Abstract
Background
Pleomorphic liposarcoma (PLS) is a rare high grade sarcoma showing lipoblastic differentiation. We evaluated PLS natural history, patient outcomes, and commonly deregulated protein biomarkers.
Patients and methods
PLS patient medical records (n=155; 1993 to 2010) were reviewed. Univariable and multivariable analyses were conducted to identify independent prognosticators. A PLS tissue microarray (TMA; n=56 human specimens) was constructed for immunohistochemical analysis of molecular markers. p53 gene sequencing (exon 5–9) was conducted.
Results
Average patient age was 57 years with primary (n=102), recurrent (n=16), and metastatic (n=37) presentations. Lower extremity was the most common site (40%); average tumor size was 11cm. Complete follow-up data were available for 83 patients with 22.6 months median follow-up; the 5Y DSS was 53%. Recurrent disease, unresectability, and microscopic positive margins predicted poor prognosis. Systemic relapse (the strongest poor prognostic determinant) occurred in 35% of localized PLS patients. IHC revealed increased expression of PPARγ (adipogenic marker), BCL2 and survivin (survival factors), VEGF (angiogenic factor), MMP2 metalloprotease, and other biomarkers. Frequent loss of Rb expression and high p53 mutation rates (~60%) were identified.
Conclusions
PLS is an aggressive metastasizing sarcoma. Identifying ubiquitous molecular events underlying PLS progression is crucial for progress in patient management and outcomes.
Keywords: Pleomorphic liposarcoma, Clinical outcome, Tissue microarray, Molecular biomarkers, p53 mutations
Introduction
Pleomorphic liposarcoma (PLS) is a rare high grade pleomorphic sarcoma; the presence of lipoblasts is required for diagnosis (1,2). Due to their rarity (less than 5% of all liposarcomas) knowledge of PLS natural history stems from anecdotal reports and small cohort analyses typically contained within larger liposarcoma studies; only three PLS-specific reports include more than 50 patients (3–5). Such caveats notwithstanding, these studies demonstrate that, as compared to other liposarcoma histological subtypes (well differentiated/dedifferentiated liposarcoma [WDLPS/DDLPS] and myxoid/round cell liposarcoma [MRC]), PLS is the most aggressive -- exhibiting avidity for systemic spread and a poor overall outcome (3–5). Surgical resection is currently the only potentially curative approach to these remarkably chemoresistant tumors; locally advanced and metastatic disease is generally non-curable. This dismal outcome of patients with PLS mandates development of improved (perhaps molecularly-based) therapeutic strategies.
PLS characteristically harbor diverse chromosomal rearrangements and genomic profiles without unifying molecular alterations, a circumstance typical of soft tissue sarcomas (STSs) with complex karyotypes; e.g., leiomyosarcoma, angiosarcoma, and myxofibrosarcoma (6–9). In contrast, WDLPS/DDLPS and MRC commonly exhibit distinctive genetic aberrations; i.e., 12q13-15 chromosomal amplification in WDLPS/DDLPS and a (12;16) translocation resulting in a FUS-DDIT3 fusion gene in MRC (10–12). This genetic complexity suggests that singular dominant molecular aberrations are unlikely to underlie PLS tumorigenesis and progression. Consequently, it may be more therapeutically relevant to elucidate the currently unknown spectrum of PLS deregulated pathways and/or processes rather than search for a possibly dominant yet non-existent locus of PLS ‘oncogenic addiction’. In light of these knowledge gaps, we chose to investigate the natural history and clinical outcome of a large PLS cohort treated at a single institution, seeking to identify disease-specific survival (DSS) prognosticators and to identify commonly deregulated molecular processes/biomarkers using human PLS specimens assembled in a tissue microarray (TMA).
Patients and methods
Clinical database
This study was conducted with institutional review board (IRB) approval from the University of Texas MD Anderson Cancer Center (UTMDACC). PLS patients seen at UTMDACC from 1/1993 through 1/2010 were identified by search of the UTMDACC prospective sarcoma database, institutional tumor registry, and pathology archives. Only patients with unequivocal PLS histology confirmed by a UTMDACC sarcoma pathologist (AJL) were included in the study (n=155); the presence of lipoblasts was mandatory for diagnosis; cases of “pleomorphic liposarcoma” in the background of well- or dedifferentiated liposarcoma or inconclusive diagnoses were excluded. An initial database was constructed including demographic and tumor associated variables. For patients with sufficient follow-up information (n=83) treatment and outcome information were included. Only patients with localized PLS were included in the univariable and multivariable analyses.
Tissue microarray (TMA) construction
After evaluation of all potential PLS FFPE samples available, 56 blocks representing tumors derived from 37 patients were selected for inclusion in the TMA. Five FFPE blocks of each of the following histologies: dedifferentiated liposarcoma (DDLPS), myxoid liposarcoma (MLPS), and unclassified pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH) were included as controls. TMA was constructed as previously described (13). In brief H&E-stained sections were reviewed from each tumor block by an institutional sarcoma pathologist (AJL) to define areas of homogeneous, viable tumor. Using an automated TMA apparatus (ATA-27, Beecher Instruments), 0.6 mm punch samples (2 per case) were obtained from each donor block and formatted into a recipient block. Sections (4μm) were cut and verified by H&E.
Immunohistochemistry
Commercially available antibodies against PPARγ (Cell Signaling, Danvers, MA), Adipophilin (Fitzgerald, Acton, MA), BCL2 (Biogenex, Fremont, CA), Survivin (Abcam, Cambridge, MA), p16 (CINtec, Heidelberg, Germany), Rb (BD Pharmingen, San Diego, CA), Cyclin D1 (Labvision, Freemont, CA), CDK4 (Invitrogen, Carlsbad, CA), VEGF (Santa-Cruz, Santa Cruz, CA), MMP2 (Millipore, Billerica, MA), MMP9 (Millipore), β-catenin (BD Biosciences, San Jose, CA), EGFR (Zymed, Carlsbad, CA), MDM2 (EMD, Gibbstown, NJ) and p53 (Dako, Carpenteria, CA) were used for immunohistochemical staining. Spots representing 40 different samples from 37 different patients were applicable for scoring analysis, in three cases both a localized and a metastatic lesion from the same patients was evaluated; labeling intensity was scored by two observers (MPG and AJL) as none, low, or medium-to-high; when pertinent, cytoplasmic and/or nuclear staining were scored separately.
p53 sequencing
Genomic DNA was extracted from paraffin-embedded tissue using a QIAamp DNA Mini Kit (Qiagen Sciences, Valencia, CA) per manufacturer’s instructions. Integrity and concentration of the extracted DNA was assessed with the NanoDrop-1000 Spectrophotometer (NanoDrop-Products, Wilmington, DE). DNA sequencing was conducted as previously described (14). In brief: primers were designed for intron sequences flanking exons 5 through 9 of the p53 gene; primers were purchased from Sigma-Genosys Technologies inc. (Woodlands, TX). 100ng of genomic DNA was used as a template for PCR amplification of exonic sequences. PCR reaction was carried out on a Eppendorf Mastercycler® pro thermal cycler (Eppendorf AG, Hamburg, Germany). PCR product sequencing was conducted on an Applied Biosystems (Foster City, CA) 373 automated DNA Sequencer. Sequence analysis was performed using “Sequence Scanner v1.0” (Applied Biosystems).
Statistical analysis
Statistical analysis was conducted as previously described (15). Patient demographics, clinical characteristics, and molecular marker expression levels were summarized using means, medians or proportions as applicable. Median overall- (OS) and disease specific-survival (DSS) were determined using the Kaplan-Meier method. 1-, and 5-year DSS rates (95% CI) were calculated for all evaluable patients. For OS analysis, death was counted as an event. For the DSS analysis, only death due to disease was counted as an event; patients who survived or died due to other causes were censored at their last follow-up (LFU) date or the date they died from other causes. Cox’s proportional hazards regression model was used to test the statistical significance of candidate prognostic factors for DSS in a univariate manner. From this model the hazard ratios for potential prognostic factors were estimated with a 95% confidence interval. All potential prognostic factors with a p-value < 0.10 were then included in a saturated model, and backward elimination was used to remove factors from the model based on the likelihood ratio test in the multiple regression analysis. Fisher’s exact and Chi-square tests were used to assess associations between molecular markers. All computations were carried out in SAS.
Results
PLS patient, tumor, and treatment variables
A total of 155 PLS patients were evaluated at UTMDACC during the investigated interval (1993–2010). Only patients with follow up (n=83) are described here. Clinicopathologic variables are summarized in Table 1A. The median presenting age was 53 (range, 14–84) years with slight male predominance. Most patients (80%) presented with localized PLS (primary or recurrent); 20% had metastatic disease, most commonly to the lungs (82%), liver (18%), and skeleton (18%). The most common sites of primary PLS were thigh (34%) and pelvis (15%). The average size of localized primary tumors was ~11cm. Average size of truncal tumors was 8.5cm±4 (1.5–16.5cm) and of extremity tumors was 11.2cm±7.2 (0.7–30cm).
Table 1.
PLS patient, tumor, treatment and outcome variables
| (1A) Patient and tumor variables | ||
|---|---|---|
| Variable | Patients with follow-up (n=83) (%) | |
| Median age (range) | 53.4 ±16.8 (14–84) | |
| F/M | 32/51 (1:1.6) | |
| Status at presentation | ||
| Primary | 60 (73%) | |
| Recurrent | 6 (7%) | |
| Metastatic1 | 17 (20%) | |
| Average tumor size (range)2 | 11.1 ±7.25 (0.7–30) | |
| Tumor site3,4 | ||
| Extremity | 49 (59%) | |
| Central | 34 (41%) | |
|
| ||
| (1B) Treatment and outcome variables | ||
| Variable | Patients with localized disease (n=66) (%) | Patients with metastatic disease (n=17) (%) |
|
| ||
| Treatment | ||
| Surgery (Yes/No) | 64 / 2 (97%) | 6 / 11 (55%)5 |
| Type of resection | ||
| Complete surgery | 62 (97%) | 3/17 (18%) |
| R0 | 56 (90%) | |
| R1 | 6 (10%) | |
| Incomplete (R2) | 2 (3%) | |
| Chemotherapy6 | 28 (45%) | 17 (100%) |
| Neoadjuvant7 | 18 (67%) | |
| Adjuvant7 | 11 (41%) | |
| Radiotherapy6 | 43 (69%) | 3 (18%)5 |
| Neoadjuvant7 | 25 (60%) | |
| Adjuvant7 | 18 (43%) | |
| Outcome | ||
| Median follow-up in months (range)8 | 31.5 (1.5–182.3) | 8.5 (2.5–47.2) |
| Local recurrence9 | 16 (26%) | NA |
| Metastasis10 | 23 (35%) | |
| Site of metastasis11 | ||
| Lungs/Thorax | 16 (70%) | NA |
| Liver | 2 (9%) | |
| Abdomen | 5 (22%) | NA |
| Skeleton | 5 (22%) | |
| 5 year disease specific survival12 | 0.65 (0.52, 0.78) | NA13 |
| Median overall survival | 7.28 (2.9, 14.7) | 0.76 (0.53, 1.58) |
Metastatic sites included: lungs 14, liver 3, skeleton 3 and others 1; 4 patients had multiple metastatic sites
Tumor size was available for 53 pts, only primary lesions were considered
Tumor location refers to the location of the primary tumor only; extremities include: upper (5%) and lower arms (3%), shoulder girdle (7%), lower (6%) and upper leg and buttock (34%), central includes: head & neck (4%), thorax (14%), abdomen (12%), pelvis (15%)
Tumor location was not available for 2 patients
Palliative surgery or radiotherapy in patients with metastatic disease
Refers to patients who underwent complete resection (n=62)
%Refer to those that underwent complete resection and received chemotherapy (n=27) or radiotherapy (n=42)
Median follow up for the entire cohort of 83 pts. was 24.7 months
Refers only to patients who underwent complete resection (n=62)
Refers to all pts. with localized disease at presentation (n=66)
Several patients had multiple site metastases; percentages refer to the group with metastatic disease only (23 pts.)
5YDSS for the entire group was 53%
1 year DSS for pts. with metastasis was 45%
All patients with metastatic PLS (n=17) received chemotherapy (doxorubicin [n=12], ifosfamide [n=12], gemcitabine [n=7], and docetaxel [n=7]). In the majority of cases (n=14) extensive and diffuse metastatic load was present precluding surgical metastasectomy; such was conducted in three cases. Palliative surgery and or/radiotherapy were utilized in six patients patients. Surgery was considered for all patients with localized (primary or recurrent) disease 9 (n=66). Only two tumors (3%) were deemed unresectable after radiation and chemotherapy. Complete macroscopic resection was achieved in 62 of 64 patients with localized disease (97%); negative microscopic margins (R0 resection) were attained in 56 (90%) complete resections. Average tumor size of patients undergoing R0 resection was 9.7cm±6.3 (1.5–29) vs. 11.7cm±6.8 (4.5–25) in patients undergoing R1/R2 resection. R0 resection was achieved in 79% (n=19) of patients with a truncal tumors as compared to 93% (n=37) of those with extremity PLS. Chemotherapy (neoadjuvant and/or adjuvant) was administered to 28 (45%) completely resected patients: agents included doxorubicin (n=24; 89%), ifosfamide (n=19; 70%), docetaxel (n=5; 19%), gemcitabine (n=5; 19%), cyclophosphamide (n=2; 7%) and dacarbazine (n=2; 7%). Radiation was delivered to 43 (69%) completely resected patients: neoadjuvant therapy was administered to 25 patients, 17 patients received post operative adjuvant radiation, and one patient received both pre- and post-operative radiotherapy.
PLS-specific survival and potential outcome-related prognosticators
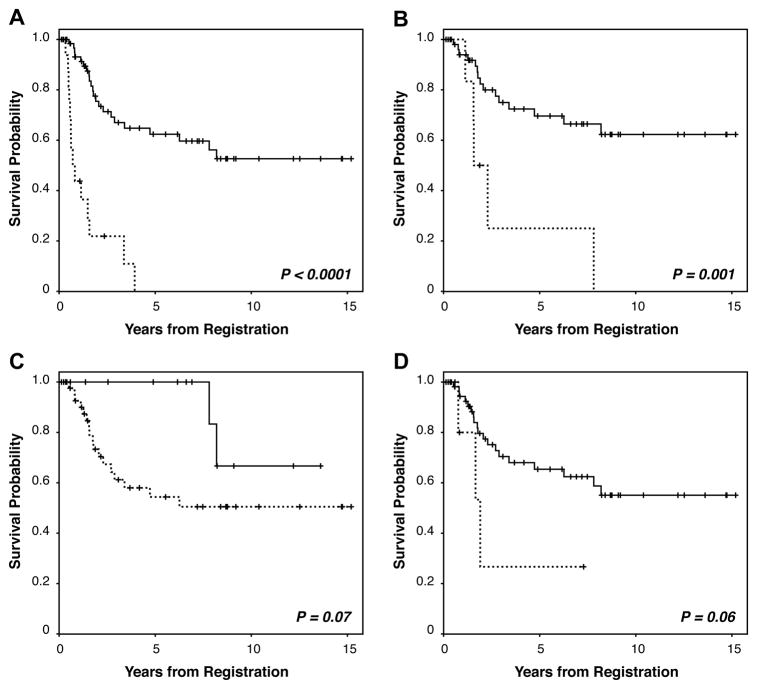
Median follow-up of patients presenting with metastasis was 8.5 months (2.5–47.2; Table 1B). The median OS for patients with metastasis was 9.1 months; 1-year DSS was 45% and none reached five years. Outcomes of patients with metastatic PLS were markedly worse than for patients with localized tumors (p<0.0001; Fig 1A). Median follow-up of patients with localized PLS was 31.5 months (1.5–182). For patients with localized PLS surviving at the end of the study (n=36), median follow up time was 47 months (2–182). Sixteen of the patients with completely resected tumors had local recurrence (25%) and 23 (35%) developed metastatic disease, mainly pulmonary spread. Within the constraints of a relatively short follow up period, the 1- and 5-year DSS of patients with localized PLS were 93% and 65%, respectively. KM analysis further demonstrated that a positive microscopic margin significantly correlated with decreased DSS (p=0.001; Fig 1B). Tumor size (<5cm) and disease status (primary vs. recurrent) did not achieve statistical significance (p=0.07 and 0.06, respectively; Fig 1C&D), possibly due to the small evaluable patient cohort.
Figure 1. Kaplan-Meier curves for PLS-specific survival.
A. stratified for patients treated for localized (solid line) versus metastatic disease (dotted line); B. stratified for R0 (solid line) versus R1 (dotted line) resection; C. stratified for tumors <5cm (solid line) versus ≥5cm; and, D. stratified for primary (solid line) versus recurrent (dotted line) disease at presentation.
A univariable DSS analysis was performed to identify factors predicting poor prognosis in localized PLS patients (Table 2A). No surgical resection, microscopically positive surgical margins, and chemotherapy treatment were significantly associated with decreased DSS. Large tumor size and recurrent disease at presentation to UTMDACC did not achieve statistical significance, possibly due to the small evaluable patient cohort. These variables (except chemotherapy) were included in a multivariate analysis: recurrent disease, no surgical resection, and positive resection margins were identified as independent poor prognostic indicators. It is of note that our analyses are limited but the relatively small patient number and combining datasets from different institutions can enhance the statistical significance of clinical information.
Table 2.
Univariable and multivariable Cox proportional hazard models for PLS disease specific survival
| (2A) Univariable analysis | |||
|---|---|---|---|
| Variable | Levels | HR (95% CI) | P value |
| Tumor size | ≥5cm versus <5cm | 3.6 (0.83–15.64) | 0.09 |
| Status of disease | recurrent versus primary | 3.04 (0.88–10.49) | 0.08 |
| Surgery | yes versus no | 0.07 (0.01–0.38) | 0.002 |
| Surgical margins | R1 versus R0 | 3.96 (1.42–11.02) | 0.008 |
| Chemotherapy | yes versus no | 2.94 (1.2–7.24) | 0.02 |
|
| |||
| (2B) Multivariable analysis | |||
| Variable | Levels | HR (95% CI) | P value |
|
| |||
| Tumor status | recurrent vs. primary | 5.05 (1.38–18.51) | 0.01 |
| Surgery | yes vs. no | 0.04 (0.001–0.23) | 0.0003 |
| Margins | R1 vs. R0 | 5.87 (1.99–17.29) | 0.001 |
PLS related molecular biomarkers
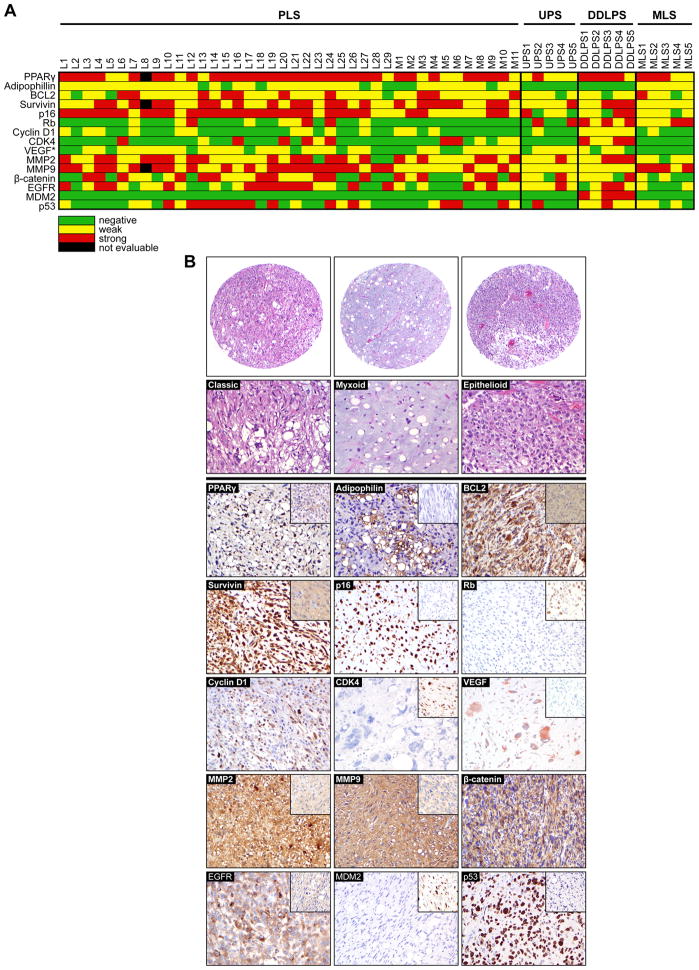
Little is known about PLS-associated molecular deregulations; such knowledge could enhance disease diagnosis, prognostication, and treatment. Hence, we evaluated protein expression of multiple cancer-related biomarkers using IHC of human PLS specimens assembled in a clinically annotated TMA which included all three PLS subtypes: classical (n=28), myxoid (n=6), and epithelioid (n=6; Figure 2). Marker selection was based on previous reports suggesting their potential relevance to PLS or malignant neoplasms per se, including markers of adipogenic differentiation (PPARγ, adipophilin), cell survival (BCL2, survivin), cell cycle regulation (p16, Rb, cyclin D1, CDK4), angiogenesis (VEGF), migration and invasion (MMP2, MMP9) and also β-catenin, EGFR, and p53. The majority of stainings were conducted at the clinical laboratory in using highly validated antibodies and in all cases positive and negative controls were utilized confirming antibody specificity. Table 3 and Figure 2 depict protein expression levels for the entire PLS cohort as well as representative specimen staining photomicrographs; as reflected in the heatmap for every antibody evaluated a spectrum from no staining up to high staining intensity could be identified across the TMA specimens.
Figure 2. Protein biomarker expression in human PLS.
A. “Heatmap” summarizing expression levels of each of the proteins evaluated in all individual scorable samples (* VEGF was only scored as positive or negative); B. Immunohistochemical images demonstrating representative levels of evaluated markers in human PLS specimens (controls included as insets: PPARγ = low expressing PLS, adipophilin = DDLPS, BCL2 = low expressing PLS, survivin = low expressing UPS/MFH, p16 = low expressing PLS, Rb = high expressing PLS, CDK4 = DDLPS, VEGF = negative expression PLS, MMP2&9 = negative expressing PLSs, EGFR = low expressing PLS, MDM2 = DDLPS, p53 = low expressing PLS). All original images were captured at ×200 magnification.
Table 3.
PLS biomarker expression analysis
| Marker | staining positive, n (%)1 | Biomarker expression level (%) | |
|---|---|---|---|
| Low | Moderate / High | ||
| PPAR γ nuclear | 39 (100%) | 23 | 77 |
| Adipophillin | 32 (80%) | 100 | 0 |
| BCL2 | 37 (93%) | 73 | 27 |
| Survivin (cytoplasmic) | 39 (100%) | 46 | 54 |
| Survivin (nuclear) | 39 (100%) | 97 | 3 |
| p16 nuclear | 40 (100%) | 30 | 70 |
| Rb | 9 (23%) | 67 | 33 |
| Cyclin D1 | 13 (33%) | 100 | 0 |
| CDK4 | 12 (30%) | 42 | 58 |
| VEGF2 | 27 (68%) | -- | -- |
| MMP2 | 37 (93%) | 49 | 51 |
| MMP9 | 39 (100%) | 44 | 56 |
| Beta catenin | 27 (68%) | 41 | 59 |
| EGFR | 28 (70%) | 50 | 50 |
| MDM2 | 0 (0%) | 0 | 0 |
| p53 | 22 (55%) | 41 | 59 |
Total number of scorable specimens for each marker varied due to different rates of sample attrition
VEGF staining was scored as positive vs. negative staining only
Interestingly, PPARγ was expressed in all PLS samples as well as in DDLPS, MLS, and UPS/MFH control specimens; 77% of PLS exhibited moderate-to-high PPARγ expression. Adipophilin expression was found in 80% of PLS, albeit mainly at low expression levels. Of controls, only MLS exhibited adipophilin positivity. Both BCL2 and survivin were commonly expressed in PLS (93% and 100% of specimens, respectively). Moderate-to-high survivin expression was identified in 54% of PLS samples; both cytoplasmic and nuclear staining were noted. p16 was expressed in all PLS samples; 70% exhibited moderate-to-high staining intensity. In contrast, 77% of PLS did not express Rb. Cyclin D1 was expressed in 33% of PLS; in contrast, all DDLPS expressed this protein. Similarly, CDK4 expression was found in 33% of PLS; all DDLPS samples variably expressed CDK4 protein. VEGF expression was found in 68% of PLS. MMP2 and MMP9 were expressed in 93% and 100% of PLS, respectively; moreover, moderate-to-high expression was noted in more than 50% of PLS. 68% of PLS variably expressed β-catenin, although expression was mainly cytoplasmic. Enhanced EGFR expression was noted at either at moderate-to-high (35%) or low (35%) expression levels.
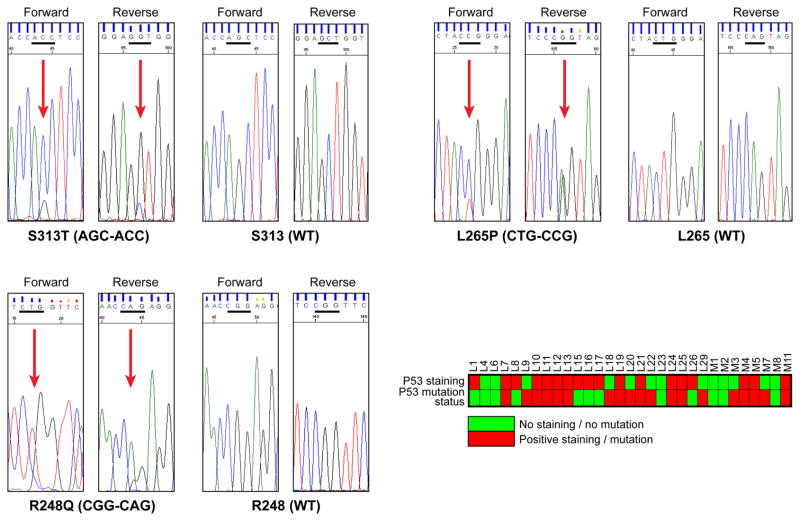
Recently, a high rate of p53 mutations was reported to occur in PLS (16). To further evaluate p53 mutational status we sequenced the p53 DNA core binding domains (exons 5–9) in DNA extracted from 31 FFPE PLS specimens. p53 mutations were identified in 19 (60%) of the samples and included: exon 5 – R158P, A159V, and R175H; exon 6 - H193R and R209T; exon 7 - S241Y, G245A, and R248Q; exon 8 – L265P and T304I; exon 9 – S313T. Of these S313T, L265P, and R248Q were the most frequent (Fig 3). We considered that this high mutation rate might be a false positive artifact due to sequencing formalin fixed tissues, so we blindly sequenced two samples whose corresponding frozen samples were available; identical p53 sequence alterations were identified. No statistical correlation between p53 mutation status and p53 protein expression levels could be identified (Fig 3).
Figure 3. p53 mutations in PLS.
Representative sequence chromatograms of the three most commonly occurring p53 mutations in PLS samples and tabulation of p53 mutational status and protein expression levels in individual PLS samples.
We next evaluated whether biomarker expression levels correlated with PLS patient outcomes. This analysis only included samples from patients with localized disease who underwent complete surgical resection and for whom follow-up information was available (n=22). Univariable analysis failed to identify prognostic value for any markers evaluated, with the caveats inherent to small sample cohorts rendering definitive conclusions problematic. Future attempts would be made to increase the number of samples retrieved from PLS patients undergoing complete resection to enable meaningful evaluation of the prognostic value of a given biomarker.
Discussion
The current study and other major series (3–5,17–20) demonstrate that PLSs exhibit unfavorable outcomes even when managed with multidisciplinary expertise at tertiary cancer centers, reflecting the aggressive biology of this malignancy. PLSs exhibit high rates of local recurrence; as per previous reports (3–5,19,20), 25% of patients undergoing complete surgical resection experienced local failure in our series. Systemic (especially pulmonary) metastases are common; 25% of our patients presented with metastatic spread and more than one-third undergoing complete resection developed distant metastasis during follow-up. This aggressive `behavior is further exemplified by five-year survival rates of ~60% for patients presenting with localized disease (Table 4). Multivariate analysis revealed only recurrent disease and positive microscopic margins as independent outcome prognosticators in this series. Other clinicopathological factors previously shown as potentially predictive include: older age, central tumor location, size larger than 10cm, tumor necrosis, high mitotic rate, and epithelioid morphology (Table 4; 3–5,19,20). A gene expression signature predicting outcome for complex karyotype STS patients was recently devised (21); the validity of this profile should be tested in the PLS patient cohort. If validated, PLS genetic profiling might possibly augment traditional staging, thereby optimizing patient treatment decisions.
Table 4.
Summary of published PLS clinical series
| Ref.# | publication | Patients with FU | 5y OS | 5y DSS | Disease prognosticators |
|---|---|---|---|---|---|
| 19 | Downes et al, 2001 | 18 | 45% | 50% | NA |
| 3 | Gebhard et al, 2002 | 48 | 57% | NA | Age, location, size, margins |
| 4 | Hornick et al, 2004 | 50 | 63% | NA | Location, size, necrosis, mitotic rate, epithelioid subtype |
| 20 | Fiore et al, 2007 | 60 | NA | 81%1 64%2 |
Size, status3 |
| 5 | Dalal et al. 2006 | 64 | NA | 59% | Age, gender, status3, Location, margins, |
| Current | Ghadimi et al. 2010 | 834 | 49% | 53%5 65%6 |
Margins, status1 |
DSS of patients with primary disease
DSS of patients with recurrent disease
Status refers to tumor status at presentation (primary versus recurrent localized disease)
Including 66 patients with localized disease and 17 with metastatic disease at presentation
DSS of all evaluable patients
DSS of patients with localized disease
Unraveling key deregulations driving PLS inception and progression is essential to establish molecular-based staging systems as suggested above; even more critical is the potential impact such knowledge might have on development of novel and effective anti-PLS targeted therapeutics. Elucidating specific PLS nodes of vulnerability awaits determination of molecular underpinnings of this malignancy; common ‘drugable’ targets are yet to be discovered. The cytogenetic complexity of PLS suggests the probability of multiple molecular aberrations rather than single “oncogenic addictions”. Strategies to disable multiple parallel and/or complementary pathways, rather than single locus “magic bullets”, are likely necessary for effective management of PLS, hence our efforts to identify biomarkers broadly reflecting different cancer-associated therapeutically-relevant processes.
Tumor suppressor pathway deregulations commonly occur in PLS, including Rb (22) and p53 (23). Our study confirms these initial observations, demonstrating loss of Rb expression in a large proportion of PLS samples. In contrast, although well characterized as a tumor suppressor inhibiting progression through the cell cycle by binding to CDK4/6 (24,25), we found increased PLS p16 expression. p16 overexpression has been previously demonstrated in various malignancies, including STS and LPS specifically (26). MDM2 and CDK4 are amplified in WDLPS/DDLPS as part of the 12q13-15 amplicon identified in these tumors (27); DDLPS samples included as controls on our TMA highly expressed these proteins. In contrast, only a 1/3 of PLS expressed CDK4 and none exhibited MDM2. Clinical trials evaluating the effects of MDM2 and CDK inhibitors (e.g., RO5045337 and PD0332991, respectively) on LPS are currently being initiated. Our findings do not support inclusion of PLS patients in the former study and call for careful PLS patient selection for inclusion in the latter. A recent study has identified p53 as more frequently mutated in PLS compared to most other STS subtypes (16). Our data recapitulate this observation, demonstrating high rates of p53 core binding domain mutations in PLS as well as varying levels of p53 protein expression levels. No correlation between p53 mutation status and p53 protein levels was identifiable, as previously shown for other malignancies (28). Therefore, p53 immunohistochemistry cannot be used as a surrogate methodology to identify p53 mutations in PLS. p53 mutations contribute to chemoresistance (29), possibly explaining PLS therapeutic resistance and suggesting that reconstituting p53 function in these tumors might be fruitful (30).
PPARγ is a nuclear hormone receptor with a critical role in adipocyte differentiation (31). While counterintuitive given its role in differentiation, data supports a PPARγ pro-tumorigenic function (32). A recent study identified enhanced PPARγ expression in LPS subtypes especially MLS, DDLPS, and PLS (33); our findings support this observation. PPARγ agonists have been shown to induce differentiation and growth inhibition in cancer cells expressing this protein including LPS (34–37). Initial clinical experience demonstrated significant adipocytic differentiation in two PLS patients treated with the PPARγ ligand troglitazone (38). A phase II clinical trial for LPS patients failed to achieve any objective clinical responses, suggesting that PPARγ activation as a solitary approach is insufficient (39); no PLS patients were included in this trial. Further investigation of PPARγ as a therapeutic target for PLS appears warranted, especially in combination with blockade of additional pathways. Molecules contributing to cancer cell survival such as BCL2 and survivin have also been of interest as novel therapeutic targets (40–42); small molecule inhibitors are being evaluated in human clinical trials. Our results demonstrate increased expression of these potential targets in PLS, especially survivin; further preclinical investigation using human cell lines and xenograft models is currently ongoing. Similarly, we identified increased PLS expression of VEGF, the metalloproteases MMP2 and MMP9, as well as the tyrosine kinase receptor EGFR; novel therapies targeting these molecules are currently available (43–45). Our study identifies several potential therapeutic targets as overexpressed in PLS. Further preclinical investigations utilizing relevant PLS experimental models are needed. As expected, we found that a single PLS may exhibit multiple molecular aberrations (see heatmap; Figure 2). This reflects the molecular complexity of PLS where a multitude of genetic and epigenetic deregulations are at play. Consequently, utilizing novel therapeutic combinations rather than single target therapies to block multiple pathways and processes might constitute the best anti-PLS approach.
Acknowledgments
We thank Ms. Vu for aid in figure preparation and Mr. Cuevas for his assistance with manuscript preparation and submission. We highly appreciate the philanthropic support of the Lobo, Margolis, and Jackson families.
Funding: This work was supported in part by National Cancer Institute at the National Institutes of Health [RO1CA138345 grant to D.L.]; Liddy Shriver Foundation seed grants [to D.L. and D.B.]; an Amschwand Foundation seed grant [to D.L.]; and a Deutsche Forschungsgemeinschaft fellowship grant (to MPG)
Footnotes
Financial Disclosures: None
Conflict of Interest: none to declare
References
- 1.Weiss SW, editor. World Health Organization Histological Classification of Tumors. Berlin, Germany: Springer; 1994. Histologic Typing of Soft Tissue Tumors. [Google Scholar]
- 2.Mentzel T, Bosemberg M, Fletcher CDM. Pleomorphic liposarcoma: Clinicopathologic and prognostic analysis of 31 cases. Mod Pathol. 1999;12:13A. [Google Scholar]
- 3.Gebhard S, Coindre JM, Michels JJ, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol. 2002;26:601–616. doi: 10.1097/00000478-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol. 2004;28:1257–1267. doi: 10.1097/01.pas.0000135524.73447.4a. [DOI] [PubMed] [Google Scholar]
- 5.Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Du X, Chen K, et al. Genetic aberrations in soft tissue leiomyosarcoma. Cancer Lett. 2009;275:1–8. doi: 10.1016/j.canlet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillou L, Aurias A. Soft tissue sarcomas with complex genomic profiles. Virchows Arch. 2010;456:201–217. doi: 10.1007/s00428-009-0853-4. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Benso R, López-Ginés C, Soriano P, et al. Cytogenetic study of angiosarcoma of the breast. Genes Chromosomes Cancer. 1994;10:210–212. doi: 10.1002/gcc.2870100311. [DOI] [PubMed] [Google Scholar]
- 9.Willems SM, Mohseny AB, Balog C, et al. Cellular/intramuscular myxoma and grade I myxofibrosarcoma are characterized by distinct genetic alterations and specific composition of their extracellular matrix. J Cell Mol Med. 2009;13:1291–1301. doi: 10.1111/j.1582-4934.2009.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedeutour F, Suijkerbuijk RF, Forus A, et al. Complex composition and co-amplification of SAS and MDM2 in ring and giant rod marker chromosomes in well-differentiated liposarcoma. Genes Chromosomes Cancer. 1994;10:85–94. doi: 10.1002/gcc.2870100203. [DOI] [PubMed] [Google Scholar]
- 11.Rubin BP, Fletcher CD. The cytogenetics of lipomatous tumours. Histopathology. 1997;30:507–511. doi: 10.1046/j.1365-2559.1997.5680797.x. [DOI] [PubMed] [Google Scholar]
- 12.Pilotti S, Della Torre G, Lavarino C, et al. Molecular abnormalities in liposarcoma: role of MDM2 and CDK4-containing amplicons at 12q13-22. J Pathol. 1998;185:188–190. doi: 10.1002/(SICI)1096-9896(199806)185:2<188::AID-PATH53>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Lazar AJ, Das P, Tuvin D, et al. Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res. 2007;13:7314–7321. doi: 10.1158/1078-0432.CCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 14.Bolshakov S, Walker CM, Strom SS, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res. 2003;9:228–234. [PubMed] [Google Scholar]
- 15.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;24:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 16.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology. Soft Tissue Sarcoma. Version 2.2008. National Comprehensive Cancer Network, Inc; 2008. [Google Scholar]
- 18.Casali PG, Jost L, Sleijfer S, et al. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann of Oncol. 2008;19:89–93. doi: 10.1093/annonc/mdn101. [DOI] [PubMed] [Google Scholar]
- 19.Downes KA, Goldblum JR, Montgomery EA, et al. Pleomorphic liposarcoma: a clinicopathologic analysis of 19 cases. Mod Pathol. 2001;14:179–184. doi: 10.1038/modpathol.3880280. [DOI] [PubMed] [Google Scholar]
- 20.Fiore M, Grosso F, Lo Vullo S, et al. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2007;109:2522–2531. doi: 10.1002/cncr.22720. [DOI] [PubMed] [Google Scholar]
- 21.Chibon F, Lagarde P, Salas S, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med. 2010;16:781–787. doi: 10.1038/nm.2174. [DOI] [PubMed] [Google Scholar]
- 22.Taylor BS, Barretina J, Socci ND, et al. Functional copy-number alterations in cancer. PLoS One. 2008;3:e3179. doi: 10.1371/journal.pone.0003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandberg AA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: liposarcoma. Cancer Genet Cytogenet. 2004;155:1–24. doi: 10.1016/j.cancergencyto.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Meye A, Würl P, Hinze R, et al. No p16INK4A/CDKN2/MTS1 mutations independent of p53 status in soft tissue sarcomas. J Pathol. 1998;184:14–17. doi: 10.1002/(SICI)1096-9896(199801)184:1<14::AID-PATH957>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto I, Mitomi H, Ohkura Y, et al. Abnormal expression of p16(INK4a), cyclin D1, cyclin-dependent kinase 4 and retinoblastoma protein in gastric carcinomas. J Surg Oncol. 2008;98:60–6. doi: 10.1002/jso.21087. [DOI] [PubMed] [Google Scholar]
- 26.Yao J, Pollock RE, Lang A, et al. Infrequent mutation of the p16/MTS1 gene and overexpression of cyclin-dependent kinase 4 in human primary soft-tissue sarcoma. Clin Cancer Res. 1998;4:1065–1070. [PubMed] [Google Scholar]
- 27.Nilbert M, Rydholm A, Mitelman F, et al. Characterization of the 12q13-15 amplicon in soft tissue tumors. Cancer Genet Cytogenet. 1995;83:32–36. doi: 10.1016/s0165-4608(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 28.Cheok CF, Verma CS, Baselga J, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8:25–37. doi: 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- 29.Hannay JA, Liu J, Zhu QS, et al. Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: a role for p53/activator protein 2 transcriptional regulation. Mol Cancer Ther. 2007;6:1650–1660. doi: 10.1158/1535-7163.MCT-06-0636. [DOI] [PubMed] [Google Scholar]
- 30.Zhan M, Yu D, Lang A, et al. Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer. 2001;92:1556–1566. doi: 10.1002/1097-0142(20010915)92:6<1556::aid-cncr1482>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 32.Ikezoe T, Miller CW, Kawano S, et al. Mutational analysis of the peroxisome proliferator-activated receptor gamma gene in human malignancies. Cancer Res. 2001;61:5307–5310. [PubMed] [Google Scholar]
- 33.Tajima T, Morii T, Kikuchi F, et al. Significance of LRP and PPAR-gamma Expression in Lipomatous Soft Tissue Tumors. Open Orthop J. 2010;4:48–55. doi: 10.2174/1874325001004010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keshamouni VG, Reddy RC, Arenberg DA, et al. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23:100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 35.Hase T, Yoshimura R, Mitsuhashi M, et al. Expression of peroxisome proliferator-activated receptors in human testicular cancer and growth inhibition by its agonists. Urology. 2002;60:542–547. doi: 10.1016/s0090-4295(02)01747-8. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K, Kawahito Y, Tsubouchi Y, et al. Expression of peroxisome proliferator-activated receptor gamma in renal cell carcinoma and growth inhibition by its agonists. Biochem Biophys Res Commun. 2001;287:727–732. doi: 10.1006/bbrc.2001.5640. [DOI] [PubMed] [Google Scholar]
- 37.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demetri GD, Fletcher CD, Mueller E, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Debrock G, Vanhentenrijk V, Sciot R, et al. A phase II trial with rosiglitazone in liposarcoma patients. Br J Cancer. 2003;89:1409–1412. doi: 10.1038/sj.bjc.6601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Cai X, Wang Y, et al. microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep. 2010;24:1363–1369. doi: 10.3892/or_00000994. [DOI] [PubMed] [Google Scholar]
- 41.Azmi AS, Wang Z, Philip PA, et al. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin Emerg Drugs. 2010 doi: 10.1517/14728214.2010.515210. Published on September 3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwasa T, Okamoto I, Takezawa K, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Meter ME, Kim ES. Bevacizumab: current updates in treatment. Curr Opin Oncol. 2010;22:586–591. doi: 10.1097/CCO.0b013e32833edc0c. [DOI] [PubMed] [Google Scholar]
- 44.Dormán G, Cseh S, Hajdú I. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010;70:949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Vivanco I, Mellinghoff IK. Epidermal growth factor receptor inhibitors in oncology. Curr Opin Oncol. 2010;22:573–578. doi: 10.1097/CCO.0b013e32833edbdf. [DOI] [PubMed] [Google Scholar]