Abstract
Background
Congenital pancytopenia is a rare and often lethal condition. Current knowledge of lymphoid and hematopoietic development in mice, as well as understanding regulators of human hematopoiesis, have led to the recent discovery of genetic causes of bone marrow failure disorders. However, in the absence of mutations of specific genes or a distinct clinical phenotype, many cases of aplastic anemia are labeled as idiopathic, while congenital immune deficiencies are described as combined immune deficiency.
Procedure
We describe the case of a 33-week gestation age male with severe polyhydramnios, hydrops, and ascites who was noted to be pancytopenic at birth. Bone marrow examination revealed a hypocellular marrow with absent myelopoiesis. An immune workup demonstrated profound B lymphopenia, near absent NK cells, and normal T cell number. Due to the similarity of the patient's phenotype with the IKAROS knockout mouse, studies were performed on bone marrow and peripheral blood to assess a potential pathogenic role of Ikaros.
Results
DNA studies revealed a point mutation in one allele of the IKAROS gene, resulting in an amino acid substitution in the DNA-binding zinc finger domain. Functional studies demonstrated that the observed mutation decreased Ikaros DNA-binding affinity, and immunofluorescence microscopy revealed aberrant Ikaros pericentromeric localization.
Conclusions
Our report describes a novel case of congenital pancytopenia associated with mutation of the IKAROS gene. Furthermore, these data suggest a critical role of IKAROS in human hematopoiesis and immune development.
Keywords: Ikaros, B lymphopenia, pancytopenia, hematopoiesis
Introduction
The IKAROS gene encodes for a C2H2 zinc finger protein whose expression is restricted to hematopoietic cells and the pituitary gland [1,2]. Proteins generated from the IKAROS gene contain two separate zinc finger domains. Four zinc fingers in the amino half of the protein take part in sequence-specific DNA binding [3]. At the C-terminus of the protein there are two additional zinc fingers that do not bind DNA but which are responsible for protein-protein interaction [4]. The C-terminal zinc-fingers enable Ikaros proteins to form dimers or multimers with different Ikaros isoforms or Ikaros family members.
Ikaros function seems to be related to its ability to localize to pericentromeric heterochromatin (PC-HC) [5]. Transcriptionally inactive genes have been shown to selectively associate with Ikaros foci in centromeric regions, while transcriptionally active genes are not associated with Ikaros complexes [5]. Mutations in Ikaros binding sites interfered with developmentally regulated shut-down of TdT [6] and λ5 [7] genes. These data further support the role of Ikaros in gene silencing. According to the current hypothesis, Ikaros binds to the DNA control elements of target genes and aids in their recruitment to centromeric foci where they are transcriptionally silenced. Ikaros associates with histone deacetylase (HDAC)-containing complexes (NuRD and Sin3) [8], although HDAC-independent gene repression via binding to transcriptional corepressor CtBP has been reported as well [9]. Ikaros directly interacts with the NuRD complex ATPase Mi-2b and with Sin3 through both its N-terminal and C-terminal regions [8,10], while interaction with the CtBP corepressor is achieved through amino acids at the N-terminal region [9].
In humans, altered expression of Ikaros isoforms has been associated with the development of different malignancies including childhood ALL [11-13] infant ALL [14], adult B cell ALL [15], myelodysplastic syndrome (MDS [16], AML [17], and adult and juvenile CML[18]. These data show an association between the loss of Ikaros function and the development of human leukemia. However, while an essential role for Ikaros in murine hematopoietic development has been described [19,20], very little information exists on the role of Ikaros in normal development of human hematopoiesis. Of importance, Ikaros mutations have not been previously reported in children with immune or hematologic deficiencies. Here we describe a novel mutation in the IKAROS gene isolated from an infant born with severe bone marrow aplasia and selective lymphopenia.
Materials and Methods
Human subjects
The patient and family members enrolled on this study signed informed consents that were approved by the University of Iowa Children's Hospital Institutional Review in accordance with the Declaration of Helsinki. Blood and bone marrow from the patient and his HLA-matched sibling were collected in heparinized tubes, and mononuclear cells were isolated by Ficoll Hypaque centrifugation. Genomic DNA was isolated from cells by DNeasy kit (Qiagen).
PCR primers, amplification and sequencing
Primers pairs derived from introns of the human IKAROS gene were used for PCR amplification of each IKAROS exon (Supplemental Appendix I) PCR was performed in 50 μl of reaction mixture containing 300 ng of genomic DNA from the patient or from MOLT-4 cells (as a control), 25 pmol of each primer, 0.2 mM each dNTP, 20 mM Tris-HCl, pH 8.8, 2 mM MgCl2, 10 MM KCl, 10 mM (NH4)2SO4, 0.1% Triton X-100, 0.1 mg/ml BSA and 1 unit of proof-reading DNA polymerase (Pfu; Stratagene). The sample mixtures were subjected to 35 cycles of PCR amplification in an automatic thermal cycler (MJ Research, PTC-200) as follows: denaturation at 95°C for 40 sec; annealing at (55°C for Exons 1, 2, 3, 6, 7-1, 7-2 and h; 62°C for Exon 5; 67°C for Exon 4) for 40 sec, and polymerization at 72°C for 40 sec. After the PCR reactions, samples were cleaned using a gel extraction kit (Wizard SV Gel and PCR Clean-up System, Promega, Madison, WI) and products were sequenced by the University of Wisconsin core facility using the dideoxy chain termination method.
Cloning and transfection
Directional cloning of exon 5 into the pcDNA3 vector
Exon-5 of the IKAROS gene from the patient was amplified by PCR using cloning primers containing EcoRI and XhoI restriction sites. Gel-purified PCR products and pcDNA3 vector were digested with EcoRI and XhoI. Ligation reactions were carried out at an insert/vector molar ratio of 3:1 (as specified below), 5 units of T4 DNA ligase and reaction mixtures were incubated at 16°C for 5 hours in ligation buffer (50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 10 mM dithiothreitol, 1 mM ATP, 25 μg/ml bovine serum albumin) (BioLabs). Transformation of competent E.coli cells was carried out in KCM buffer (0.5M KCL, 0.15M CaCl2, 0.25M MgCl2). Transformed E.coli cells were plated onto an LB agar plate that contained ampicillin (100 μg/ml).
Biochemical experiments
A full-length IKAROS gene harboring the same mutation detected in the patient's IKAROS gene (amino acid change 210 cysteine (C) to phenylalanine (F)) was generated from wild type Ikaros previously cloned into pcDNA3 vector (Invitrogen) using the QuickChange method (Stratagene, La Jolla, CA). 293T cells were transfected via the calcium phosphate method. Nuclear extracts were prepared as described from 293T cells transfected with the wild type and mutant IKAROS constructs in the pcDNA3 expression vector [21]. The level of Ikaros protein was normalized by Western blotting as previously reported [22]. Electro-mobility shift assays (EMSA) were performed as described previously [22]. All probes were derived from synthetic double-stranded oligonucleotides and labeling by filling in with Klenow and 32P labeled nucleotides. The probes derived from pericentromeric heterochromatin (PC-HC) have been described previously [22]. Sequences of the probes are as shown in Supplemental Appendix II. Differences in DNA-binding ability were quantified by measuring the strength of shifted bands by phosphoimaging using the ImageQuant 5.1 program.
Immunofluorescence microscopy
Peripheral blood-derived mononuclear cells from the patient, his sister and Jurkat cells (control) were analyzed by immunofluorescence microscopy and stained as previously described [23]. Images were acquired at room temperature by a Nikon immunofluorescent microscope using ×100 oil immersion with numerical aperture of 1.4
Results
Case report
A 33-week-old for gestation age premature male infant weighing 3,063 grams was born to a 31-year-old Caucasian female. Maternal history was remarkable for previous premature twin gestations who were still born, but was otherwise incomplete due to mother having been adopted. During the last week of pregnancy there was severe polyhydramnios as well as moderate fetal hydrops, with ascites noted by ultrasound examination. Cordocentesis revealed fetal anemia with a hemoglobin of 6.3 g/dl and the fetus subsequently received a transfusion of 105 ml of packed red blood cells via umbilical vein two days prior to delivery. Following unsuccessful attempts to stop premature labor, the patient was delivered by an emergency C-section due to fetal distress, with 1 and 5 minute APGAR scores of 6 and 9. The initial complete blood count (CBC) revealed a white blood cell count of 2,400/mm3, a hemoglobin of 14.5g/dl, and a platelet count of 20,000/mm3. The differential showed 99% lymphocytes, with an absolute neutrophil count (ANC) of 24/mm3 and an absolute lymphocyte count (ALC) of 2,300/mm3. The patient was given granulocyte colony stimulating factor (G-CSF) and intravenous immunoglobulin (IVIG), broad spectrum IV antibiotics, as well as multiple blood and platelet transfusions over the first five days of life. Blood cultures for bacteria and fungus remained negative. A work-up for viral causes of cytopenia was initiated, and blood, urine, and tracheal aspirate cultures were negative for CMV, adenovirus, and enterovirus. TORCH titers were also negative, and subsequent PCR testing for CMV was also negative. Imaging studies excluded skeletal malformations, intracellular calcifications and intracranial hemorrhage. Bone marrow aspiration of the tibia revealed significant marrow aplasia, absent myelopoiesis with a normal karyotype 46XY and no other cytogenetic abnormalities. Over the course of the next three weeks, the patient continued to require frequent red blood cell and platelet transfusions, and despite giving high doses of G-CSF (20 μg/kg), the ANC persisted at zero.
Due to the findings of pancytopenia, immunological abnormalities, and the presence of an HLA-matched sibling donor, an allogeneic bone marrow transplant was recommended. In preparation for transplant, a double lumen central venous catheter was placed at three weeks of age, and shortly thereafter cellulitis developed at the exit site, with cultures positive for Pseudomonas aeroginosa. Granulocyte transfusions were given, in concert with broad spectrum antibiotics, resulting in significant clinical improvement. On day of life 47 the patient underwent an allogeneic bone marrow transplant following a reduced intensity myeloablative preparative regimen consisting of fludarabine (120 mg/m2), melphalan (140 mg/m2) and horse anti-thymocyte globulin (150 mg/kg). Cyclosporine plus methotrexate were given for graft versus host disease prophylaxis. Four days following transplant the patient developed severe renal insufficiency requiring hemodialysis. Due to concerns of delayed engraftment and infection, a second infusion of marrow cells (2 × 108 total nucleated cells/kg) was given at day 14. An elevation of peripheral blood neutrophil count to greater than 1,000/mm3 ensued on day 21 post BMT, seven days following the second stem cell infusion. However, the patient's clinical condition continued to deteriorate with subsequent pulmonary failure, persistent renal dysfunction and death of multi-organ failure at 40 days post BMT. An autopsy was not performed.
Assessment of pancytopenia and immune findings
The initial bone marrow aspiration done at five days of age demonstrated marked hypoplasia, with scattered erythroid precursors, an abundance of lymphocytes, and an absence of myeloid precursors. Due to the size of the patient a bone marrow biopsy could not be performed, making it difficult to quantitate marrow cellularity. Given the findings of a normal lymphocyte count despite evidence of impaired hematopoiesis, an extensive immunological evaluation was undertaken. Initial immunoglobulin levels were obtained on day five of life, after the patient had already received IVIG for presumed sepsis on day 2, and of note IgM was markedly decreased (19 mg/dl) while IgG was within the normal range (761 mg/dl). Immunophenotyping revealed the absence of B cells (<1%), minimal numbers of NK cells (1%), and an abundance of T lymphocytes (99%) with a normal CD4:CD8 ratio of 2.5:1. Further immunophenotyping analyses revealed near complete absence of CD45RO on CD3+ cells (<1%), and nearly all CD3+ T cells expressed TCR αβ (95%) (Table I). While T cell clonality was not directly determined, there was heterogeneous expression of other cell surface markers, including CD5 and CD7, and approximately 20% of CD4 and CD8 T cell subsets expressed activation markers CD25 and CD69. T cell function was determined by a blastogenic assay, and absent responses to mitogens (PHA and ConA) and anti-CD3 was noted. To further evaluate the patient for congenital bone marrow failure syndromes and primary immune deficiencies, a number of other tests were performed. No abnormalities were detected as noted in Table 2,
Table I. Immunophenotype of Ikaros deficient subject at three weeks of age.
| Lymphocyte Subset | Patient Absolute Count (%)* | Control Absolute Count (%)** |
|---|---|---|
| Total Lymphocytes | 2350 | 3400-7600 |
| CD3 | 2328 (99%) | 2500-5500 (53-84%) |
| CD3/TcR αβ | 2281 (97%) | 2900-8500 (89-97%) |
| CD3/TcR γδ | 19 (1%) | 10-120 (1-5%) |
| CD3/4 | 1482 (63%) | 1600-4000 (35-64%) |
| CD3/8 | 870 (37%) | 560-1700 (12-28%) |
| CD4/45RA | 1435 (61%) | 1200-3700 (64-95%) |
| CD8/45RA | 894 (38%) | 450-1500 (80-99%) |
| CD4/45RO | 33 (1%) | 60-900 (2-22%) |
| CD8/45RO | 23 (1%) | 30-330 (1-9%) |
| CD3-/16+56+ | 35 (1%) | 170-1100 4-18%) |
| CD19 | 0 (0%) | 120-2100 (14-76%) |
absolute count expressed as cells/mm3, determined by multiplying total lymphocyte count × % of lymphocyte subset, with (%) representing percent of lymphocyte subset within the lymphocyte gating parameter from peripheral blood;
values presented as 10th and 90th percentiles, total and percentage of cells expressing indicated markers for the lymphocyte population, based on established values for infants age 0-3 months
Table II. Laboratory assessment of neonatal pancytopenia and congenital immune deficiency.
| Disease | Inheritance | Testing modality | Result |
|---|---|---|---|
| Fanconi anemia | Autosomal recessive | DEB induced chromosome breakage study | No increase in chromosome breakage |
| Kostmann disease | Autosomal recessive/sporadic | Gene mutation analysis | Normal ELA2, HAX1 gene sequence |
| Bruton agammaglobulinemia | X-linked recessive | BTK protein expression, BTK gene sequencing | Normal BTK expression |
| Dyskeratosis congenita | X-linked recessive/ autosomal dominant/recessive | Telomere length by flow-FISH | Normal lymphocyte telomere length |
| Hemophagocytic lympho-histiocytosis HLH | Autosomal recessive | Perforin/granzyme studies, NK function, tissue biopsy | Normal granzyme expression, lack of bone marrow erythrophagocytosis |
| Osteopetrosis | Autosomal recessive | Bone marrow biopsy, plain x-rays | Normal bone density on x-ray |
Ikaros protein in patient's hematopoietic cells exhibits abnormal subcellular localization
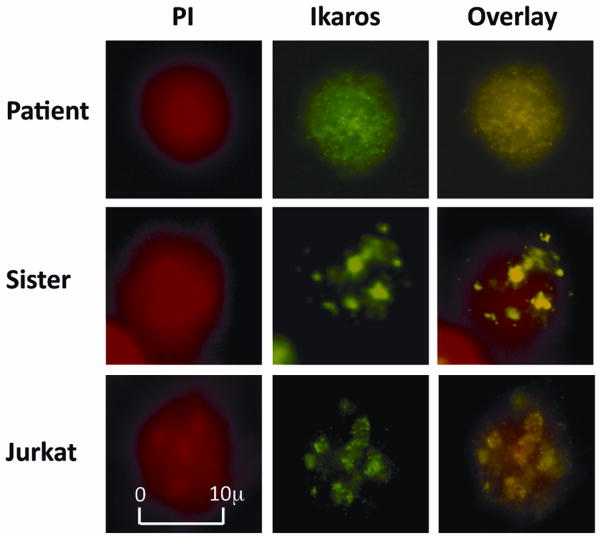
Loss of Ikaros expression or activity in the mouse results in a phenotype that resembles the hematopoietic defects observed in our patient; thus we tested the function of Ikaros in our patient's lymphocytes. Physiologically, in normal hematopoietic derived-cells, Ikaros localizes to pericentromeric heterochromatin (PC-HC) in the nucleus where it is hypothesized to recruit genes destined for activation or repression [5,24]. Localization of Ikaros to PC-HC is essential for its normal function in chromatin remodeling and regulation of gene expression. We used immunofluorescence microscopy to compare the subcellular localization of Ikaros protein in the patient's cells to that observed in cells isolated from his unaffected sister, and in the Jurkat T cell line (as a control). Subcellular localization of Ikaros was detected using anti-IK-CTS antibodies (green) that are specific to the C-terminal end of the Ikaros protein, while DNA was labeled with propidium iodine (red). In the peripheral blood-derived mononuclear cells of the patient's sister, as well as in Jurkat cells, Ikaros exhibits the typical punctate staining pattern that is consistent with localization at PC-HC (Figure 1 middle and bottom panel). In contrast, in the patient's peripheral blood-derived mononuclear cells, Ikaros exhibits an abnormal, diffuse nuclear localization (Figure 1 top panel), indicating that Ikaros protein does not localize to PC-HC. These data suggest Ikaros is functionally impaired in the patient's hematopoietic cells.
Fig. 1.
Abnormal subcellular localization of Ikaros. Immunofluorescence microscopy was used to detect and compare Ikaros' subcellular localization in lymphocytes of the patient (top panel); with those from his unaffected sister (middle panel) and Jurkat T cells—as a control (bottom panel). DNA was labeled with propidium iodine (red)—left panel. Subcellular localization of Ikaros was detected by anti-IK-CTS antibodies (green)—middle vertical panel. The right panel shows the combined images with yellow indicating co-localization of Ikaros and DNA.
Identification of a point mutation in the Ikaros gene
Experiments were undertaken to identify the cause of the abnormal subcellular localization of Ikaros in the patient's hematopoietic cells. Previous reports indicate that post-translational modifications (e.g., phosphorylation) or mutation of the IKAROS gene can result in the loss of Ikaros localization to PC-HC [21,25]. To determine if an IKAROS mutation was responsible for the loss of PC-HC localization observed in the patient's hematopoietic cells, we sequenced the patient's IKAROS gene. Genomic DNA was isolated from peripheral blood of our patient and his parents. IKAROS exons were amplified by PCR and sequenced. Sequence analysis of the patient's IKAROS gene revealed an ambiguous sequence in exon–5 at position 629 (Figure 2A). Repeated sequencing with different primers did not resolve this ambiguity, suggesting that one of the IKAROS alleles has a point mutation. Sequencing of the same exon in the patient's parents unambiguously identified wild type IKAROS sequence. The sequences obtained for the other IKAROS exons in the patient and in his parents were identical to the wild type IKAROS gene.
Fig. 2.
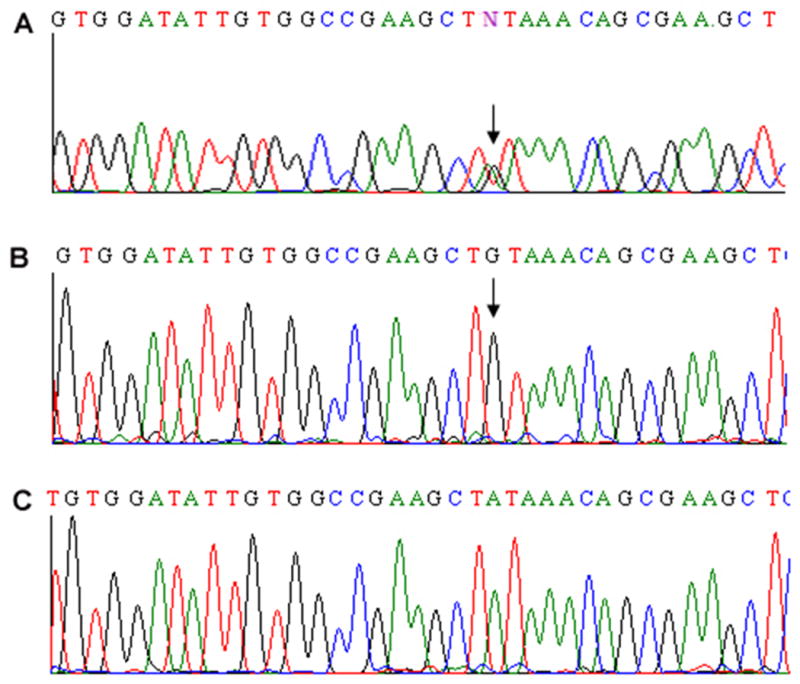
Sequencing of Ikaros. Bidirectional sequencing of Ikaros exon 5 was performed from genomic DNA isolated from the peripheral blood of the patient (A). Arrow indicates an ambiguous sequence in exon 5 at position 629. Bidirectional sequencing of cloned Ikaros' alleles reveals the presence of an Ikaros' mutated allele, with the point mutation indicated by an arrow at position 629 (B), and the wild-type allele (C).
To clarify whether one of the patient's IKAROS alleles harbors a point mutation we used directional cloning and sequencing of colonies transfected with one amplicon. The rationale for this was that each amplicon would contain one of the two alleles. Sequencing of multiple colonies would then allow us to obtain the sequences of both alleles. For directional cloning we amplified Exon-5 from the patient's genomic DNA by PCR, cloned it into the pcDNA3 vector, and transfected into E. coli. Amplicons from individual colonies (each containing one of the Ikaros alleles) were sequenced. Two types of sequences were observed following directional cloning: 1) sequence containing the wild-type IKAROS exon 5 (Figure 2C), or 2) sequence containing a point mutation in exon 5. This mutation (A to G) at position 629 in the coding mRNA results in the substitution of tyrosine210 (UAU) for cysteine (UGU) (Figure 2B). These data provide evidence that the patient has a mutation in exon 5 of one IKAROS allele.
Mutation of Ikaros decreased its ability to bind DNA probes derived from pericentromeric heterochromatin (PC-HC)
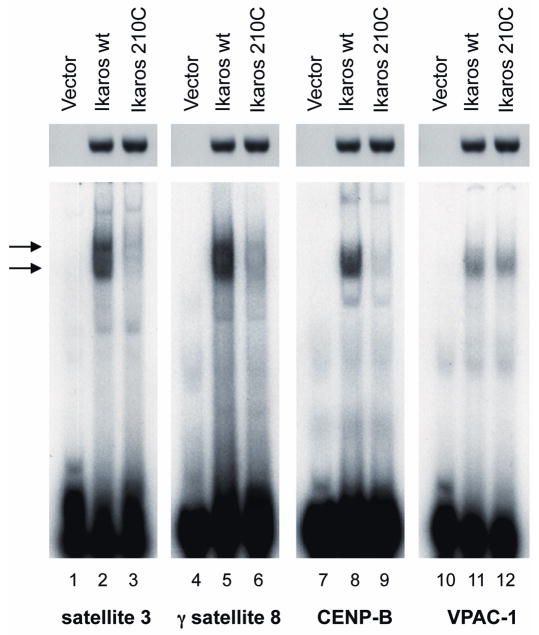
The observed mutation results in the substitution of tyrosine 210 within the beta-turn of the 4th DNA-binding zinc finger of the Ikaros protein with a cysteine. The beta sheet is an integral, evolutionarily conserved part of the structure of the C2H2 zinc finger, containing a bulky amino acid (phenylalanine or tyrosine) at that site [26]. We hypothesized that the substitution of tyrosine with cysteine alters the structure of this C2H2 zinc finger unit and changes the DNA-binding affinity of the Ikaros protein. To test this hypothesis, the embryonal kidney carcinoma cell line, 293T, was transfected with an expression vector containing the wild type IKAROS, or a mutant IKAROS gene containing the mutation observed in the patient. The 293T cell line does not express endogenous Ikaros, and it has been demonstrated that transfected IKAROS has identical DNA-binding abilities in 293T cells as in hematopoietic cells [25,27]. Thus, 293T cells are an established model for studying the DNA-binding of the Ikaros protein [25]. Results showed that Ikaros proteins harboring the mutation observed in the patient have decreased DNA-binding affinity (4-5 fold) toward probes derived from human PC-HC when compared to the wild type Ikaros (Figure 3, lanes 1-9). Mutant Ikaros did not exhibit decreased DNA-binding affinity to the known Ikaros target gene VPAC-1 (Figure 3, lanes 10-12).
Fig. 3.
Mutated Ikaros protein has altered DNA binding affinity. 293T cells were transfected to express wild-type or mutated Ikaros and analyzed by EMSA. Loading was verified by Western blotting with IK-CTS antibody as shown above each EMSA. EMSA was performed with three probes derived from human PC-HC (g satellite 8, satellite 3 and CENP-B, lines 1–9) and with a probe derived from the upstream regulatory region of Ikaros target gene VPAC-1 (lines 10–12). Ikaros containing complexes are indicated by arrows.
These data suggest that the point mutation, observed in one IKAROS allele in the patient, results in decreased DNA-binding affinity of Ikaros toward PC-HC. Previous reports indicate that strong DNA-binding affinity of Ikaros toward the probes derived from PC-HC are essential for Ikaros localization to PC-HC [25]. Taken together with results from immunofluorescence microscopy, these data suggest that the patient's hematopoietic cells have impaired Ikaros protein function due to its inability to localize to PC-HC, and that this loss of function is likely due to a point mutation in one IKAROS allele that decreases Ikaros DNA-binding affinity toward PC-HC.
Discussion
Neonatal pancytopenia is rare and often secondary to other pathologic conditions, including infection and metabolic disturbances. Here we report a newborn with pancytopenia and selective B/NK lymphopenia. An extensive hematologic and immunological evaluation initially failed to reveal the etiology of the patient's condition. Since the patient's laboratory findings closely mirrored those described in the IKAROS knockout mouse, a mutational analysis and molecular localization studies of the IKAROS gene and its protein products was performed in the patient's hematopoietic cells.
Several murine knockout models have given insight into the biological function of Ikaros in hematopoiesis. IKAROS null knockout mice lack B and NK cells, but T cells are present in adult mice, although in reduced numbers [28]. It has been suggested that Ikaros may play a key role in early regulation of hematopoiesis, as Ikaros knock-out mice displayed hypocellular marrows with diminished numbers of competitive repopulating units and hematopoietic progenitors [19,29]. In Ikaros dominant negative (DN) mutants, deletion of the DNA-binding zinc fingers results in the production of shorter Ikaros isoforms that retain the C terminal protein-binding domain and act as DN mutants, affecting the function of other Ikaros-associated proteins [19]. Homozygous DN knock-out mice completely lack T, B, and NK cells and antigen-presenting dendritic cells and have a defect in erythropoiesis and severe anemia [29]. The more severe effects observed in the IKAROS DN mutants likely arise from the combined DN effect on the function of Ikaros and its binding partners. Heterozygous DN knockout mice develop T cell leukemias and lymphomas [30]. A third IKAROS -targeted mouse, IKL/L, has the β-galactosidase (βgal) reporter gene inserted in-frame into exon 2 that is present in all Ikaros isoforms. These mice produce very low levels of Ikaros proteins [20,31,32]. The IKL/L mice exhibit T lineage defects that are identical to those reported in IKAROS null mice along with reduced numbers of B cells, and defects in myelopoiesis.
IKAROS encodes a zinc finger DNA-binding protein that is involved in regulating gene expression via chromatin remodeling. In hematopoietic cells, Ikaros binds upstream of regulatory regions of target genes and aids in their recruitment to PC-HC [5]. This process leads to either activation or repression of Ikaros target genes [24]. Thus, localization to PC-HC is the essence of Ikaros activity, and inability to localize to PC-HC reflects a loss of Ikaros function. Localization to PC-HC is detected by punctate staining in the nucleus of hematopoietic cells using immunofluorescent or confocal microscopy [5]. Ikaros normally localizes in PC-HC and produces punctate staining in the nucleus of all hematopoietic cells. Our result demonstrated that in the patient's hematopoietic cells, Ikaros exhibits a diffuse nuclear distribution. This contrasts with the normal localization pattern that is predominantly in PC-HC, as seen in the patient's sister hematopoietic cells and in Jurkat cells. These data suggest that the function of the Ikaros protein may be severely impaired in our patient's hematopoietic cells.
It has been demonstrated by Cobb et al. that the ability of Ikaros to localize to PC-HC is directly dependent on its DNA-binding affinity towards DNA sequences located at PC-HC [25]. In that study, mutations of the DNA-binding zinc fingers #2 and #3 of Ikaros were found to inhibit its ability to bind DNA probes. Phosphorylation of particular serine or threonine residues were also reported to control Ikaros' pericentromeric localization to both human and murine PC-HC [21]. Sequencing analysis of our patient's IKAROS gene revealed an ambiguous signal at amino acid 210, suggesting the presence of a mutated IKAROS allele. PCR-based cloning of IKAROS alleles confirmed the presence of a point mutation at position 629. This results in the replacement mutation of amino acid 210 from tyrosine to cysteine within the DNA-binding zinc finger #4. Functional analysis of the mutated Ikaros protein demonstrated that the observed mutation severely impairs the DNA-binding affinity of Ikaros to the DNA sequences derived from human PC-HC, suggesting that this mutation may be responsible for the loss of pericentromeric localization of Ikaros protein observed in the patient's hematopoietic cells.
Our analysis has several limitations. Since the exons were amplified by PCR prior to sequencing and cloning, there is a remote possibility that the observed mutation has been artificially introduced by PCR. We do not believe this is the case since the PCR was performed with a proof-reading polymerase pfu that has a low frequency of polymerase infidelity (2 × 10-4 nucleotides/cycle) and because of the short size of the amplified exon. The observed mutation might present a physiological polymorphism. We do not believe this is the case, for several reasons. First, sequences of the IKAROS gene from the patient's parents revealed an absence of this mutation providing evidence that this is a de novo mutation. In addition, previously published results that included sequencing of the IKAROS gene from 80 different patients did not reveal the mutation observed in our patient [11,33]. We also sequenced the IKAROS gene from blood of 12 different control subjects without observing this mutation. Another limitation of our analysis is that it does not distinguish whether the observed data are due to mosaicism or true heterozygosity. Further testing will be required to determine whether loss of Ikaros function is a direct consequence of the observed mutation, though it may be impossible to prove that the observed defect in Ikaros function (loss of PC-HC localization) was the main cause of the patient's clinical condition. However, the presence of a point mutation that results in decrease in Ikaros binding affinity toward the sequences that are derived from PC-HC and that are bound by Ikaros in vivo, suggests that this mutation is responsible for the loss of PC-HC localization of Ikaros in the patient's cells. Ikaros binds DNA poorly as a monomer, and it has been demonstrated that Ikaros localizes to PC-HC as a multimer [6]. Thus, the presence of a mutated allele could act as a dominant negative mutant in terms of ability to bind to PC-HC and inactivate the function of the wild type Ikaros in hematopoietic cells. Due to the limited availability of patient tissue, our analysis is restricted to the presented results.
The laboratory and clinical findings observed in the patient – lack of B and NK cells, normal number of T cells with impaired function, impaired erythropoiesis, megakaryopoiesis, and myelopoiesis – resemble the phenotype of Ikaros-deficient mice, although defects in megakaryopoiesis and myelopoiesis are more pronounced in our patient than in murine knockout models [20,34-38]. A possible explanation for this is that mutant IKAROS acts as a dominant negative with a deleterious effect not only on Ikaros protein, but also on other proteins that belong to the Ikaros family and which normally form a complex with Ikaros. Ikaros has been shown to repress expression of the λ5 gene [39], and to control expression of RAG1 and RAG2 during B lineage differentiation [40]. This suggests that Ikaros is essential for B cell lineage development. Interestingly, homozygous DN Ikaros knock-out mice have αβ T cells, yet lack γδ T cells NK cells and the B cell lineage, closely resembling the immunologic pattern observed in our patient. An alternative explanation is that an additional, unidentified defect has a compound effect that when combined with the inactivation of Ikaros function, produces the observed clinical picture.
In summary, we present a novel case of congenital pancytopenia and B/NK cell lymphopenia that is associated with the loss of Ikaros function and the presence of a mutation of one IKAROS allele. This case illustrates the importance of examining regulators of hematopoiesis as possible causes of unexplained congenital pancytopenia, and should prompt assessment of Ikaros in this setting.
Supplementary Material
Acknowledgments
The work has been supported in whole or in part by National Institute of Health grants R01 HL095120 (to S.D.) This work was also supported by a Four Diamonds Fund of the Pennsylvania State University, College of Medicine, John Wawrynovic Leukemia Research Scholar Endowment, Midwest Athletes Against Childhood Cancer Award, St. Baldrick's Foundation Career Development grant and by the University of Wisconsin Institute for Clinical and Translational Research, funded through National Institute of Health Clinical and Translational Science Award 1UL 1RR025011 (to S.D.)
Footnotes
Conflict of Interest Statement: None of the authors have any conflict of interest or affiliations with any organization that has a direct interest, financial or otherwise, in the subject matter detailed in this manuscript.
References
- 1.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 2.Yu S, Asa SL, Ezzat S. Fibroblast growth factor receptor 4 is a target for the zinc-finger transcription factor Ikaros in the pituitary. Mol Endocrinol. 2002;16(5):1069–1078. doi: 10.1210/mend.16.5.0832. [DOI] [PubMed] [Google Scholar]
- 3.Molnar A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994;14(12):8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996;15(19):5358–5369. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KE, Guest SS, Smale ST, et al. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91(6):845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 6.Trinh LA, Ferrini R, Cobb BS, et al. Down-regulation of TDT transcription in CD4(+)CD8(+) thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 2001;15(14):1817–1832. doi: 10.1101/gad.905601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbattini P, Lundgren M, Georgiou A, et al. Binding of Ikaros to the lambda5 promoter silences transcription through a mechanism that does not require heterochromatin formation. EMBO J. 2001;20(11):2812–2822. doi: 10.1093/emboj/20.11.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koipally J, Renold A, Kim J, et al. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18(11):3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275(26):19594–19602. doi: 10.1074/jbc.M000254200. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Sif S, Jones B, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 11.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 12.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper RP, Schoenmakers EF, van Reijmersdal SV, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–1266. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Heerema N, Crotty L, et al. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1999;96(2):680–685. doi: 10.1073/pnas.96.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakase K, Ishimaru F, Avitahl N, et al. Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res. 2000;60(15):4062–4065. [PubMed] [Google Scholar]
- 16.Crescenzi B, La Starza R, Romoli S, et al. Submicroscopic deletions in 5q- associated malignancies. Haematologica. 2004;89(3):281–285. [PubMed] [Google Scholar]
- 17.Yagi T, Hibi S, Takanashi M, et al. High frequency of Ikaros isoform 6 expression in acute myelomonocytic and monocytic leukemias: implications for up-regulation of the antiapoptotic protein Bcl-XL in leukemogenesis. Blood. 2002;99(4):1350–1355. doi: 10.1182/blood.v99.4.1350. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama H, Ishimaru F, Avitahl N, et al. Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukemia. Cancer Res. 1999;59(16):3931–3934. [PubMed] [Google Scholar]
- 19.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 20.Dumortier A, Kirstetter P, Kastner P, et al. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219–2226. doi: 10.1182/blood-2002-05-1336. [DOI] [PubMed] [Google Scholar]
- 21.Gurel Z, Ronni T, Ho S, et al. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283(13):8291–8300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronni T, Payne KJ, Ho S, et al. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282(4):2538–2547. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- 23.Popescu M, Gurel Z, Ronni T, et al. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–13880. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberg D, Smale ST, Merkenschlager M. Upstream of Ikaros. Trends Immunol. 2003;24(11):567–570. doi: 10.1016/j.it.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Cobb BS, Morales-Alcelay S, Kleiger G, et al. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14(17):2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 27.Dovat S, Ronni T, Russell D, et al. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16(23):2985–2990. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5(6):537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Nichogiannopoulou A, Shortman K, et al. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7(4):483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 30.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 31.Kirstetter P, Thomas M, Dierich A, et al. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32(3):720–730. doi: 10.1002/1521-4141(200203)32:3<720::AID-IMMU720>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Dumortier A, Jeannet R, Kirstetter P, et al. Notch activation is an early and critical event during T-Cell leukemogenesis in Ikaros-deficient mice. Mol Cell Biol. 2006;26(1):209–220. doi: 10.1128/MCB.26.1.209-220.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichogiannopoulou A, Trevisan M, Neben S, et al. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190(9):1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avitahl N, Winandy S, Friedrich C, et al. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10(3):333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 36.Winandy S, Wu L, Wang JH, et al. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J Exp Med. 1999;190(8):1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dijon M, Bardin F, Murati A, et al. The role of Ikaros in human erythroid differentiation. Blood. 2008;111(3):1138–1146. doi: 10.1182/blood-2007-07-098202. [DOI] [PubMed] [Google Scholar]
- 38.Pulte D, Lopez RA, Baker ST, et al. Ikaros increases normal apoptosis in adult erythroid cells. Am J Hematol. 2006;81(1):12–18. doi: 10.1002/ajh.20507. [DOI] [PubMed] [Google Scholar]
- 39.Thompson EC, Cobb BS, Sabbattini P, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26(3):335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Reynaud D, Demarco IA, Reddy KL, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9(8):927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.