Abstract
Purpose
To determine whether breast cancer subtype is associated with patterns of ipsilateral breast tumor recurrence (IBTR), either true recurrence (TR) or elsewhere local recurrence (ELR), among women with pT1-T2 invasive breast cancer (IBC) who receive breast-conserving therapy (BCT)
Methods and Materials
From 1/1998 to 12/2003, 1223 women with pT1-T2N0-3 IBC were treated with BCT (lumpectomy + whole breast radiation). Ninety percent of patients received adjuvant systemic therapy, but none received trastuzumab. Biologic subtype was approximated using estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2): luminal A (ER+ or PR+ and HER-2-), luminal B (ER+ or PR+ and HER-2+), HER-2 (ER- and PR- and HER-2+), and basal (ER- and PR- and HER-2-). Imaging, pathology and operative reports were reviewed by two physicians independently, including an attending breast radiologist. Readers were blinded to subtype and outcome. TR was defined as IBTR within the same quadrant and within three centimeters of the primary tumor. All others were ELR.
Results
At median follow-up of 70 months, 24 patients developed IBTR (5-year cumulative incidence 1.6%), including 15 TR and 9 ELR. At 5 years, basal (4.4%) and HER-2 (9%) subtypes had a significantly higher incidence of TR compared with luminal B (1.2%) and luminal A (0.2%) subtypes (p<0.0001). On multivariate analysis, basal subtype (HR 4.8, p=0.01), younger age at diagnosis (HR 0.97, p=0.05), and increasing tumor size (HR 2.1. p=0.04) were independent predictors of TR. Only younger age (HR 0.95, p=0.01) significantly predicted for ELR.
Conclusions
Basal and HER2 subtypes are significantly associated with higher rates of TR among women with pT1-T2 IBC after BCT. Younger age predicts for both TR and ELR. Strategies to reduce TR in basal breast cancers, such as increased boost doses, concomitant radiation and chemotherapy, or targeted therapy agents, should be explored.
Keywords: breast cancer, biologic subtype, basal, triple negative, local recurrence, true recurrence, elsewhere recurrence
Introduction
While breast-conserving therapy (BCT), consisting of conservative surgery followed by whole breast radiation, has become the standard of care for early-stage breast cancer, (1–3) ipsilateral breast tumor recurrence (IBTR) continues to be of clinical concern with an overall risk of 2–8% by 10 to 15 years in modern series (4–6). IBTR can occur as two different entities: true recurrence (TR) which is thought to arise in the vicinity of the original tumor from residual cancer cells, and elsewhere local recurrence (ELR) which is thought to occur as a de novo cancer or from previously undetected microscopic disease in a different area of the ipsilateral breast (7).
Clinical studies of conservative breast surgery alone report that 80–85% of IBTR occur in the vicinity of the original tumor (8, 9), and studies of IBTR after BCT report that 44–79% are TR (10–16). These findings have implications for treatment, as the rationale for considering accelerated partial breast irradiation (APBI) is based on the notion that most IBTR after BCT occur within the original tumor vicinity. Published consensus guidelines for APBI have attempted to identify those patients with a low risk of clinically-occult disease remote from the lumpectomy cavity who would be suitable for this treatment (17).
DNA microarray profiles can be used to classify breast tumors into distinct biologic subtypes (18, 19). Because this testing is often not feasible in the clinical setting, these subtypes can be approximated using commonly available markers of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) as follows: luminal A (ER+ or PR+ and HER-2-), luminal B (ER+ or PR+ and HER-2+), HER-2 (ER- and PR- and HER-2+), and basal (ER- and PR- and HER-2-) (20). Recent studies show that biologic subtypes are prognostic for local-regional recurrence and distant metastasis after BCT (21–25), with the basal and HER-2 subtypes associated with higher rates of both. In a subset analysis of the Danish post-mastectomy trials, these two subtypes were also associated with little to no benefit in overall survival after post-mastectomy radiation therapy (26).
The impact of biologic subtype on the pattern of IBTR in the breast is unclear. Further understanding of this relationship can help inform therapeutic decision-making, in particular with respect to the radiation field to the breast following conservative surgery. The purpose of this study was to determine whether breast cancer subtype, as approximated by ER, PR, and HER-2, is associated with the type of IBTR among women with early-stage breast cancer who receive BCT.
Methods and Materials
Patient selection
The study cohort included 1223 consecutively treated women with early stage invasive breast cancer who received breast conserving surgery followed by whole breast radiation from January 1, 1998 through December 31, 2003 at Dana-Farber Cancer Institute/Brigham and Women’s Hospital (n=692) or Massachusetts General Hospital (n=531) in Boston, MA. All patients had newly diagnosed, nonmetastatic 2002 American Joint Committee on Cancer (AJCC) (27) pathologic stage T1-T2 invasive breast cancer. Patients with prior malignancy (except non-melanoma skin cancers), synchronous bilateral breast cancer, or treatment with preoperative systemic therapy were excluded. The study was approved by the Institutional Review Board of the affiliated hospitals.
Classification of subtype groups
Patients were categorized based on the receptor status of their primary tumor: luminal A (ER-positive or PR-positive and HER-2–negative), luminal B (ER-positive or PR-positive and HER-2–positive), HER-2 (ER-negative, PR-negative, and HER-2–positive), and basal (ER-negative, PR-negative, and HER-2–negative). ER and PR status were assessed by immunohistochemical (IHC) staining. Tumors were considered HER-2– positive if they were scored 3 on IHC staining or if they were scored 2 or greater by IHC staining and showed HER-2 amplification (ratio >2.0) based on fluorescence in situ hybridization (FISH). Tumors that scored 2 or greater by IHC staining in the absence of FISH amplification were considered HER-2 negative (28).
Treatment
All patients underwent local excision of the primary breast cancer and 1130 patients (92%) underwent surgical lymph node evaluation. Adjuvant chemotherapy was given to 46% of patients, hormonal therapy to 77% of patients, and both to 32% of patients. None of the patients received pre-operative chemotherapy, and none received adjuvant trastuzumab. All patients received external beam radiation therapy to the whole breast. Median dose was 60 Gy (range 20–72). The most common doses were 45 Gy in 1.8-Gy fractions to the whole breast followed by tumor-bed boost to 61 Gy, or 50 Gy in 2.0-Gy fractions to the whole breast followed by tumor-bed boost to 60 Gy. One patient chose to stop treatment after 20 Gy, despite counseling by the treating physician to complete the entire course of radiation. A separate supraclavicular or axillary field was typically added after axillary dissection only if there were four or more involved axillary lymph nodes.
Follow-Up
Patients were generally seen in follow-up 4 to 6 weeks after radiation therapy was completed, and then every 6 months thereafter with yearly breast imaging. For patients who discontinued follow-up, attempts were made to obtain follow-up information from their primary care physician. Follow-up time was counted from date of diagnosis to the date of the first event, or to the last known confirmed date of breast cancer disease-free status. In total, 64 patients (5%) were lost to follow-up.
Local recurrence and coding as true versus elsewhere
Patients from the cohort with IBTR as their first event were identified. All of these patients had yearly breast imaging and original images accessible. For each recurrence, imaging of the primary and recurrent tumor were reviewed with pathology reports to compare histology and operative notes to better characterize location of the recurrent tumor with respect to excision site and scars. Mammograms, magnetic resonance imaging (MRI), if available, and ultrasounds were reviewed for each case by an attending breast radiologist (PF, DGS) and resident radiation oncologist (JAH). Each IBTR was coded as a TR if the recurrence was in the same quadrant of the breast and within three centimeters of the primary tumor bed. All other IBTR were coded ELR. Readers were blinded to biologic subtype.
Outcomes and statistical methods
The primary study endpoint was type of IBTR, either TR or ELR. Time to TR or ELR was a secondary endpoint. The primary study variable was biologic subtype. Additional covariates included age (continuous), grade (1 or 2 vs. 3), margin status (positive vs. <2mm but not on ink vs. ≥ 2mm), nodal status (positive vs. negative), tumor size (continuous), pathologic tumor stage (T, T1 vs. T2), and lymphovascular invasion (LVI present vs. absent or unknown).
Chi-square test was performed to compare baseline characteristics among the four subtype groups. A nonparametric K-sample test was used to compare medians across groups. The five-year actuarial estimates of TR and ELR were calculated by the Kaplan-Meier method and a log-rank test was used to compare incidence functions. Cox regression univariable analysis (UVA) was performed for TR and ELR. The association between subtype and time to TR or ELR as a first event was analyzed using Gray’s competing risks multivariable analysis (MVA), and each significant factor on UVA was put into the model. Competing events included death and distant failure. All analyses were performed in Stata 11.1 (StataCorp, College Station, TX). All statistical tests were two-sided and statistical significance was defined as a p-value of 0.05.
Results
Baseline characteristics and association with biologic subtype
Patient characteristics of the cohort are shown in Table 1. The majority of patients (81%) had pT1 tumors and most (73%) were node negative. Margins were close (< 2 mm but not on ink) or negative (≥ 2 mm) in 96% of patients. The luminal A subtype made up 77% of the patient cohort and HER-2 only 4.3%. One patient received less than 50 Gy and did not have any disease recurrence.
Table 1.
Baseline Patient Characteristics
| All patients (n=1223) | ||
|---|---|---|
| Characteristic | No. | % |
| Age at diagnosis, median, years (range) | 55 (23–89) | |
| Age, years | ||
| <40 | 90 | 7.4 |
| 40–50 | 307 | 25.2 |
| 50–60 | 414 | 33.9 |
| >60 | 408 | 33.5 |
| Menopausal Status | ||
| Pre | 372 | 30.4 |
| Post | 742 | 60.6 |
| Peri | 102 | 8.3 |
| Unknown | 7 | 0.7 |
| Pathologic T stage* | ||
| T1mic | 6 | 0.5 |
| T1a | 144 | 11.8 |
| T1b | 294 | 24.0 |
| T1c | 544 | 44.5 |
| T2 | 235 | 19.2 |
| Pathology | ||
| Invasive ductal | 958 | 78.3 |
| Invasive lobular | 138 | 11.3 |
| Ductal + lobular | 116 | 9.5 |
| Tubular | 5 | 0.4 |
| Medullary | 1 | 0.1 |
| Mucinous | 4 | 0.3 |
| Unknown | 1 | 0.1 |
| Pathologic N stage† | ||
| N0 | 888 | 72.6 |
| N1 | 260 | 21.3 |
| N2 | 51 | 4.2 |
| N3 | 14 | 1.1 |
| Nx | 8 | 0.7 |
| Grade | ||
| 1 | 311 | 25.4 |
| 2 | 509 | 41.6 |
| 3 | 386 | 31.6 |
| Unknown | 17 | 1.4 |
| Margins | ||
| Negative (≥ 2mm) | 1013 | 82.8 |
| Close (< 2mm but not on ink) | 160 | 13.2 |
| Positive (on ink) | 48 | 3.9 |
| Unknown | 1 | 0.1 |
| LVI positive | 318 | 26 |
| LVI negative | 900 | 73.6 |
| Unknown | 4 | 0.3 |
| Subtype | ||
| Luminal A | 937 | 76.6 |
| Luminal B | 98 | 8.0 |
| Her 2 | 52 | 4.3 |
| Basal | 136 | 11.1 |
| RT dose | ||
| < 50 Gy | 1 | 0.1 |
| 50– 60 Gy | 710 | 57.8 |
| > 60 Gy | 517 | 42.1 |
| Chemotherapy | ||
| Yes | 558 | 45.7 |
| No | 663 | 54.3 |
| Unknown | 0 | 0 |
| Hormonal Therapy | ||
| Yes | 942 | 77.1 |
| No | 277 | 22.7 |
| Unknown | 2 | 0.2 |
Pathologic T and N stage are based on AJCC 2002 6th edition guidelines (27)
LVI=lymphovascular invasion
RT=radiation therapy
Gy= Gray
As shown in Table 2, among the subtypes there were significant differences in median age (p < 0.0001), pT1 disease (p<0.0001), presence of LVI (p=0.001), node positivity (p<0.0001), and histologic grade (p<0.0001). HER-2 and basal subtypes were more frequently associated with younger age, more advanced pathologic T stage, lymph node involvement, higher grade, and LVI.
Table 2.
Baseline Patient Characteristics Stratified by Subtype
| Characteristic | All pts (n=1223) |
Luminal A (n=937) |
Luminal B (n=98) |
Her-2 (n=52) |
Basal (n=136) |
p-value |
|---|---|---|---|---|---|---|
| Median age (years) | 55 | 56 | 54 | 50 | 53 | <0.0001 |
| T1 (%) | 80 | 84 | 76 | 67 | 64 | <0.0001 |
| LVI (%) | 26 | 24 | 40 | 39 | 27 | 0.001 |
| Node positive (%) | 27 | 24 | 36 | 46 | 32 | <0.0001 |
| Grade 3 (%) | 32 | 20 | 47 | 71 | 84 | <0.0001 |
| Systemic treatment, (%) | 90 | 93 | 92 | 73 | 80 | <0.0001 |
| Chemotherapy | 46 | 38 | 67 | 69 | 76 | <0.0001 |
| Hormonal therapy | 77 | 89 | 90 | 10 | 10 | <0.0001 |
| Margins positive, % | 4 | 4 | 6 | 2 | 4 | NS |
| Median RT Dose, Gy | 60 | 60 | 60 | 60 | 61 | NS |
| Post-menopausal, % | 60 | 62 | 55 | 46 | 58 | NS |
LVI=lymphovascular invasion
RT=radiation therapy
Gy= Gray
Rates of TR and ELR
At a median follow-up of 70.4 months, 24 patients developed IBTR as the site of first recurrence (5-year cumulative incidence = 1.6%). Of these, 15 (63%) were coded as TR and 9 (37%) were ELR. Mean time to TR was 3.6 years compared with 4.2 years to ELR which was not statistically significant (p=0.5).
Factors associated with TR
Table 3 summarizes results of the UVA of factors associated with TR. When compared with all other subtypes, basal subtype was associated with a seven-fold increased risk of TR (p<0.0001). Age at diagnosis was also associated with TR with a HR of 0.96 or a 4% decrease in risk per 1 year increase in age (p=0.003). Tumor size was associated with a two-and-a-half fold increase in TR per centimeter increase in size (p=0.002). Grade 3 disease and node positivity were also associated with an increased risk of TR. Margin status and LVI were not significant on UVA.
Table 3.
Univariable Analysis of factors associated with TR
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| Basal vs. all other subtypes | 7.2 | 2.6–19.7 | p<0.0001 |
| Age at diagnosis, years (continuous) | 0.96 | 0.93–0.99 | p=0.003 |
| Tumor size, cm (continuous) | 2.5 | 1.4–4.5 | p=0.002 |
| Grade 3 vs. Grade 1/2 | 14.7 | 3.3–64.3 | p<0.0001 |
| Node positive vs. negative | 4.1 | 1.5–11.7 | p=0.008 |
HR= hazard ratio
CI=confidence interval
cm=centimeter
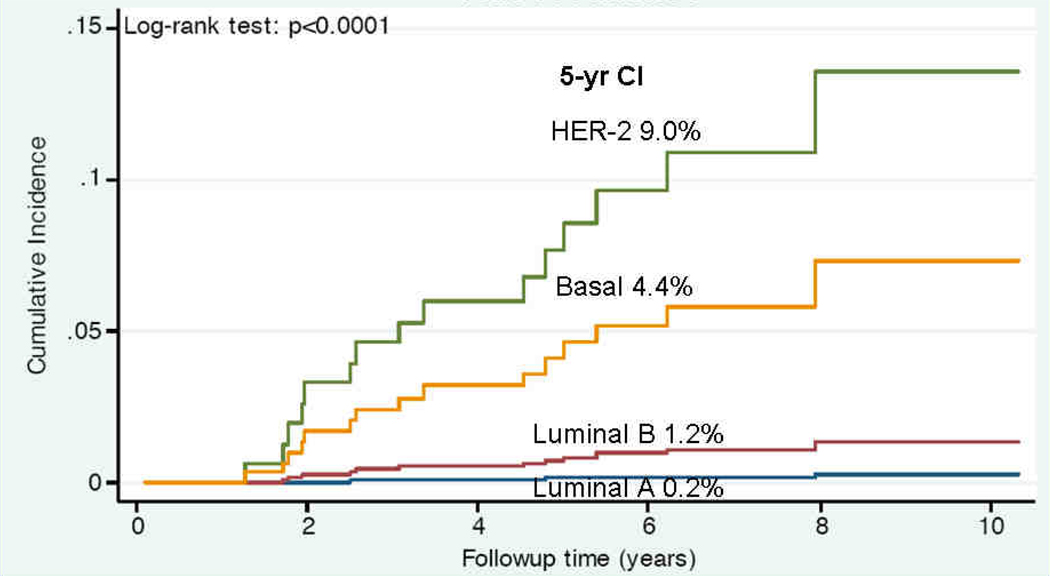
Multivariable analysis (MVA) of factors associated with TR is shown in Table 4. Basal subtype remained significantly associated with increased risk of TR (HR 4.8, p=0.01). Younger age and larger tumor size also predicted for TR. Figure 1 shows the cumulative incidence plot of TR stratified by subtype. At 5 years, basal (4.4%) and HER-2 (9%) subtypes had a higher incidence of TR compared to luminal B (1.2%) and luminal A (0.2%) subtypes (p<0.0001).
Table 4.
Multivariable Analysis of Factors Associated with TR
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| Basal subtype | 4.8 | 1.4–15.8 | p=0.01 |
| Age at diagnosis, years (continuous) | 0.97 | 0.94–0.99 | p=0.05 |
| Tumor size, cm (continuous) | 2.1 | 1.1–4.2 | p=0.04 |
HR= hazard ratio
CI=confidence interval
cm=centimeter
Figure 1.
Cumulative incidence plot of TR stratified by breast cancer subtype. Five-year rates of TR are shown, p<0.0001 (log-rank test).
Factors associated with ELR
On UVA, age at diagnosis was the only factor associated with ELR, with a HR of 0.95 (95% CI 0.91–0.98, p=0.01) or a 5% decrease in risk of ELR for every 1 year increase in age. Biologic subtype was not associated with ELR (p=0.59). The 5-year cumulative incidence of ELR was 0.48% (95% CI 0.2–1.2%).
Actuarial analysis of TR and ELR by age group
Table 5 shows five-year actuarial rates of TR and ELR by age group. Age cutpoints were chosen based on even distribution across quartiles, and by dividing the cohort by median age (55 years). The 5-year rate of TR was highest in patients less than 47 years of age (2.3%), with no events in patients older than 64. Similarly, rate of ELR was highest in patients less than 47 (1.6%), with no events in patients older than 55.
Table 5.
Five-year Rates of TR and ELR by Age Groups *
| Age (years) | 5 year rates of TR or ELR, % (95% CI) |
|
|---|---|---|
| TR | ELR | |
| < 47 | 2.3 (1.1 – 5.2) | 1.6 (0.6 – 4.2) |
| 47–55 | 1.2 (0.4 – 3.8) | 0.3 (0.05– 2.4) |
| 55–64 | 1.0 (0.3 – 3.2) | 0 |
| > 64 | 0 | 0 |
| < 55 | 1.8 (0.9 – 3.5) | 1.0 (0.4 – 2.3) |
| > 55 | 0.5 (0.2 – 1.6) | 0 |
Cutpoints were chosen based on even distribution across quartiles, and divided by median age (55 years)
CI=confidence interval
Discussion
We assessed whether biologic subtype, as approximated by ER, PR, and HER-2, is associated with the pattern of IBTR in women who underwent BCT for early-stage breast cancer. Basal subtype, when compared with all other subtypes, was an independent predictor of TR (HR 4.8, p=0.01). Younger age and increasing tumor size were also significantly associated with TR on MVA. Younger age was the only predictor of ELR, while subtype was not. Margin status was not a significant predictor of TR in the present study, but only 4% of patients had positive margins and all patients with IBTR had negative margins, thus limiting our ability to detect this association. We also found a high rate of TR among HER-2 subtype patients, although they made up only 4.3% of the cohort. The prevalence of HER-2 subtype patients in the Carolina Breast Cancer Study was only 6.7% so the small proportion of HER-2 patients appears similar to population-based studies (29).
The majority of IBTR in the present study were at or near the primary site (TR 63%) compared with 37% away from the primary site (ELR). These numbers are similar to those reported in other studies, with rates of TR generally ranging from 59–79% (4, 10–16). We also found the mean time to TR was 3.6 years compared with 4.2 years to ELR though the difference was not statistically significant. Longer time to ELR compared to TR is consistent with other studies (4, 11, 14–16, 30). In a study by Smith and colleagues of 130 patients with IBTR, 51% were considered new primary tumors compared with 44% TR (15). New primary tumors were distinctly different from the original primary with respect to location, histology or flow cytometry. This lower TR rate is likely because of the extended follow up period of 14.2 years over which time the number of TR levels off and ELR or “new primary” tumors increase.
There is no clear consensus on the ideal way to designate TR from ELR. Most studies take the location of the primary and recurrent tumor into account by quadrant when making this designation (4, 10, 11, 31). Komoike et al. used location and surgical margin to classify IBTR (13), Smith et al. also looked at change in histology and DNA flow cytometry (14), while Huang et al. designated a TR as one located within 3 cm of the primary tumor bed and with the same histology (12). In a study by Recht et al. IBTR were classified as TR, marginal miss (MM), and ELR based on the location of the recurrence with respect to the radiation field and boost volume (15, 32). Kurtz et al. defined “new tumors” as occurring at least 5 cm away from the primary tumor (16). We designed our methodology after reviewing the studies above.
To our knowledge, the present study appears to be the only one to specifically look for association of different breast cancer subtypes with type of IBTR. Smith et al. reported that ER status was not related to location of IBTR, but their group did not specifically look at subtype (14). Huang et al. reported that more patients with ER+ tumors developed new primary tumors rather than TR (p=0.05) (12). This is similar to what we see in the present study where 6 of the 9 ELR were luminal A tumors. However, we have too few ELR events for this to establish statistical significance.
We found that basal subtype was associated with an increased risk of TR. Some studies have shown that the basal or triple negative subtype of breast cancer is associated with an increased risk of IBTR (22, 33), and a shorter median time to recurrence (31). Our results suggest that these recurrences are more often within or close to the primary tumor site. This finding is supported by evidence showing the biologic aggressiveness of basal or triple negative breast cancer (34, 35), and may even suggest some inherent resistance to radiation. There was also a high rate of TR among the HER-2 subtype patients in the current study. Voduc and colleagues determined molecular subtypes of 2,985 tumors, and found that the HER-2 and basal subtypes demonstrated an increased risk of regional recurrence after BCT (24). Another study found a higher rate of local-regional recurrence among ER/PR negative or HER-2 positive patients with T1a,b N0 breast cancer (21). In these studies, like the present study, none of the HER-2 positive patients received adjuvant trastuzumab.
The results of this study may have implications for the use of APBI. Had we found that a particular subtype predicted for ELR, this group could potentially be less suitable for APBI and possibly better treated with whole breast radiation. However, this was not the case in the present study. Given the small number of events and limited follow-up time, subtype was not associated with ELR. Also, the basal group had a higher rate of distant metastases (15.4% vs. 4.8% among all other subtypes, p<0.0001) and death (16.2% vs. 4.1% among all other subtypes, p<0.0001). Thus, in a competing risks analysis, more basal patients would have been censored prior to developing ELR. Longer follow up will be needed to better characterize the association of subtype and ELR. Nevertheless, evidence of the unsuitability of a specific subtype to APBI should come primarily from analyzing APBI trials and evaluating patterns of failures.
Younger age was associated with increased risk of ELR, which is consistent with findings reported by Komoike et al (13). However, Recht et al. showed that the incidence of TR/MM was higher in age ≤ 34 years compared with age > 34 years (17% vs. 8%), but there was little difference noted between rates of elsewhere recurrences (15). In our study, age was an independent risk factor for TR and ELR, particularly in women under 55 years of age. Young women with breast cancer are more likely than those with later-onset disease to carry a genetic predisposition to breast carcinoma, including mutations in BRCA1/BRCA2 tumor suppressor genes (36). Haffty et al. showed that carriers of BRCA1/BRCA2 mutations have significantly higher risks of late IBTR when compared with sporadic breast carcinoma patients (37). This may be consistent with the possible development of second primary tumors. Regardless of mediating factors, it is well reported in the literature that young age is an independent risk factor for local recurrence after BCT (38–41). The consensus statement from the American Society for Radiation Oncology (ASTRO) recommends against treating women <50 years with APBI outside of a clinical trial, and few women in this age group have been treated with APBI in prospective studies (17). Our results support this recommendation.
The different patterns of recurrence among breast cancer subtypes could partially be explained by “early” versus “late” IBTR among the different subtypes. Luminal tumors tend to have a more indolent course (24), and it is possible that the rates of TR among the subtypes might equalize over time. Longer follow-up will be required to fully understand the pattern of IBTR among breast cancer subtypes over time.
There are several potential limitations to this study. Firstly, it is retrospective and may be subject to inherent biases. In addition, there were a limited number of events. Longer follow-up of this cohort will be needed to better characterize ELR or new primary tumors. Lack of adjuvant trastuzumab, which had not yet become the standard of care for patients with HER-2 positive early stage breast cancer (28, 42), tempers the findings for the luminal B and HER-2 groups. Subtypes were approximated with ER, PR, and HER-2 markers, rather than the gold standard of genotyping. However, given that receptor information is readily available in the clinic, this method may have more general applicability. Lastly, while our methodology for discerning TR from ELR was robust and consistently applied to each case, future research on quantitative DNA fingerprinting techniques may more objectively distinguish recurrent tumor from new primary breast cancer (43).
In conclusion, we found that basal subtype was a significant independent predictor of TR in women with T1-T2 breast cancer who receive BCT. Age was significantly associated with both TR and ELR. These results may have implications with respect to the radiation field, in particular whole breast compared with partial breast treatment. Nevertheless, further studies on the patterns of failure for patients treated with APBI with regard to biological subtypes are needed. Furthermore, future research towards reducing TR in basal or triple negative breast cancers, such as the use of targeted systemic agents, higher boost doses, or concomitant chemotherapy and radiation, is warranted.
Acknowledgments
The study was supported in part by: Award Numbers R01CA139118 (AGT) and P50CA089393 (AGT) from the National Cancer Institute (NCI); Grant CA50628 (AN) from the National Institutes of Health (NIH); Jane Mailloux Research Fund (AGT); Blanche Montesi Fund (AGT); Tim Levy Fund for breast cancer research (AGT). Content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at: 51st Annual American Society for Radiation Oncology (ASTRO) Meeting, Chicago, IL (November 1-5, 2009)
Conflicts of Interest Notification:
None
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Freedman GM, Anderson PR, Hanlon AL, et al. Pattern of local recurrence after conservative surgery and whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2005;61:1328–1336. doi: 10.1016/j.ijrobp.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Schnitt SJ, Hayman J, Gelman R, et al. A prospective study of conservative surgery alone in the treatment of selected patients with stage I breast cancer. Cancer. 1996;77:1094–1100. [PubMed] [Google Scholar]
- 9.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 10.Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833–842. doi: 10.1016/0360-3016(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 11.Gujral DM, Sumo G, Owen JR, et al. Ipsilateral Breast Tumor Relapse: Local Recurrence Versus New Primary Tumor and the Effect of Whole-Breast Radiotherapy on the Rate of New Primaries. Int J Radiat Oncol Biol Phys. 2011;79:19–25. doi: 10.1016/j.ijrobp.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 12.Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 13.Komoike Y, Akiyama F, Iino Y, et al. Analysis of ipsilateral breast tumor recurrences after breast-conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer. 2005;12:104–111. doi: 10.2325/jbcs.12.104. [DOI] [PubMed] [Google Scholar]
- 14.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 15.Recht A, Silen W, Schnitt SJ, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1988;15:255–261. doi: 10.1016/s0360-3016(98)90002-5. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz JM, Amalric R, Brandone H, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer. 1989;63:1912–1917. doi: 10.1002/1097-0142(19890515)63:10<1912::aid-cncr2820631007>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 21.Albert JM, Gonzalez-Angulo AM, Guray M, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 2010;77:1296–1302. doi: 10.1016/j.ijrobp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 23.Wo JY, Taghian AG, Nguyen PL, et al. The association between biological subtype and isolated regional nodal failure after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2010;77:188–196. doi: 10.1016/j.ijrobp.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 25.Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 26.Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 27.Greene FLPD, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 28.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 29.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 30.Gage I, Recht A, Gelman R, et al. Long-term outcome following breast-conserving surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:245–251. doi: 10.1016/0360-3016(95)02001-R. [DOI] [PubMed] [Google Scholar]
- 31.Parikh RR, Housman D, Yang Q, et al. Prognostic value of triple-negative phenotype at the time of locally recurrent, conservatively treated breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:1056–1063. doi: 10.1016/j.ijrobp.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 32.Recht A, Silver B, Schnitt S, et al. Breast relapse following primary radiation therapy for early breast cancer. I. Classification, frequency and salvage. Int J Radiat Oncol Biol Phys. 1985;11:1271–1276. doi: 10.1016/0360-3016(85)90241-x. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee S, Reis-Filho JS, Ashley S, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45 Suppl 1:27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 35.Gluz O, Liedtke C, Gottschalk N, et al. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 36.Robson M, Gilewski T, Haas B, et al. BRCA-associated breast cancer in young women. J Clin Oncol. 1998;16:1642–1649. doi: 10.1200/JCO.1998.16.5.1642. [DOI] [PubMed] [Google Scholar]
- 37.Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359:1471–1477. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 38.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 39.Jobsen JJ, van der Palen J, Ong F, et al. The value of a positive margin for invasive carcinoma in breast-conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys. 2003;57:724–731. doi: 10.1016/s0360-3016(03)00644-8. [DOI] [PubMed] [Google Scholar]
- 40.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 41.Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer. 2004;101:1264–1274. doi: 10.1002/cncr.20523. [DOI] [PubMed] [Google Scholar]
- 42.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 43.Schlechter BL, Yang Q, Larson PS, et al. Quantitative DNA fingerprinting may distinguish new primary breast cancer from disease recurrence. J Clin Oncol. 2004;22:1830–1838. doi: 10.1200/JCO.2004.05.123. [DOI] [PubMed] [Google Scholar]