakic, 1989; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Goldman-Rakic and Schwartz, 1984; Goldman-Rakic et al., 1984; Lewis and Van Essen, 2000; Mesulam and Mufson, 1982; Neal et al., 1990; Petrides and Pandya, 1984, 2002a, 2006, 2009; Rozzi et al., 2006; Schwartz and Goldman-Rakic, 1984; Shiwa, 1987; Suzuki and Amaral, 1994).
Area 45
This area has connections with auditory-related areas TS1, TS2, TS3, TAa, paAlt and Tpt of the superior temporal gyrus, and paI and ProK of the supratemporal plane (Fig. 3G). Area 45 also has connections with visually-related areas TE1, TE2 and TEa of the inferior temporal region, and FST of the superior temporal sulcus. It is related to somatosensory area SII-PV of the inferior parietal region. This area has connections with multimodal areas TPO, PGa and IPa of the superior temporal sulcus, and POa and PG of the inferior parietal lobule. Area 45 has paralimbic connections with the insula and with areas TF and TL of the parahippocampal gyrus (Boussaoud et al., 1990; Cavada and Goldman-Rakic, 1989; Cipolloni and Pandya, 1999; Gerbella et al., 2010; Lavenex et al., 2002; Lewis and Van Essen, 2000; Maioli et al., 1998; Martin-Elkins and Horel, 1992; Mesulam et al., 1977; Mesulam and Mufson, 1982; Petrides and Pandya, 1984, 2002a, 2009; Romanski et al., 1999b; Saleem et al., 2008; Schall et al., 1995; Schwartz and Goldman-Rakic, 1984; Seltzer and Pandya, 1989; Shiwa, 1987; Suzuki and Amaral, 1994; Webster et al., 1994).
Area 8Av
This area has connections with visually-related areas TE3, TEm, TEa and TEO of the inferior temporal region, MT, FST and MST of the superior temporal sulcus, and V4t and V4 of the lateral preoccipital region (Fig. 3H). Area 8Av is related to multimodal areas TPO, PGa and IPa of the superior temporal sulcus, and POa, IPd, PG and Opt of the inferior parietal lobule (Andersen et al., 1990; Baizer et al., 1991; Ban et al., 1991; Barbas and Mesulam, 1985; Barbas and Pandya, 1989; Blatt et al., 1990; Borra et al., 2008, 2010; Bullier et al., 1996; Cavada and Goldman-Rakic, 1989; Distler et al., 1993; Gerbella et al., 2010; Goldman-Rakic et al., 1984; Lewis and Van Essen, 2000; Maioli et al., 1983, 1998; Medalla and Barbas, 2006; Neal et al., 1990; Petrides and Pandya, 1984, 1999, 2006, 2009; Schall et al., 1995; Schwartz and Goldman-Rakic, 1984; Seltzer and Pandya, 1989; Shiwa, 1987; Stanton et al., 1995; Stepniewska et al., 2005; Suzuki and Amaral, 1994; Ungerleider et al., 1986; Webster et al., 1994; Yeterian and Pandya, 2010).
Like the local connections, the long association connections of the prefrontal cortex are largely reciprocal. By and large the long connections inter-relate dorsal and ventral trend areas with dorsal and ventral post-Rolandic regions, respectively, albeit with some related to the opposite trend.
Corticocortical Fiber Pathways of the Prefrontal Cortical Areas
The prefrontal cortical areas, as detailed above, receive afferents from, and send efferents to post-Rolandic sensory association areas, as well as to multimodal and paralimbic regions. These afferent and efferent post-Rolandic connections are conveyed to and from the prefrontal cortex by specific fiber pathways. With the availability of more precise anatomical methodologies in the 1970's, in particular the autoradiographic tract tracing method (Cowan et al., 1972), it became possible to discern not only fiber trajectories in greater detail but also their specific origins and terminations (Petrides and Pandya, 2002b, 2006; Schmahmann and Pandya, 2006). We will here describe these pathways in view of the concept of dual trends in the prefrontal cortex.
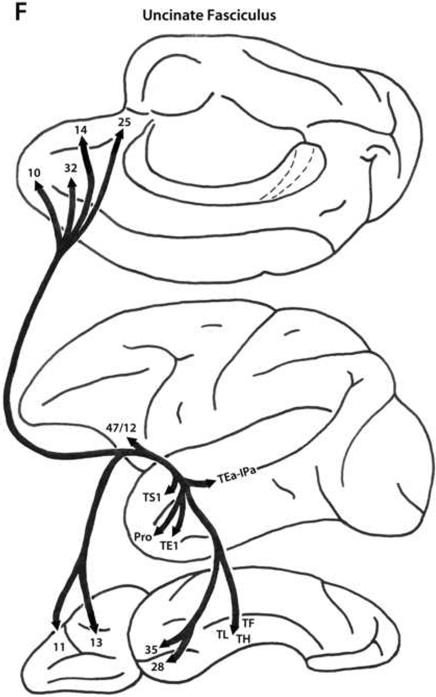
It is important to point out that the white matter fasciculi are composed of axons that link major post-Rolandic regions with particular frontal regions (Petrides and Pandya, 2002b, 2006; Schmahmann and Pandya, 2006; Schmahmann et al., 2007). For instance, the uncinate fasciculus links the anteriormost part of the temporal lobe (lateral, medial and ventral surfaces) with the orbital and ventral medial frontal region. However, this does not mean that every area found on the anterior temporal lobe is linked with every area found on the orbital and ventromedial frontal cortex. The origins and terminations of the axons that travel in this fasciculus are quite specific for the different areas that are found in the anterior temporal and orbital and ventromedial frontal region. Thus, in the schematic figures that illustrate the fasciculi (see Fig. 4), only the major points of origin and termination of the axons running in a particular fasciculus are illustrated. For the details of connections between architectonic areas, the reader should refer to the text and figures that appear in the first part of this article.
Figure 4.
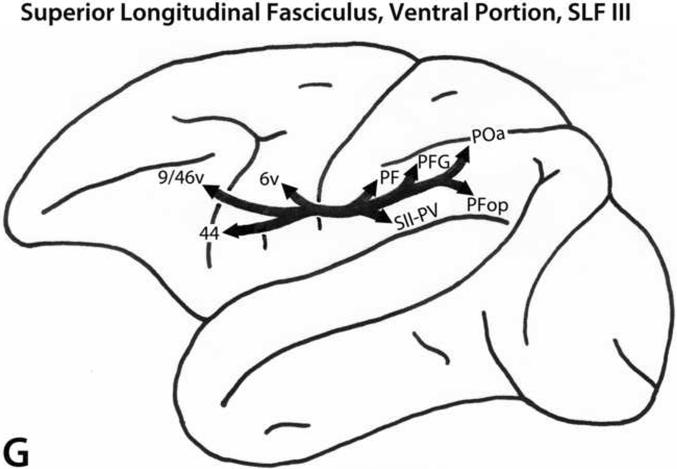
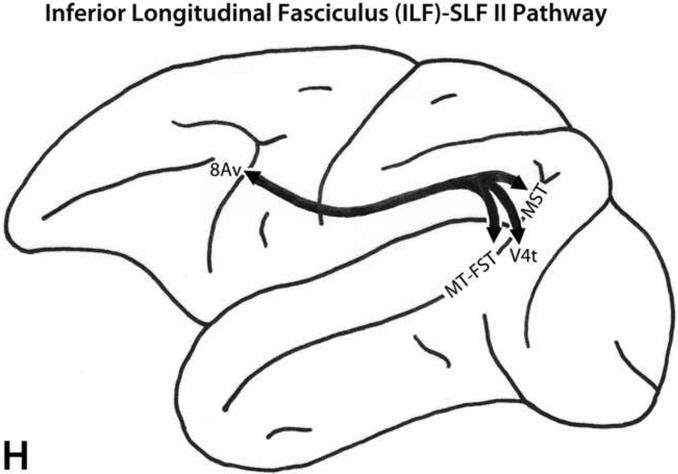
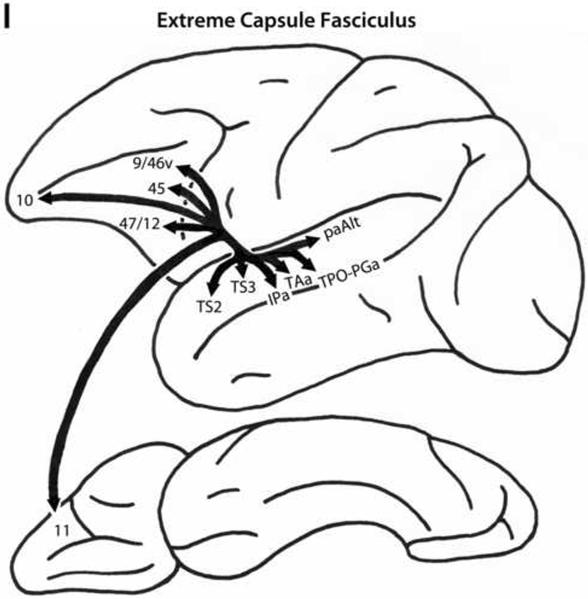
A. Schematic diagrams of the medial, lateral and ventral surfaces of the macaque monkey cerebral hemisphere depicting the trajectories of the long prefrontal fiber pathways. A: Cingulate fasciculus (dorsal trend) limbic pathway. B: Superior longitudinal fasciculus, dorsal portion (SLF I, dorsal trend) somatosensory pathway. C: Superior longitudinal fasciculus, middle portion (SLF II, dorsal trend) multimodal pathway. D: Occipitofrontal (dorsal trend) visual pathway. E: Arcuate fasciculus (dorsal trend) auditory pathway. F: Uncinate fasciculus (ventral trend) limbic pathway. G: Superior longitudinal fasciculus, ventral portion (SLF III, ventral trend) somatosensory pathway. H: Inferior longitudinal fasciculus - superior longitudinal fasciculus, middle portion (ILF - SLF II, ventral trend) visual pathway. I: Extreme capsule fasciculus (ventral trend) auditory and multimodal pathway. Note that the arrows and their trajectories indicate connectivity between areas, and do not represent the magnitude of those connectional relationships. For more detailed descriptions of these pathways, see Schmahmann and Pandya (2006).
Prefrontal Afferent Fiber Pathways
Dorsal trend afferent pathways
There are five distinct fiber pathways connecting post-Rolandic regions with the dorsal trend areas of the prefrontal cortex: the cingulate fasciculus (limbic), the dorsal (somatosensory) and middle (multimodal) portions of the superior longitudinal fasciculus (SLF I and SLF II, respectively), the occipitofrontal fasciculus (OFF, visual), and the arcuate fasciculus (auditory and multimodal).
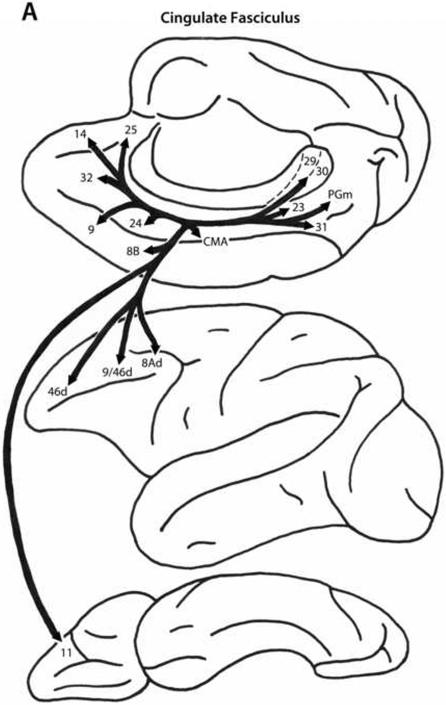
Cingulate Fasciculus
As the name implies the cingulate fasciculus (Fig. 4A) originates from the region of the cingulate gyrus, especially the caudal portion, and from the adjoining retrosplenial area. It also includes some contingents of fibers from the inferior parietal and medial preoccipital regions. These fibers occupy a ventral position within the cingulum bundle and course to the frontal lobe up to the genu of the corpus callosum. From there the fibers enter the white matter of the prefrontal region and terminate in dorsal trend areas 9, 46d, 9/46d, 8B and 8Ad, and in ventral trend area 11 of the orbital surface (Mufson and Pandya, 1984; Petrides and Pandya, 2002b, 2006; Schmahmann and Pandya, 2006; Schmahmann et al., 2007).
Superior Longitudinal Fasciculus
The association fibers connecting the frontal cortex with the superior and inferior parietal lobules are known collectively as the superior longitudinal fasciculus (SLF). This major pathway was divided into three branches, i.e., SLF I, SLF II, and SLF III, by Petrides and Pandya (1984). This subdivision is now widely accepted and has been confirmed in the human brain with diffusion tensor imaging (e.g., Frey et al., 2008; Makris et al., 2005; Rushworth et al., 2006; Schmahmann and Pandya, 2006). The first two branches, namely the SLF I and SLF II, link primarily frontal areas of the dorsal trend with post-Rolandic cortex and are described here. The third branch, namely the SLF III, links primarily areas of the ventral frontal trend and is described later.
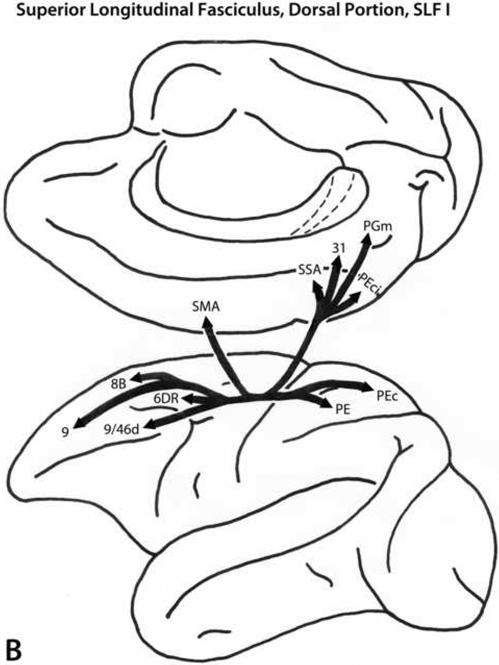
SLF I
The SLF I fiber pathway (Fig. 4B) originates from the medial and dorsal (superior) parietal regions and courses through the white matter of the superior parietal and superior frontal regions to the frontal cortex. This pathway terminates in dorsal trend areas 9, 9/46d and 8B, dorsal premotor area 6, and in the SMA (Petrides and Pandya, 1984; Schmahmann and Pandya, 2007).
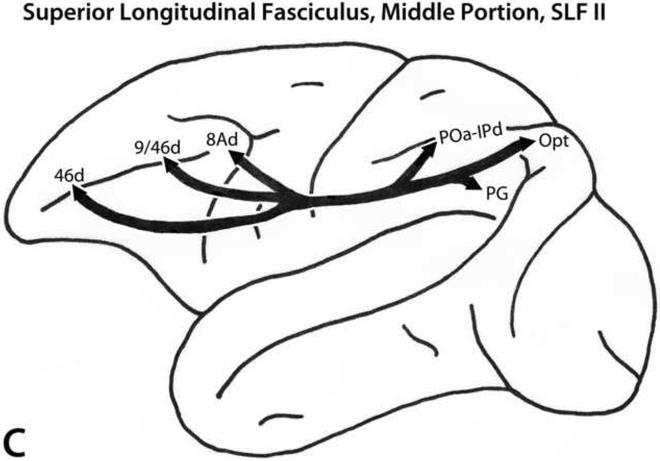
SLF II
The SLF II fiber pathway (Fig. 4C) arises from the caudal portion of the inferior parietal lobule, including the middle and caudal parts of the lateral bank of the intraparietal sulcus. It courses initially in the white matter of the inferior parietal lobule above the Sylvian fissure, remains in a location medial and dorsal to the fissure, and then continues in the white matter of the frontal lobe to terminate in various architectonic areas of the caudal prefrontal region. This pathway terminates in dorsal trend areas 8Ad, 9/46d and 46d, and in dorsal premotor area 6 (Petrides and Pandya, 1984, 2002b, 2006).
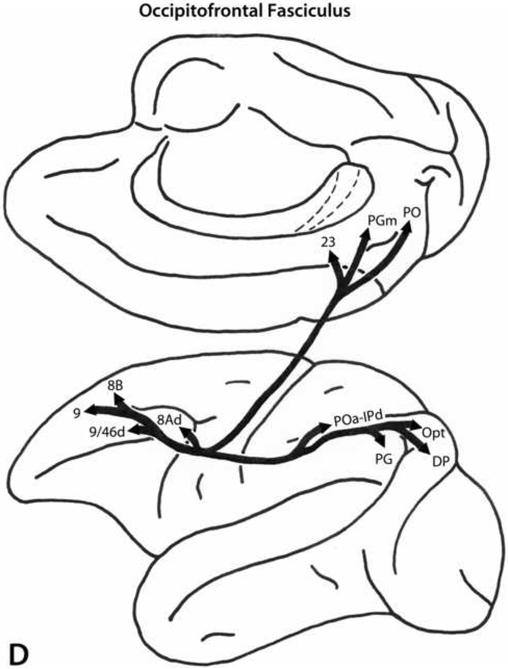
Occipitofrontal Fasciculus
The OFF (Fig. 4D), although often documented in the neuroanatomical literature, has been controversial regarding not only its precise course in the brain but also its existence in the human brain. In experimental material the OFF is clearly identifiable and originates from the medial and dorsal preoccipital regions as well as from the caudalmost inferior parietal lobule where it merges with the dorsal preoccipital region. This fiber bundle courses in the white matter above the caudate nucleus and Muratoff's bundle and medial to the corona radiata. It remains in this position up to the frontal horn of the lateral ventricle. From there the fibers proceed to terminate in dorsal trend areas 8B, 9, 9/46d, and 8Ad (Petrides and Pandya, 2002b, 2006; Yeterian and Pandya, 2010).
It should be pointed out that the fibers of the SLF II and those of the OFF (superior fronto-occipital fascicle) run in close juxtaposition in the white matter of the inferior parietal lobule but differ in terms of the origins: those of the SLF II originate from posterior parietal cortical areas, while those of the OFF originate from occipital cortical areas. Thus, the fundamental difference lies in the cells of origin of those axons. Although these differences are clear in experimental preparations in the macaque monkey, they are difficult to distinguish with diffusion tensor imaging (DTI) in the human given the limitations of the method (see Petrides et al., this issue).
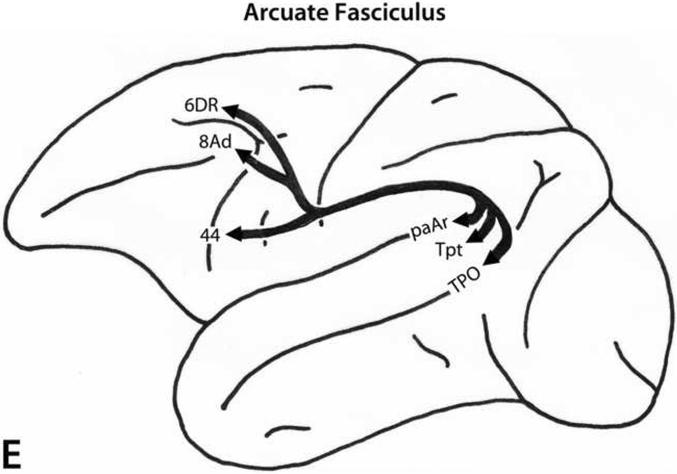
Arcuate Fasciculus
The arcuate fasciculus (Fig. 4E) originates from the caudalmost part of the superior temporal gyrus (area Tpt) and the adjacent cortex in the caudal superior temporal sulcus (area TPO). It arches around the caudal end of the Sylvian fissure (hence its name) and runs in the white matter of the inferior parietal lobule immediately below the superior longitudinal fasciculus (SLF II). It remains in this location up to the frontal lobe where one contingent of the fibers begins to turn dorsally to terminate in areas 8Ad and in dorsal premotor area 6 (Petrides and Pandya, 1988, 2002b, 2006, 2009) and another branch continues ventrally to terminate in area 44 (Petrides and Pandya, 2009).
Ventral trend afferent pathways
There are four distinct fiber pathways from post-Rolandic regions to the ventral trend areas of the prefrontal cortex: the uncinate fasciculus (limbic), the ventral portion of the superior longitudinal fasciculus (SLF III, somatosensory), the inferior longitudinal fasciculus (ILF)-SLF II pathway (ILF-SLF II, visual), and the extreme capsule fasciculus (auditory, multimodal).
Uncinate Fasciculus
The uncinate fasciculus (Fig. 4F) arises from the rostral part of the temporal lobe and the parahippocampal gyrus. More specifically, these fibers originate in the rostral part of the superior temporal gyrus and the superior temporal sulcus, the rostral part of the inferior temporal region, areas TH, TL and TF of the parahippocampal gyrus, and the perirhinal and entorhinal cortex. The uncinate fasciculus courses dorsally in the white matter of the limen insulae, in the narrow space above the amygdala and just below the base of the claustrum (Klinger and Gloor, 1960; Petrides and Pandya, 2007). It is important to note that these fibers are distinct from those of the extreme capsule. The uncinate fasciculus fibers terminate in ventral and medial portions of the prefrontal cortex: in ventral trend areas 13, 11, and 47/12, in areas 10 and 14, and in areas 25 and 32 (Muñoz et al., 2009; Petrides and Pandya, 1988, 2002b, 2007, 2009; Schmahmann et al., 2007; Ungerleider et al., 1989).
SLF III
The SLF III pathway (Fig. 4G) originates from the cortex of the anterior portion of the inferior parietal lobule (areas PF and PFG), including the anterior portion of the lateral bank of the intraparietal sulcus, as well as from the parietal opercular region (areas SII-PV and PFop). The fibers course in the white matter of the inferior parietal and frontal regions to terminate in ventral premotor area 6v, in area 44 of the caudal bank of the lower limb of the arcuate sulcus, and in area 9/46v of the caudoventral portion of the lower bank of the principal sulcus (Petrides and Pandya, 1984, 2002b, 2006).
ILF-SLF II
The ILF-SLF II pathway (Fig. 4H) arises from the lateral preoccipital area, including areas V4t, MT, MST and FST, with a minor contingent from the ventral preoccipital area. This pathway travels first via the ILF and then ascends to become part of the SLF II. It terminates mainly in ventral trend prefrontal area 8Av up to the caudal tip of the principal sulcus (Yeterian and Pandya, 2010).
This fiber bundle has been recently described on the basis of experimental investigation in the macaque monkey in which the labeled fibers were traced following isotope injections limited to a specific region of the lateral preoccipital area. These fibers terminated in area 8Av. Connections between this part of the lateral preoccipital cortex and the region of area 8 in the macaque monkey have been shown by Ungerleider and Desimone (1986). Moreover, Petrides and Pandya (2006), following isotope injections in area 8Av, observed fibers coursing via the SLF II and the ILF, and terminating in the lateral preoccipital region. This pathway should not be confused with the occipitofrontal fascicle since the latter pathway originates from the medial and dorsomedial preoccipital region and terminates in area 8Ad. Although the inferior fronto-occipital fascicle has not been delineated in the macaque monkey, the ILF-SLF II pathway in the macaque brain is located more dorsally than the inferior fronto-occipital pathway as shown by post-mortem dissection in the human brain (Trolard, 1906; Curran, 1909; see also Schmahmann and Pandya, 2006).
Extreme Capsule Fasciculus
The extreme capsule fibers (Fig. 4I) arise from the mid-portion of the superior temporal gyrus including areas TS2, TS3 and paAlt, from areas IPa, TAa and TPO-PGa of the superior temporal sulcus, from areas TH and TL of the parahippocampal gyrus, and from the insula. These fibers travel between the claustrum and the insula to reach the ventrolateral part of the frontal lobe. The extreme capsule terminates in ventral trend areas 11, 45, 47/12, and 9/46v, as well as in area 10 (Petrides and Pandya, 1988, 2002b, 2007, 2009; Schmahmann et al., 2007).
Prefrontal Efferent Fiber Pathways
The prefrontal areas give rise to long association connections that travel via the aforementioned fiber pathways. Thus, in the dorsal prefrontal trend, area 32 sends fibers via the cingulate fasciculus to areas 24 and 23. It also sends fibers via the extreme capsule and uncinate fasciculus to terminate in temporal areas Pro, TS1, TS2, TAa, and TPO (Petrides and Pandya, 2007). Area 9 also sends fibers via the cingulate fasciculus to areas 24 and 23, and to the cingulate motor area and areas 30 and 31. In addition, this area sends fibers via the extreme capsule to areas TPO, TAa and the insula (Morris et al., 1999; Petrides and Pandya, 2007). Area 8B sends fibers via the cingulate fasciculus to terminate in areas 24, 23 and the CMA. It also sends fibers via the SLF I to terminate in areas PGm and 31 as well as in the SMA and dorsal area 6 (Petrides and Pandya, 2006). Area 10 sends connections via the cingulate fasciculus to areas 24, 23, 31, 29 and 30. It also sends fibers via the extreme capsule and uncinate fasciculus to temporal areas Pro, TS1, TS2, TS3, TAa and TPO (Petrides and Pandya, 2006). Area 9/46d sends fibers via the cingulate fasciculus to areas 24 and 23, the CMA, and areas 29 and 30. This area also projects via the SLF I to areas 31, PGm, PEc, and PEci. In addition, area 9/46d sends fibers via the SLF II to intraparietal areas POa and IPd and to areas PG and PGop (Petrides and Pandya, 2006). Rostral area 8Ad sends projections via the cingulate fasciculus to areas 23 and 31, and via the OFF to areas IPd, PGm, and PO. This area projects via the SLF-II to areas POa and PG-Opt, and via the arcuate fasciculus to caudal area TPO and areas Tpt and paAc. Rostral area 8Ad also sends fibers via the extreme capsule to area paAlt, rostral area TPO, and area PGa (Petrides and Pandya, 2006). In contrast, the caudal part of area 8Ad sends fibers via the OFF to areas IPd, PGm and PO, and via the SLF-II to areas PG, Opt and POa (Petrides and Pandya, 2006).
In the ventral prefrontal trend, area 11 sends fibers via the uncinate fasciculus to terminate in the temporal proisocortex and in areas TE1, TEa, IPa and 35 (Petrides and Pandya, 2007). The ventral portion of area 10 sends fibers only via the extreme capsule to terminate in areas TS1, TAa and TPO (Petrides and Pandya, 2007). Area 9/46v sends fibers via the SLF III to terminate in the gustatory cortex, and in areas SII-PV, 1 and 2, and PF, PFG and PG of the inferior parietal lobule. In addition, this area projects via the cingulate fasciculus to areas 6DR, 24, the SMA and the CMA (Petrides and Pandya, 2006). Finally, area 8Av sends fibers via the OFF to areas IPd and POa of the intraparietal sulcus. It also sends fibers via the ILF-SLF II to areas MST, MT and FST of the superior temporal sulcus (Petrides and Pandya, 2006).
Thus it seems that these distinct fiber pathways inter-relate the prefrontal cortex with post-Rolandic sensory-specific association (dorsal [SLF I] and ventral [SLF III] somatosensory; caudodorsal [arcuate fasciculus] and rostral [extreme capsule] auditory; dorsomedial [OFF] and ventrolateral [ILF-SLF II] visual), multimodal (SLF II, arcuate fasciculus, extreme capsule), and paralimbic (cingulate fasciculus, uncinate fasciculus) areas. Like the local and long connections, these pathways are largely reciprocal in nature and appear to be organized in a manner consistent with the concept of dual trends in the prefrontal region.
DISCUSSION
We have reviewed the connectional organization of the prefrontal cortex in terms of both local connections within the frontal lobe and long association connections with post-Rolandic cerebral cortical regions, including sensory-specific, multimodal, and paralimbic areas. We have also described the distinct association fiber pathways that inter-relate the prefrontal cortex with post-Rolandic cortical areas.
In terms of local connections, these appear to be organized in a manner consistent with the concept of dual prefrontal architectonic-connectional trends, with one trend (and its predominant local connections) occupying the medial and dorsolateral portions of the prefrontal cortex and the other involving the orbital and ventrolateral portions. Within each trend, a given area is connected with both less and more architectonically differentiated regions within the frontal lobe. These connections, overall, may allow for stepwise integration between less differentiated medial and orbital prefrontal areas and highly differentiated lateral areas.
The long association connections can also be interpreted in the context of the overall dorsal-ventral dichotomy in the prefrontal cortex. Thus, the dorsal and medial post-Rolandic visual association areas, the dorsal and medial somatosensory association areas, and the caudodorsal auditory association areas tend to relate via specific pathways preferentially with the dorsal and medial prefrontal regions. The dorsal and medial multimodal and limbic areas also seem to relate more strongly to the dorsal and medial prefrontal regions. In contrast, the lateral and ventral post-Rolandic visual association areas, the ventral somatosensory areas, and the rostroventral auditory association region relate preferentially to the lateral and orbital, and also medial, prefrontal areas. Similarly, the ventral multimodal and limbic areas relate more strongly to ventrolateral and orbital prefrontal, as well as to medial, prefrontal areas.
One feature of the long association connections of the prefrontal region is the specific fiber pathways relating prefrontal with post-Rolandic areas. These pathways seem to be organized in a manner consistent with dual prefrontal trends. Thus, the OFF (visual), the SLF I (somatosensory) and the arcuate fasciculus (auditory) as well as the cingulate fasciculus (limbic) and SLF II (multimodal) convey fibers from dorsal and medial post-Rolandic regions preferentially to medial and dorsolateral prefrontal regions. In contrast, the SLF III (somatosensory), the ILF-SLF II pathway (visual), the extreme capsule (auditory, multimodal) and the uncinate fasciculus (auditory, visual, limbic) convey information from lateral and ventral post-Rolandic regions preferentially to ventrolateral and orbital prefrontal regions.
The anatomical trends discussed above of a fundamental difference between dorsal and ventral regions of the prefrontal cortex are consistent with several proposals regarding dichotomous functional organization within the prefrontal cortex (e.g., Goldman-Rakic, 1996a, 1996b; Macko et al., 1982; O'Reilly, 2010; Pandya and Yeterian, 1985; Petrides, 1994, 2005). Some of these proposals entail broad conceptualizations such as a dorsal-ventral division of the prefrontal cortex into sectors dealing, respectively, with spatial vs. object information processing (e.g., Goldman-Rakic, 1996a, 1996b) by analogy with the dorsal occipito-parietal stream dealing with spatial information and the ventral occipito-temporal stream dealing with object information. More recently, O'Reilly (2010) has modified this conceptualization somewhat to emphasize a “how vs. what” distinction by analogy to the emphasis that Goodale and Milner (1992) have placed on the dorsal occipito-parietal stream as processing the how of action, in addition to spatial processing. The proposal by Petrides (1994, 2005) has conceptualized a mid-dorsolateral prefrontal system for the monitoring and manipulation of information and a mid-ventrolateral prefrontal system for the active retrieval of information from memory. The present review of the local and long connections of the prefrontal cortex indicates that such proposals of dichotomous prefrontal functional organization are tenable from a morphological perspective.
There are some common functions that are often ascribed to the prefrontal cortex, such as planning and sequencing, working memory, decision-making, response inhibition, emotional control, and communication. Although certain areas of the prefrontal cortex have been emphasized as having particularly important roles for these different processes (see Fuster, 2008 for review), we must remember that the various prefrontal areas beginning with the least architectonically differentiated and proceeding to the most architectonically differentiated are interlinked within each of the two cortical trends so that the specific functions in each trend ultimately are tied together in order to serve the overall integrative function of the trend, i.e., the “how-where-spatial” function of the dorsal trend and the “what-object” function of the ventral trend (e.g., Romanski, 2004; O'Reilly, 2010).
Much has been said about the existence of two distinct trends in the prefrontal cortex, supported by morphological and functional observations. However, one of the consistent features of prefrontal connectivity is that certain areas within each cortical trend are related to areas in the opposite trend in terms of both local and long connections, albeit to a lesser extent than within each trend. This is not surprising in that areas belonging to the two different trends ultimately must work together in order to effect complex behaviors that call for the integration of the processes served by each trend. It is of course evident that, in addition to individual prefrontal areas having specific functional roles, these areas must interact to effect overall cognitive, emotional and executive integration (Petrides, 1994, 2005; Wilson et al., 2010). The local connections of the prefrontal areas permit functional interactions between them and their long connections permit interactions with post-Rolandic regions which themselves may provide a substrate for such broad, integrative prefrontal function.
The importance of interruption of frontal cortical connectivity with various post-Rolandic regions has been invoked to explain various aspects of pathological behavior and cognition. For instance, a number of studies have attempted to explain some aspects of spatial neglect in terms of lesions of specific fasciculi linking post-Rolandic cortical regions with the frontal cortex. Mesulam (1981) highlighted the importance of connections between parietal and frontal areas to explain various aspects of neglect. More recently, Doricchi and Tomaiuolo (2003) have provided evidence that extrapersonal spatial neglect (without hemianopia) can be the consequence of damage to the superior longitudinal fasciculus. Thiebaut de Schotten et al. (2005) stimulated the white matter of the inferior parietal lobule during neurosurgical procedures and observed visuo-spatial neglect. These authors originally interpreted the visuospatial neglect as being due to disruption of the occipitofrontal fasciculus, although they later pointed out that it might have been also the disruption of the superior longitudinal fasciculus. More recently, He et al. (2007) observed chronic visual spatial neglect following damage of the superior longitudinal fasciculus.
In conclusion, we have reviewed, from the perspective of dual prefrontal architectonic trends, the local and long association connections of the prefrontal cortex and the major fiber pathways relating prefrontal and post-Rolandic cortical areas. A detailed knowledge of prefrontal cortical connectivity in the monkey brain may help to illuminate further connectional relationships in the human brain, which, in turn, may aid in understanding human brain function.
Acknowledgments
The research of D.P. was supported by the Edith Nourse Rogers Memorial Veterans Administration Medical Center and NIH Grants NS 16842 and NS 20967, of E.Y. by the Colby College Presidential Research Fund, and of M.P. by CIHR grant MOP-14620 and NSERC Grant RGPIN 7466. We would like to thank Mr. Jason Parkhill for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Asanuma C, Cowan WM. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: A study using retrogradely transported fluorescent dyes. Journal of Comparative Neurology. 1985;232(4):443–455. doi: 10.1002/cne.902320403. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. Journal of Comparative Neurology. 1990;296(1):65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Watanabe K, Kubota K. Connections of area 8 with area 6 in the brain of the macaque monkey. Journal of Comparative Neurology. 1988;277(1):21–40. doi: 10.1002/cne.902770103. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Sako H, Murata A. Ipsilateral connections of the anterior cingulate cortex with the frontal and medial temporal cortices in the macaque monkey. Neuroscience Research. 1994;21(1):19–39. doi: 10.1016/0168-0102(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Meunier M, Lu MX, Ungerleider LG. Thalamic and temporal cortex input to medial prefrontal cortex in rhesus monkeys. Experimental Brain Research. 1997;115(3):430–444. doi: 10.1007/pl00005713. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. Journal of Neuroscience. 1991;11(1):168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleydier C, Mauguière F. The duality of the cingulate gyrus in monkey. Brain. 1980;103(3):525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Ban T, Shiwa T, Kawamura K. Cortico-cortical projections from the prefrontal cortex to the superior temporal sulcal area (STs) in the monkey studied by means of HRP method. Archives Italiennes de Biologie. 1991;129(4):259–272. [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1988;276(3):313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56(4):841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. Journal of Comparative Neurology. 1999;410(3):343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam M-M. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. Journal of Comparative Neurology. 1981;200(3):407–432. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam M-M. Cortical afferent input to the principalis region of the rhesus monkey. Neuroscience. 1985;15(3):619–637. doi: 10.1016/0306-4522(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. Journal of Comparative Neurology. 1987;256(2):211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. Journal of Comparative Neurology. 1993;336(2):211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Benton AL. The prefrontal region: Its early history. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Dysfunction. Oxford University Press; New York: 1991. pp. 3–32. [Google Scholar]
- Bianchi L. The functions of the frontal lobes. Brain. 1895;18(4):497–522. [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. Journal of Comparative Neurology. 1990;299(4):421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: An anatomical and neurophysiological study. Journal of Comparative Neurology. 2003;466(2):161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Bonin G von, Bailey P. The Neocortex of Macaca Mulatta. University of Illinois Press; Urbana: 1947. [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cerebral Cortex. 2008;18(5):1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Borra E, Ichinohe N, Sato T, Tanifuji M, Rockland KS. Cortical connections to area TE in monkey: Hybrid modular and distributed organization. Cerebral Cortex. 2010;20(2):257–270. doi: 10.1093/cercor/bhp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: Cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. Journal of Comparative Neurology. 1990;296(3):462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Broca P. Sur la faculté du language articulare. Bulletins et Memoires de la Societé D'Anthropologie de Paris. 1865;6:377–393. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationlehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth; Leipzig: 1909. [Google Scholar]
- Bullier J, Schall JD, Morel A. Functional streams in occipito-frontal connections in the monkey. Behavioural Brain Research. 1996;76(1–2):89–97. doi: 10.1016/0166-4328(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P, Ferraina S, Mitsuda T, Giannetti S, Squatrito S, Maioli MG, Molinari M. Early coding of reaching: Frontal and parietal association connections of parieto-occipital cortex. European Journal of Neuroscience. 1999;11(9):3339–3345. doi: 10.1046/j.1460-9568.1999.00801.x. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivisions of the orbital and medial prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 1994;346(3):366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995a;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1995b;363(4):642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal (1996) Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology. 1989;287(4):393–421. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. Journal of Comparative Neurology. 1999;403(4):431–458. [PubMed] [Google Scholar]
- Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Research. 1972;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Curran EJ. A new association fiber tract in the cerebrum with remarks on the fiber tract dissection method of studying the brain. Journal of Comparative Neurology and Psychology. 1909;19(6):645–656. [Google Scholar]
- Distler C, Boussaoud D, Desimone R, Ungerleider LG. Cortical connections of inferior temporal area TEO in macaque monkeys. Journal of Comparative Neurology. 1993;334(1):125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? NeuroReport. 2003;14(17):2239–2243. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Economo C von, Koskinas GN. Die Cytoarchitektonik der Hirnrinde der erwachsenen Menschen. Springer; Berlin: 1925. [Google Scholar]
- Ferrier D. The Functions of the Brain. Smith, Elder; London: 1876. [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion tensor fiber tractography. Journal of Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. Academic Press; New York: 2008. [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cerebral Cortex. 2010;20(1):141–168. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gattera R. A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosensory and Motor Research. 1995;12(3–4):359–378. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Kuypers HGJM, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: An anatomical and electrophysiological study. Experimental Brain Research. 1984;56(3):410–424. doi: 10.1007/BF00237982. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1996a;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academic of Sciences. 1996b;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Schwartz ML. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982;216(4547):755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Research. 1999;817(1–2):45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Passage of an iron rod through the head. Boston Medical and Surgical Journal. 1848;39(20):389–393. [PMC free article] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: Connections in macaques. Journal of Comparative Neurology. 1990;293(2):299–330. doi: 10.1002/cne.902930211. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: II. Cortical connections. Journal of Comparative Neurology. 1987;265(3):332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. Journal of Comparative Neurology. 2008;509(5):608–641. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. Journal of Comparative Neurology. 1987;264(3):356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Trojanowski JQ. Prefrontal granular cortex of the rhesus monkey: I. Intrahemispheric cortical afferents. Brain Research. 1977;132(2):209–233. doi: 10.1016/0006-8993(77)90417-6. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. Journal of Comparative Neurology. 1976;168(2):197–248. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, Margulies DS, Castellanos FX, Milham MP, Petrides M. Broca's region: Linking human brain functional connectivity data and non-human primate tracing anatomy studies. European Journal of Neuroscience. 2010;32(3):383–398. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. Journal of Comparative Neurology. 1960;115(3):333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in rhesus monkeys. Journal of Comparative Neurology. 2005;493(4):479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2007;465(4):499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. Journal of Neuroscience. 1995;15(5):3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: Areal, modular and retinotopic patterns. Visual Neuroscience. 1990;5(2):165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Szwarcbart MK, Mishkin M, Rosvold HE. Occipitotemporal corticocortical connections in the rhesus monkey. Experimental Neurology. 1965;11(2):245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Projections to the neocortex. Journal of Comparative Neurology. 2002;447(4):394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Inferior frontal eye field projections to the pursuit-related dorsolateral pontine nucleus and middle temporal area (MT) in the monkey. Visual Neuroscience. 1989;3(2):171–180. doi: 10.1017/s0952523800004478. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. Journal of Comparative Neurology. 2000;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Experimental Brain Research. 1990;82(1):214–218. doi: 10.1007/BF00230855. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The organization of the frontal motor cortex. News in Physiological Sciences. 2000;15(5):219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rozzi S, Calzavara R, Matelli M. Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. European Journal of Neuroscience. 2003;17(3):559–578. doi: 10.1046/j.1460-9568.2003.02476.x. [DOI] [PubMed] [Google Scholar]
- Luria AR. The Working Brain: An introduction to neuropsychology. Basic Books; New York: 1973. [Google Scholar]
- Macko KA, Jarvis CD, Kennedy C, Miyaoka M, Shinohara M, Sololoff L, Mishkin M. Mapping the primate visual system with [2–14C]deoxyglucose. Science. 1982;218(4570):394–397. doi: 10.1126/science.7123241. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd Edition Academic Press; New York: 2008. [Google Scholar]
- Maioli MG, Squatrito S, Samolsky-Dekel BG, Sanseverino ER. Corticocortical connections between frontal periarcuate regions and visual areas of the superior temporal sulcus and the adjoining inferior parietal lobule in the macaque monkey. Brain Research. 1998;789(1):118–125. doi: 10.1016/s0006-8993(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Maioli MG, Squatrito S, Galletti C, Battaglini PP, Sanseverino ER. Cortico-cortical connections from the visual region of the superior temporal sulcus to frontal eye field in the macaque. Brain Research. 1983;265(2):294–299. doi: 10.1016/0006-8993(83)90345-1. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Martin-Elkins CL, Horel JA. Cortical afferents to behaviorally defined regions of the inferior temporal and parahippocampal gyri as demonstrated by WGA-HRP. Journal of Comparative Neurology. 1992;321(2):177–192. doi: 10.1002/cne.903210202. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. Journal of Comparative Neurology. 1986;251(3):281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar projections linking periarcuate and lateral intraparietal areas depends on cortical structure. European Journal of Neuroscience. 2006;23(1):161–179. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ. Insula of the old world monkey: III. Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Research. 1977;136(3):393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Meyer A. The frontal lobe syndrome, the aphasias and related conditions. Brain. 1974;97(1):565–600. doi: 10.1093/brain/97.1.565. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences. 1983;6:414–417. [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Arroyo-Jiminez MM, Artacho-Perula E, Insausti AM, Marcos P, Cebada-Sanchez S, Martinez-Ruiz J, Munoz M, Blaizot X, Martinez-Marcos A, Amaral DG, Insausti R. Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. Journal of Anatomy. 2007;211(2):250–260. doi: 10.1111/j.1469-7580.2007.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán MA, Mufson EJ, Mesulam M-M. Neural inputs into the temporopolar cortex of the rhesus monkey. Journal of Comparative Neurology. 1987;256(1):88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stillwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. Journal of Comparative Neurology. 2004;469(1):37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Guela C, Mesulam M-M. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology. 1992;323(3):341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam M-M. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Archives of Neurology. 1993;50(3):279–285. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. Journal of Comparative Neurology. 1993;337(4):669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. (1998) Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Research Bulletin. 1998;45(2):209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) European Journal of Neuroscience. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam M-M. Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. Journal of Comparative Neurology. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. (1984) Some observations on the course and composition of the cingulum bundle in the rhesus monkey. Journal of Comparative Neurology. 1984;225(1):31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Mishkin M, Saunders R. (2009) Resection of the medial temporal lobe disconnects the rostral superior temporal gyrus from some of its projection targets in the frontal lobe and thalamus. Cerebral Cortex. 2009;19(9):2114–2130. doi: 10.1093/cercor/bhn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH. Some efferent connections of the prefrontal cortex in the monkey. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 397–409. [Google Scholar]
- Neal JW, Pearson RCA, Powell TPS. The ipsilateral corticocortical connections of area 7 with the frontal lobe in the monkey. Brain Research. 1990;509(1):31–40. doi: 10.1016/0006-8993(90)90305-u. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC. The what and how of prefrontal cortical organization. Trends in Neurosciences. 2010;33(8):355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN. Anatomy of the auditory cortex. Revue Neurologique. 1995;151(8–9):486–494. [PubMed] [Google Scholar]
- Pandya DN, Dye P, Butters N. Efferent cortico-cortical projections of the prefrontal cortex in the rhesus monkey. Brain Research. 1971;31(1):35–46. doi: 10.1016/0006-8993(71)90632-9. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HGJM. Cortico-cortical connections in the rhesus monkey. Brain Research. 1969;13(1):13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey, and its projection pattern. Zeitschrift für Anatomie und Entwicklungsgeschichte. 1973;139(2):127–161. doi: 10.1007/BF00523634. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology. 1982;204(2):196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam M-M. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research. 1981;42(3–4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex, Volume 4, Association and Auditory Cortices. Plenum Publishing; New York: 1985. pp. 3–61. [Google Scholar]
- Pandya DN, Yeterian EH. Comparisons of prefrontal architecture and connections. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1996;351(1346):1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Parvizi J. Corticocentric myopia: Old bias in new cognitive sciences. Trends in Cognitive Sciences. 2009;13(8):354–359. doi: 10.1016/j.tics.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio AR. Neural connections of the posteromedial cortex in the macaque. Proceedings of the National Academy of the United States of America. 2006;103(5):1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; New York: 2000. [Google Scholar]
- Petrides M. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Volume 9. Elsevier; Amsterdam: 1994. pp. 59–82. [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology. 1984;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. Journal of Comparative Neurology. 1988;273(1):52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Volume 9. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2002a;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; New York: 2002b. pp. 31–50. [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 2006;498(2):227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biology. 2009;7(8):e1000170. doi: 10.1371/journal.pbio.1000170. doi:10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. Cortex. 2011 in preparation. [Google Scholar]
- Potter H, Nauta WJH. A note on the problem of olfactory associations of the orbitofrontal cortex in the monkey. Neuroscience. 1979;4(3):361–367. doi: 10.1016/0306-4522(79)90099-x. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: Anatomical evidence for somatic representation in primate frontal association cortex. Journal of Comparative Neurology. 1989;282(2):293–316. doi: 10.1002/cne.902820210. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cerebral Cortex. 2000;10(9):851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Domain specificity in the primate prefrontal cortex. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(4):421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1999a;403(2):141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature Neuroscience. 1999b;2(12):1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Pandya DN. Architectonics and connections of the posterior parahippocampal gyrus in the rhesus monkey. Society for Neuroscience Abstracts. 1983;9:222. [Google Scholar]
- Rozzi S, Calzavara R, Belmaih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cerebral Cortex. 2006;16(10):1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cerebral Cortex. 16(10):1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. Journal of Comparative Neurology. 2008;506(4):659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sanides F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Annals of the New York Academic of Sciences. 1969;167(1):404–423. [Google Scholar]
- Sarkissov SA, Filimonoff IN, Preobrashenskaya NS. Cytoarchitecture of the Human Cortex Cerebri [in Russian] Medgiz; Moscow: 1949. [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. Journal of Neuroscience. 1995;15(6):4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Weeden VJ. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in the rhesus monkey: Relationship between intraparietal and principal sulcal cortex. Journal of Comparative Neurology. 1984;226(3):403–420. doi: 10.1002/cne.902260309. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Cola MG, Gutierrez C, Massee M, Weldon C, Cusick CG. Overlapping and nonoverlapping cortical projections to cortex of the superior temporal sulcus in the rhesus monkey: Double anterograde tracer studies. Journal of Comparative Neurology. 1996;370(2):173–190. doi: 10.1002/(SICI)1096-9861(19960624)370:2<173::AID-CNE4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Research. 1978;149(1):1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. Journal of Comparative Neurology. 1989;281(1):97–113. doi: 10.1002/cne.902810108. [DOI] [PubMed] [Google Scholar]
- Shiwa T. Corticocortical projections to the monkey temporal lobe with particular reference to the visual processing pathways. Archives Italiennes de Biologie. 1987;125(2):139–154. [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections of the frontal lobe from the macaque frontal eye fields. Journal of Comparative Neurology. 1993;330(2):286–301. doi: 10.1002/cne.903300209. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. Journal of Comparative Neurology. 1995;353(2):291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cerebral Cortex. 2005;15(6):809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; New York: 2002. [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309(5744):2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Trolard P. Le faisceau longitudinal inférieur du cerveau. Revue Neurologique. 1906;14:440–446. [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. Journal of Comparative Neurology. 1986;248(2):190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Experimental Brain Research. 1989;76(3):473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of V4 in the macaque. Cerebral Cortex. 2008;18(3):477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey: II. Frontal lobe afferents. Brain Research. 1975;95(1):25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the monkey: II. Cortical afferents. Journal of Comparative Neurology. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DR. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. Journal of Comparative Neurology. 1987;262(2):256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204(4389):205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeine Ergebnisse unserer Hirnforschung. Journal of Psychology and Neurology. 1919;25(1):279–461. [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area in the macaque monkey. Journal of Comparative Neurology. 1940;73(1):59–86. [Google Scholar]
- Wang Y, Shima K, Isoda M, Sawamura H, Tanji J. Spatial distribution and density of prefrontal cortical cells projecting to three sectors of the premotor cortex. Neuroreport. 2002;13(19):1341–1344. doi: 10.1097/00001756-200207190-00025. [DOI] [PubMed] [Google Scholar]
- Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw Hill; New York: 1964. [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cerebral Cortex. 1994;4(5):470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Wilson CRE, Gaffan D, Browning PGF, Baxter MG. Functional localization within the prefrontal cortex: Missing the forest for the trees? Trends in Neurosciences. 2010;33(12):533–540. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys. Journal of Comparative Neurology. 2010;518(18):3725–3751. doi: 10.1002/cne.22420. [DOI] [PubMed] [Google Scholar]