Abstract
Tea is the second most consumed beverage in the world reported to have multiple health benefits. Preventive and therapeutic benefits of tea polyphenols include enhanced general well being and anti-neoplastic effects. The pharmacologic action of tea is often attributed to various catechins present therein. Experiments conducted in cancer cell lines and animal models demonstrate that tea polyphenols protect against cellular damage caused by oxidative stress and altered immunity. Tea polyphenols modify various metabolic and signaling pathways in the regulation of proliferation, apoptosis, angiogenesis, and metastasis and therefore restrict clonal expansion of cancer cells. Tea polyphenols have been shown to reactivate tumor suppressors, block the unlimited replicative potential of cancer cells, and physically bind to nucleic acids involved in epigenetic alterations of gene regulation. Remarkable interest in green tea as a potential chemopreventive agent has been generated since recent epigenetic data showed that tea polyphenols have the potential to reverse epigenetic modifications which might otherwise be carcinogenic. Like green tea, black tea may also possess chemopreventive and chemotherapeutic potential; however, there is still not enough evidence available to make any conclusive statements. Here we present a brief description of tea polyphenols and discuss the findings of various in vitro and in vivo studies of the anticancer effects of tea polyphenols. Detailed discussion of various studies related to epigenetic changes caused by tea polyphenols leading to prevention of oncogenesis or cancer progression is included. Finally, we discuss on the scope and development of tea polyphenols in cancer prevention and therapy.
Keywords: Apoptosis, Angiogenesis, Cancer, Epigenetics, Green tea, Tea catechins, Metastasis
INTRODUCTION
Cancer is a complex biologic disorder resulting from integrated effects of environmental, physical, metabolic, and genetic factors [1, 2]. Despite the progress made in scientific research, additional studies are necessary to determine how to prevent cancer at early stages and reduce morbidity and mortality [2–4]. The current treatment options available are limited because they do not differentiate between cancer and normal cells and thus kill both causing adverse side effects and early termination of therapy ultimately impairing patient outcome.
The fundamental traits acquired by primary cells to transform into cancer cells require the ability to evade apoptosis by inhibiting proapoptotic signaling and stimulating survival factors pathways. Cancer cells attain self-sufficient growth through uncontrolled activation of oncogenes and attain unlimited replicative potential through increased telomerase activity. These changes render cancer cells unresponsive to antigrowth signals with the loss of tumor suppressor gene activity. Over activation of invasion-related proteases in cancer cells offer a distinct advantage in tissue invasion and metastasis. Cancer cells also acquire the ability to sustain angiogenesis and build an extensive network of blood vessels to maintain continuous supply of nutrients [1]. Hence, rational anticancer treatments would disrupt these mechanisms and cause cancer cell death. The ideal chemopreventive agent would be one which can inhibit these processes in neoplastic cells or reverse them and thus inhibit the cell from converting to a malignant phenotype.
In view of these concerns about the existing treatment modalities for cancer, researchers are putting considerable efforts toward finding new therapeutic strategies which can protect normal cells and efficiently kill cancer cells [2]. In the past two decades, nutraceuticals or 'natural' substances isolated from food, developed as medicines have attracted considerable interest in the field of cancer. The major advantages of using nutraceuticals are that these agents are part of the daily diet and can be consumed within a fairly broad concentration range without significant side effects. Natural polyphenols are group of compounds which are widely prevalent in fruits, beverages (such as tea), vegetables, and spices, and have been reported to participate in a wide range of signaling and metabolic pathways that may lead normal cells to neoplastic transformation if left unchecked.
Tea a popular beverage consumed since ancient times which provides health benefits and reduces the risk of several human diseases including cancer [2–5]. Next to water, it is most widely consumed beverage with a per capita worldwide consumption of approximately 0.12 liters per day. It is produced from the leaves of Camellia sinensis. Based on the manufacturing process used tea is available in four different forms such as green, black, oolong, and white tea. The process of preparation of green tea prevents the oxidation of green leaf polyphenols; in black tea most of these substances are oxidized; and in oolong tea they are partially oxidized. White tea is made from newly growth buds and young leaves by inactivating polyphenol oxidation through steaming and drying. Out of all tea produced only 20% is green tea and less than 2% is oolong tea. Green tea is consumed primarily in China and Japan and most widely studied for its health benefits. The polyphenolic composition of green tea includes catechins (30–42%), flavonols (5–10%), and other flavonoids such as theogallin (2–3%), gallic acid (0.5%), quinic acid (2%), theanine (4–6%), and methylxanthins (7–9%). The major catechins present in green tea are (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallate, (−)-epigallocatechin, and (−)-epicatechin. EGCG accounts for 50–65% of the total catechin content in green tea. The major polyphenols in black tea are catechins (3–10%), flavanols (6–8%), methylxanthines (8–11%), theaflavins (3–6%) and thearubigens (12–18%) [2].
Green tea polyphenols have gained increasing attention from researchers in the field of cancer biology since it was discovered that polyphenols could affect cancer cell growth. Initial evidence from epidemiologic studies suggest reduced risk of some cancers in a region of the world where green tea was regularly consumed [2 and references therein]. Recent review on clinical studies critically assessed association between green tea consumption and the risk of cancer incidence and mortality. Reports indicate that 51 prospective controlled interventional and observational studies of 1.6 million participants conducted assessed either the associations between green tea consumption and risk of cancer incidence or cancer mortality. These results assessed associations between green tea and risk of digestive tract cancer incidence which was inconclusive and conflicting. Although, there was limited evidence that green tea could reduce the incidence of liver cancer, the evidence for esophageal, gastric, colon, rectum, and pancreatic cancers were contradictory. Observational studies and one randomized controlled trial on prostate cancer suggested a decrease risk in men consuming higher quantities of green tea or green tea extracts. Moreover, there was limited to moderate evidence that the consumption of green tea reduced the risk of lung cancer, especially in men. There was also evidence that green tea consumption could increase the risk of bladder cancer [3].
Studies on black tea polypheols and its extract have been conducted in rodent models using chemical carcinogens or ultra-violet (UV) radiation. Limited studies which include studies on skin, lungs, buccal pouch, liver, colon, esophagus, small intestine, prostate and mammary gland cancers have been reported [6]. Results of these studies demonstrate that black tea possesses chemopreventive and chemotherapeutic potentials. Studies with black tea indicated that the mechanisms of chemopreventive actions are multi-factorial and include effects on xenobiotic phase I, phase II and antioxidant enzymes, xenobiotic-induced DNA damage, cellular kinases, transcription factors, and oncogenes. However, because of limited available data on black tea and cancer this review will focus on studies conducted with green tea polyphenols.
EFFECTS OF TEA POLYPHENOLS
Effect of tea polyphenols on the survival pathways in cancer cells
The cell cycle is controlled by cyclic activation and inactivation of cyclin-dependent kinases (CDKs) and their inhibitors. The cell monitors and regulates the cell cycle at checkpoints to ensure that damaged or incomplete DNA is not passed on to daughter cells. The tumor suppressor proteins p53 and retinoblastoma (Rb) play an important role in triggering the control mechanisms at both G1/S and G2/M checkpoints. p53, the major regulator of cell cycle checkpoints can cause cell arrest through induction of p21, therefore allowing time for DNA repair before the cell progresses into the cell cycle. Overexpression of cyclins and CDKs, and inactivation of tumor suppressor proteins p53 and Rb lead to cell cycle deregulation, which is a hallmark of cancer [4]. Tea polyphenols have been demonstrated to induce cell cycle arrest in several different cancer cell lines. In vitro studies demonstrated that EGCG causes cell cycle arrest through modulation on the levels and activity of cyclins, CDKs, CDK inhibitors, and tumor suppressors: p53 and Rb in human breast, prostate, cervical, pancreatic, bladder, and head and neck cancer cells [7–8]. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK and inhibition of mammalian target of rapamycin (mTOR) in p53-positive and p53-negative human hepatoma cells [9].
In response to DNA damage, p53 can initiate cell death via apoptosis if DNA damage is irreparable. Tea polyphenols cause induction of apoptosis in many cancer cell lines such as melanoma, leukemia, neuroblastoma, hepatocellular carcinoma and cancers of pancreas, colon, prostate, lung, and breast [10]. EGCG causes apoptosis through induction of p53 and by influencing the ratio of pro- to anti- apoptotic factors in favor of apoptosis [7,11]. Apoptosis induced by polyphenols in cancer cells is caspase 3-dependent, which is concurrent with the ability of cancer cells to inhibit proteasome activity and accumulate the proapoptotic protein, Bax [8,12]. Studies have shown that tea polyphenols have the ability to stimulate H2O2 generation and induce apoptosis of lung cancer cells and H-ras-transformed bronchial epithelial cells which can by prevented by introducing catalase [13]. In colon cancer cells, EGCG induces apoptosis via inhibition of NAG-1 in p53-independent manner [14]. However, another study showed that tea polyphenols can impart protective effects on PC12 cells through inhibition of the 6-hydroxydopamine-induced cell apoptosis [15]. Tea polyphenols including EGCG can induce cell cycle arrest and apoptosis in cells lacking functional p53 by activating p73, a closely related p53 family member expressing p53 target genes p21 and MDM2 [16]. Recent studies demonstrate that EGCG can sensitize tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in prostate, malignant glioma, pancreatic, and hepatocellular carcinoma cells [7,8,17]. Upregulation of proapoptotic BH3-only protein p53-upregulated modulator of apoptosis (PUMA) by tea polyphenols cause both p53 dependent and independent apoptosis of colorectal cancer cells [18]. Tissue factor pathway inhibitor-2 (TFPI-2) overexpression in renal cell carcinoma [13]; or down-regulation of inhibitor of DNA binding 2, a dominant negative helix-loop-helix protein in prostate cancer cells inhibits cell growth and induces apoptosis after EGCG treatment [19]. In preclinical studies, infusion of green tea significantly reduced the incidence of chemical-induced lung carcinoma or mouse skin carcinogenesis, upregulated p53 and Bax expression, and dowregulated Bcl2 and survivin [20–21]. However, in a recent study EGCG protected non-small cell lung carcinoma cells from apoptosis induced by serum deprivation via Akt activation [22]. This effect may limit the clinical use of EGCG in treating and preventing non-small cell lung carcinoma.
Effect of tea polyphenols on self-sufficiency growth signals in cancer cells
The insulin-like growth factor/insulin growth factor-1 receptor (IGF/IGF-IR) system plays important role in the development and growth of various malignancies and remains an important target in cancer. EGCG inhibited IGF-IR levels and activity, increased expression of TGF-beta2 and insulin growth factor binding protein-3 (IGFBP-3) and decreased levels of matrix metalloproteinase’s (MMPs)-7 and MMP-9 mRNA in colon cancer cells [23]. EGCG also caused apoptosis of malignant brain tumors and hepatocellular carcinoma cells through inhibition of IGF-I [24,25]. Oral infusion of green tea polyphenol inhibited development and progression of prostate cancer in a transgenic adenocarcinoma of the mouse prostate (TRAMP), reduced IGF-I levels, decreased activation of Akt and ERK, and inhibited vascular endothelial growth factor (VEGF); MMP-2 and MMP-9 levels in the dorso-lateral prostate of these mice. IGF-I signaling was significantly inhibited only when intervention was initiated at early stages of cancer development [7,23,25]. In a clinical study, twenty-six men with positive prostate biopsies and scheduled for radical prostatectomy received Polyphenon E supplementation until the time of surgery demonstrated a significant reduction in serum levels of prostate-specific antigen (PSA), HGF, and VEGF [26]. Furthermore, inhibition of protein expression and activity of VEGF, MMP-2 and MMP-9 and elevation of TIMP1 expression was observed after administration of GTPs in the drinking water of mice bearing UVB-induced skin tumors. [27].
Tea polyphenols modulate the activity of membrane-associated receptor tyrosine kinases which play important role in the control of many fundamental cellular processes. Overexpression of the human epidermal growth factor receptor-2 (HER-2/neu) is associated with poor prognosis in patients with breast cancer and head and neck squamous-cell carcinoma. EGCG inhibits activation of these receptors and exerts anti-proliferative and anti-angiogenic activities by inhibiting STAT3 and NF-κB activation. EGCG and Polyphenon E have shown to cause a decrease in phosphorylation of epidermal growth factor receptor (EGFR) and HER-2 proteins resulting in activation of Akt and extracellular signal-regulated kinase (ERK) proteins, and transcriptional activity of AP-1 and NF-κB promoters in HT29 human colon cancer cells. EGCG inhibited HER-3 signaling, cyclooxygenase-2 (COX-2) transcription, and prostaglandin E2 (PGE-2) production in human colon cancer cell lines [23]. EGCG causes downregulation of EGFR by inducing mitogen-activated protein kinase (MAPK) which phosphorylates EGFR at sites critical for receptor internalization [28]. EGCG also inhibits the activation of platelet-derived growth factor receptors (PDGFR) and fibroblast growth factor receptors (FGFR) in human epidermoid carcinoma cell line, and PDGFR in human glioblastoma and hepatic stellate cell lines [23, 29]. EGCG has shown responsiveness to cancer cells by affecting the 67KDa laminin receptor present in the lipids raft on the membrane thereby modulating the activity of tyrosine kinases [30–31].
Effect of tea polyphenols on the replicative potential of cancer cells
In most somatic human cells telomeres shorten in length with every cell division and eventually reach a critical length. This shortening causes a cell to loose its proliferative ability, and the cell undergoes permanent cell cycle arrest or senescence. Unlike somatic cells, hematopoietic stem cells, keratinocytes in the basal layers of the epidermis, uterine endometrial cells, germ cells, and various tumors possess unlimited replicative potential because of their ability to maintain constant telomere length and presence of active telomerase activity. Increased telomerase activity correlated with upregulation of the human telomerase reverse transcriptase gene (hTERT) and increased levels of hTERT mRNA. Studies demonstrated that green tea polyphenols block telomerase activity as a major mechanism for limiting the growth of human cancer cells. Tea polyphenols significantly inhibited telomerase activity of HepG2 cells compared with the control group. EGCG treatment caused inhibition of proliferation and telomerase activity and induction of apoptosis in cervical adenocarcinoma cells. Tea polyphenols cause repression of hTERT mRNA expression in lung, oral cavity, thyroid and liver carcinoma cells. Another study using tongue cancer cell lines indicated that tea polyphenols reduced hTERT activity in a time- and dose-dependent manner, disabling telomerase activity and terminating unlimited cell proliferation [32]. EGCG induced telomere fragmentation in HeLa and 293 cells but not in MRC-5 cells [33]. This could be relevant to the apoptosis-inducing effect of EGCG on cancerous cells but not on normal cells.
Effect of tea polyphenols on invasion, metastasis, and angiogenesis of cancer cells
Malignant cells require continuous supply of nutrients to maintain high metabolic activity which could be achieved through chemotaxis toward a preexisting vascular network, or by infiltration of vascular endothelial cells leading to neo-vascularization. In this context, urokinases, VEGF, FGF, transforming growth factor-beta (TGF-β), PGDF, endothelin-1, extracellular matrix (ECM) proteolytic enzymes, MMPs and tissue inhibitor of metalloproteinases (TIMPs) play critical roles. Tea polyphenols inhibit angiogenesis and cell invasion by affecting the expression of these molecules. Urokinase-type plasminogen activator (uPA) is over-expressed in breast, ovarian, and prostate malignancies and was implicated in cell invasion and metastasis. By inhibiting uPA, EGCG reduced the size or caused complete remission of tumors in mice. GTP inhibited invasion of breast cancer cells by inhibiting AP-1 and NF-κB and suppressing uPA secretion. Invasion of human oral cancer cells was blocked by EGCG by inhibiting uPA, MMP-2 and MMP-9 [34]. EGCG inhibited MMP-2 and MMP-9 while inducing the activity of their inhibitors TIMP-1 and TIMP-2 in neuroblastoma, fibrosarcoma, glioblastoma, prostate, endothelial, and human gastric cancer cells [8,13,34,35]. In TRAMP mice, green tea polyphenol infusion resulted in marked inhibition of effectors of angiogenesis and metastasis, notably VEGF, uPA, MMP-2, and MMP-9 [7]. EGCG has been shown to directly inhibit metallothionein-1 (MT1)-MMP activity in HT-1080 human fibrosarcoma cells and human umbilical vein endothelial cells (HUVEC) cells, leading to accumulation of non-activated MMP-2 at the cell surface. EGCG treatment of human breast cancer cells reduced MMP-2 activity and expression at the translational and transcriptional level. EGCG treatment reduced the expression of focal adhesion kinase (FAK), membrane type-1-matrix metalloproteinase (MT1-MMP), NF-κB, and VEGF, and reduced adhesion of cells to ECM, fibronectin, and vitronectin [36]. In oral squamous cell carcinoma cells, EGCG significantly enhanced the expression of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) mRNA and inhibited MMP-2 and MMP-9. Administration of black tea polyphenols also reduced the incidence of DAB-induced hepatomas as demonstrated by the markers of invasion viz. MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinase, TIMP-2, and RECK; angiogenesis viz. hypoxia-inducible factor (HIF)-1alpha, VEGF, and its receptor, VEGFR-1, and the expression of histone deacetylase (HDAC)-1 [13].
In addition to the inhibitory effects of tea polyphenols on migration, invasion, and metastasis of cancer cells it also modulates the expression of adhesion molecules. EGCG inhibited melanoma cell migration and spreading by inhibiting tyrosine phosphorylation of FAK and MMP-9 activity. EGCG can prevent the metastatic spread of breast, colon and hypopharyngeal carcinoma cells by inhibiting HGF/Met signaling [13,37,38]. Tea polyphenols also mediate its anti-metastatic activity at nanomolar concentrations by interacting with laminin receptors [30]. EGCG inhibited the migration/invasion of breast carcinoma cells by suppressing the HRG-stimulated activation of ErbB2/ErbB3/Akt pathway [39]. EGCG inhibited invasion of melanoma cells by up-regulation the expression of E-cadherin and inhibited growth, invasion, angiogenesis, and metastasis of human pancreatic cancer by inhibiting proliferation (Ki-67 antigen and proliferating cell nuclear antigen (PCNA), angiogenesis (von Willebrand factor, VEGF, and CD31), metastasis (MMP-2, MMP-7, MMP-9, and MMP-12), inducing apoptosis, and growth arrest in tumor xenograft model [40]. Furthermore, EGCG administration reduced primary tumor growth and lung metastasis in mice bearing B16-F3m melanomas and increased their survival [41]. EGCG binds to and inhibit phosphorylation of vimentin thereby limiting the tumor promotion and proliferation [13]. On the contrary, EGCG enhanced the production of pro-MMP-7 via generation of reactive oxygen species and activation of JNK1/2 and c-JUN/c-FOS induction, as well as AP-1 transactivation, in colon cancer cells [42].
Epigenetic regulation by tea polyphenols
Until recently, it was hypothesized that mutations in genes consequent to internal or external insult to the cell, leads to generation of structurally and functionally abnormal proteins progressing to cancer. Because of an improved understanding of epigenetic processes, it is now evident that gene expression can be switched “on” or “off” without abrupt changes in the nucleotide sequences [43]. This can be achieved by modifying either the functional moieties on the nucleotides or on histone proteins or activity of enzymes, especially DNA methyltransferases (DNMTs), histone acetyltransferases, and/or histone deacetylases. These changes control higher organizational genetic information and determine gene expression changes which explain how a cell can differentiate into many different cell types and organs by epigenetic switching. These processes are highly regulated in normal cells, but loss of this regulation leads to genesis of disease processes including carcinogenesis.
DNA methylation is the only genetically programmed DNA modification in mammals and perhaps the best studied epigenetic mechanism. This post-replication modification is almost exclusively found on the 5’ position of the pyrimidine ring of cytosine in the context of the dinucleotide sequence CpG [43]. 5-methylcytosine accounts for 1% of all bases, varying slightly in different tissue types; and the majority (75%) of CpG dinucleotides are methylated in mammalian genomes. The composition of the genome is reflected in and dictates the epigenetic machinery to establish particular local and global epigenetic patterns using CpG spacing, sequence motifs, and DNA structure [44]. A global hypomethylation of the genome is also observed in cancers [45]. This decreased methylation may initiate oncogenesis by causing chromosome instabilities and transcriptional activation of oncogenes and genes involved in metastasis [46]. A region and gene specific increase in methylation of multiple CpG islands is observed along with global genomic hypomethylation in malignant cells [43,47]. In contrast, hypermethylation of CpG islands in the promoter region of a tumor suppressor or otherwise cancer-related gene is often associated with transcriptional gene silencing. A number of genes involved in DNA repair, cell cycle regulation, apoptosis, and other physiologic processes are modulated by hypermethylation of respective CpG islands present on promoter regions. A recent study suggested that methylation of multiple genes plays an important role in prognosis of patients with breast cancer. This study not only described the association of methylation mediated silencing of multiple genes with the severity of disease, but also speculated that the molecular crosstalk between genes or genetic pathways is individually regulated [48].
Several studies indicate that DNA methylation can be reversed by intake of multiple food components including tea polyphenols. For example, methyl deficient diets have lead to changes in the methylation patterns consistent with alterations observed during transformation of normal cells to neoplasms [43]. Thus, changes in these methylation patterns by tea polyphenols may be responsible for their chemopreventive action. Together, silencing and unsilencing of genes can occur through modification of histones, as well as by changes in the DNA methylation [49–51]. In addition to factors that govern the overall recruitment and release of histones (histone occupancy), there is a complex interplay of reversible histone modifications that govern gene expression, including histone acetylation, methylation, phosphorylation, ubiquitination and biotinylation. Modification of histone deacetylase has also surfaced as a strategy for changing tumor behavior [49–51].
Earlier studies demonstrated that tea polyphenols bind to DNA and RNA, accumulate through regular consumption playing a significant role in cancer prevention [13,52]. It was reported that catechol-containing dietary polyphenol inhibited enzymatic DNA methylation in vitro largely by increasing the formation of S-adenosyl-L-homocysteine (a potent noncompetitive inhibitor of DNMTs) during the catechol-O-methyltransferase-mediated O-methylation of the dietary catechol [43]. EGCG and (−)-epigallocatechin repressed telomerase mRNA in lung, oral cavity, thyroid, and liver cancer cells might be linked to inhibition of cell growth [32]. EGCG also demonstrated anti-neoplastic activity by suppressing the telomerase activity of digestive cancer cells [53]. EGCG can inhibit DNMT activity and reactivate methylation silenced genes p16INK4a, retinoic acid receptor β (RAR β), O6-methylguanine methyltransferase (MGMT), and human mutL homologue 1 (hMLH1) genes in human colon, esophageal, and prostate cancer cells [13]. In another study, methylation of CDX2 and other genes involved in gastric carcinogenesis was investigated in relation to the clinico-pathologic and selected lifestyle factors of patients with gastric cancer. An inverse association of CDX2 methylation was observed with the intake of green tea [43]. Decreased annexin-I expression is a common event in early-stage bladder cancer development. In part, green tea induced the expression of mRNA and protein levels of annexin-I through demethylation of its promoter and actin remodeling [54]. EGCG, an efficient inhibitor of human dihydrofolate reductase, altered the p16 methylation pattern from methylated to unmethylated after folic acid deprivation resulting in growth inhibition of human colon carcinoma cells. This same study demonstrated that through disruption of purine metabolism, EGCG caused adenosine release from the cells modulating different signaling pathways via binding to adenosine-specific receptors [43]. Treatment of oral cancer cells with EGCG partially reversed the hypermethylation status of the RECK gene significantly enhanced RECK mRNA expression. In another study, tissue factor pathway inhibitor-2 (TFPI-2), a member of the Kunitz-type serine proteinase inhibitor family, is inversely related to an increasing degree of malignancy. EGCG inhibited growth and induced apoptosis in renal cell carcinoma through TFPI-2 mRNA and protein overexpression [13]. Epigenetic silencing of glutathione-S-transferase pi (GSTP1) by hypermethylation is recognized as being a molecular hallmark of human prostate cancer. Recently our laboratory reported that exposure of LNCaP cells to GTP at physiologically attainable concentrations caused demethylation in the proximal GSTP1 promoter and regions distal to the transcription factor binding sites. GTP exposure caused a concentration- and time- dependent re-expression of GSTP1 and DNMT1 inhibition. GTP exposure also increased mRNA and protein levels of MBD1, MBD4 and MeCP2; HDAC 1–3 whereas levels of acetylated histone H3 (LysH9/18) and H4 decreased. In addition, GTP reduced MBD2 association with accessible Sp1 binding sites causing increased binding and transcriptional activation of the GSTP1 gene. Importantly, GTP treatment did not result in global hypomethylation and promoted maintenance of genomic integrity. Unlike 5-aza-2'deoxycitidine treatment, GTP exposure did not activate prometastatic gene S100P. This study demonstrates the dual potential of tea polyphenols at physiologically attainable non-toxic doses to alter DNA methylation and chromatin modeling, the two global epigenetic mechanisms of gene regulation. Another report demonstrated a significant reduction in the number of newly formed tumors in Apc (Min/+) mice treated with azoxymethane-treatment and supplementation with a solution of green tea caused downregulation RXR alpha. These results correlated with decreased CpG methylation in the promoter region of the RXR alpha gene [43].
Recent reports demonstrated that treatment of breast cancer and promyelocytic leukemia cells with EGCG resulted in a decrease in E2F-1 binding sites, hTERT promoter methylation and ablation of histone H3Lys9 acetylation causing an increase in binding of E2F-1 repressor at the hTERT promoter resulting in cell death. The Polycomb Group (PcG) proteins are epigenetic repressors of gene expression and their repression is achieved via action of two multi-protein PcG complexes-PRC2 (eed) and PRC1 (Bmi-1). These complexes increase histone methylation and reduce acetylation that leads to a closed chromatin conformation. Bmi-1 is over-expressed in breast, prostate, colon, pancreatic and non-small cell lung cancers. EGCG treatment caused suppression of two key PcG protein, Bmi-1 and Ezh2 and lead to global reduction in histone H3-K27-trimethylation. This caused reduced expression of key cell cycle regulated proteins viz. cdk1, cdk2, cdk4, cyclin D1, cyclin E, cyclin A, and cyclin B1 and increased expression of cell cycle kinase inhibitors viz. p21/waf1 and p27/kip1. EGCG treatment resulted in induction of apoptosis through increased expression of Bax, caspase -9, -8 and -3 and poly ADP-ribose polymerase (PARP) cleavage along with decrease Bcl-xL expression [43].
Another important epigenetic regulation occurs via modifications of microRNA (miRNA) expression. Not many studies are available in the literature that explored the influence of tea polyphenols on the expression of miRNAs in human cancers. A recent study demonstrated that EGCG treatment altered the expression of miRNAs in human hepatocellular carcinoma HepG2 cells. Thirteen miRNAs were upregulated and 48 were downregulated. Among the miRNAs upregulated by EGCG, some target genes included RAS, Bcl2, E2F, TGFBR2 and c-Kit. Among those miRNAs downregulated by EGCG include the target genes comprised of HOX family proteins, including PTEN, SMAD, MCL1, SLC16A1, TTK, PRPS1, ZNF513, and SNX19 with diversified functions. Treatment with EGCG down-regulated Bcl-2, an anti apoptotic protein, and transfection with anti-miR-16 inhibitor suppressed miR-16 expression and counteracted the EGCG effects on Bcl-2 down-regulation and induced apoptosis in these cells [43]. These results suggest that EGCG may exert its biologic functions through modulation of miRNA expression.
In another study of 42 non-smoking healthy individuals supplementation of green tea polyphenols significantly increased both the activity and the levels of glutathione S- transferase Pi (GSTP1), a phase 2 enzyme, in individuals with low baseline enzyme activity/level suggesting tea polyphenol intervention may enhance the detoxification of carcinogens in individuals with low baseline detoxification capacity [13]. Though such studies are limited, the results are encouraging and might be indicative of epigenetic modification imparted by regular consumption of tea to decrease the risk of certain cancers. However, well planned and controlled studies are needed.
POTENTIAL LIMITATIONS
The concentrations of tea polyphenols used for in vitro studies range from 20µM to100µM or even higher. These levels cannot be achieved in vivo, especially inside or surrounding cancer cells. Therefore, it may be unwise to extrapolate the results of in vitro studies to in vivo situations. The levels which can be achieved in blood after 2 to 3 cups of tea range from 0.1µM to 0.6µM and even after 7 to 9 cups, it is less than 1µM. Furthermore, the concentrations of catechins in the tissue depend on the duration of tea intake. This might be the reason that the results from clinical trials have not been encouraging. Tea polyphenols are partially absorbed as demonstrated through a study that 0.012% of EGCG was absorbed after 30 minutes of an oral dose of 56 mg in rats and 0.32% after 60 min of an oral dose 97 mg in human [55]. This low absorption of polyphenols is probably because of increased breakdown in the intestines at high pH as tea catechins have been shown to be more stable at low pH [56]. Tea polyphenols have a half-life of less than 2 hrs which can be extended multiple folds by addition of superoxide dismutase or catalase. Tea polyphenols are considered natural antioxidants but have been shown to oxidize to form prooxidant species depending on the physiologic conditions. The extent of this conversion depends on the partial pressure of oxygen in in vivo and in vitro conditions. Hence, elucidation of in vitro results to in vivo situations needs further investigation, especially in light of reports describing that under physiologic conditions, biotransformation reactions, such as methylation, can modify green tea polyphenols and therefore limit their in vivo cancer-preventive activity [57].
To overcome these potential problems, derivatives of tea polyphenols have been developed to increase their bioavailability and potency. Peracetate protection groups on the reactive hydroxyls of (−)-EGCG are 6 times more stable than natural (−) EGCG under slightly alkaline conditions. Hydroxylated EGCG increased proteasome-inhibitory activity in intact leukemic cells more than natural (−)-EGCG, but not in bioassays indicating an intercellular conversion [56]. Ester bond-containing tea polyphenols potently and selectively inhibit the proteasomal chymotrypsin-like but not trypsin-like activity in vitro and in vivo [7]. A trimethoxy derivative of epicatechin-3-gallate showed high anti-proliferative and proapoptotic activity against melanoma in a mouse melanoma model by stably and strongly inhibiting dihydrofolate reductase after conversion to a stable quinone methide product by specific melanocyte enzyme tyrosinase [58]. Reports suggest that non-gallated flavon-3-ols in green and black teas have higher bioavailability compared with gallated flavan-3-ols [59]. Numerous reports confirmed that a mixture of catechins is more effective in anti-carcinogenic and chemotherapeutic potential compared with EGCG alone indicating that other catechins in tea also have a significant role in anticancer activities either by modifying the effectiveness of EGCG or through complementary activities [60 and references therein]. Therefore, further experiments are needed to address the most effective composition of various catechins or their derivative to design effective formulations.
Tachibanam and co-workers determined an effective concentration of EGCG to inhibit the biologic activity of many proteins by competitive or noncompetitive mechanisms. Interestingly, these effective concentrations ranged from 0.003µM to 10mM [30]. To achieve higher concentrations of polyphenols in the tissues either higher intake of tea polyphenols or derivatives of tea polyphenols with increased stability and bioavailability may be required. However, the increased bioavailability and effectiveness of tea polyphenols derivatives and increased concentrations of natural tea polyphenols are difficult to achieve and may be toxic to normal cells. Therefore, development of synthetic derivatives is needed or new technologies to achieve higher concentrations of tea polyphenols in the systemic circulation and target tissue is required. One such effort using encapsulated green tea polyphenol epigallocatechin-3-gallate (EGCG) in polylactic acid-polyethylene glycol nanoparticles showed that encapsulated EGCG retains its biologic effectiveness with a more than 10-fold dose advantage for exerting its proapoptotic and angiogenesis inhibitory effects [61].
SYNERGISM WITH TEA POLYPHENOLS
Many studies have shown that tea polyphenols reversed the cancer multi-drug resistance and may reduce their effective doses and prevent side effects [62]. A study demonstrated that EGCG enhanced chemoresistance to both doxorubicin and paclitaxel by increasing Nrf2 and detoxification enzyme levels in breast cancer cell lines and this effect was more prominent in cell lines with very low basal Nrf2 levels [63]. Tea polyphenols have been shown to modulate the activity of various xenobiotic metabolic enzymes. Decreased activity of CYP3A4, 2A6, 2C19, and 2E1 and increased activity of the CYP1A2 and 2B isoforms in humans and rodents were observed after infusion with tea polyphenols [64–66]. A study demonstrated EGCG-mediated apoptosis in multiple myeloma cells was potentiated by bortezomib [67]. However, in another study green tea polyphenols blocked the anticancer activity of bortezomib and boronic acid in multiple myeloma and glioblastoma cell lines [68]. Therefore, the use of tea polyphenols in synergistic studies requires further scrutiny in view of these recent reports. In addition, tea polyphenols upregulate phase II enzymes in neoplastic cells affecting xenobiotic metabolism which can alter the efficacy and toxicity of cancer chemotherapeutic agents. Further studies are required with tea polyphenols complemented with radio or chemotherapy to reduce the dose and increase its efficacy.
CONCLUSIONS AND FUTURE DIRECTIONS
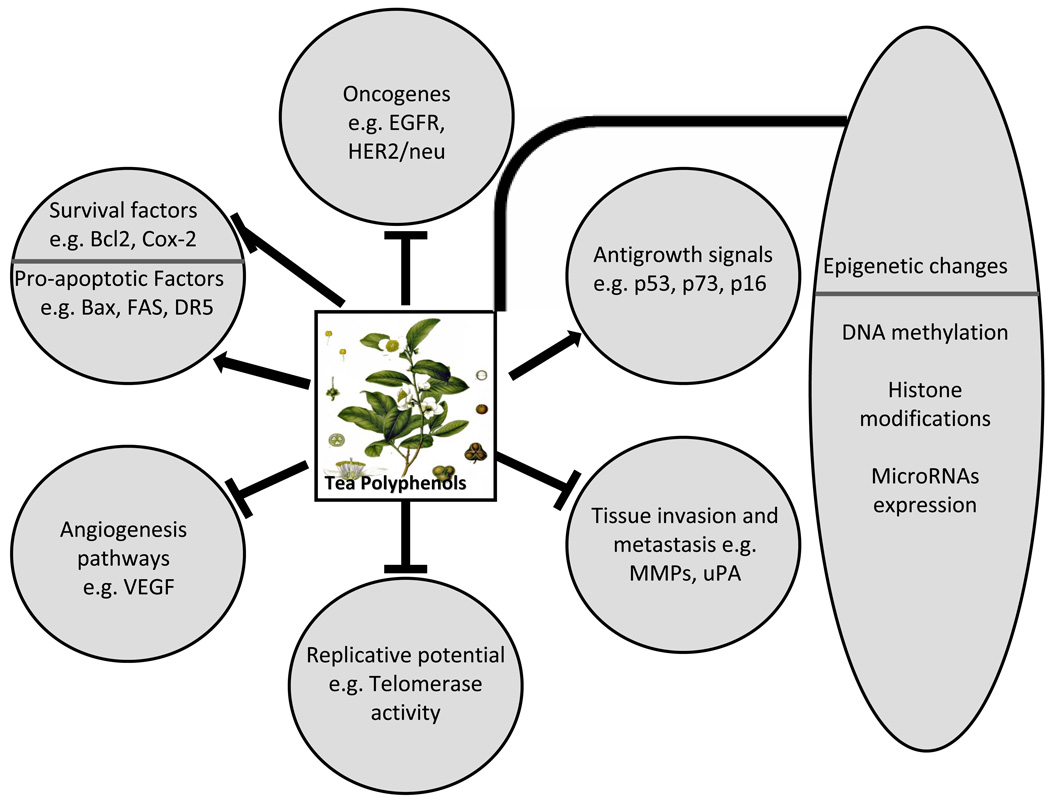
Green tea polyphenols have shown potential to decrease the risk of various human diseases including cancer by targeting various signaling pathways. Therefore, tea polyphenols remain an excellent candidate for chemoprevention and further research is warranted to understand the involvement of particular mechanisms and/or pathways. A schematic representation of targets and pathways modulated by green tea polyphenols is shown in Fig. (1). A better understanding of the mechanisms of epigenetic changes with tea polyphenols is required for the precise use in chemoprevention regimens. Recent reports on the additive or synergistic effects of tea polyphenols to increase the efficacy of chemo- or radiation therapy is a promising area of research and emphasis on more well-designed and well-planned studies is needed. This approach has the potential to enhance the efficacy of conventional therapies by adjustment of dose and time periods needed to achieve optimum results. In addition, development of new derivatives of tea polyphenols with improved bioavailability and efficacy are required. Studies to develop techniques like nanotechnology should be encouraged which could lead to sustained and precise delivery of polyphenols to the target site(s), thereby achieving improved efficacy and effective treatments to improve patient outcomes as an ultimate goal.
Figure 1. Pathways affected by tea polyphenols.
Numerous pathways are deregulated in cancer cells that include i) evasion of apoptosis by activating survival or inhibiting proapoptotic factors, ii) achieve self-sufficiency by modulating oncogenes, iii) attain insensitivity to antigrowth signals by modulating tumor suppressors, iv) gaining replicative potentials by modulating telomerase activity, v) invasion of neighboring tissues through increase activity of metastatic and invasion molecules, and vi) enhancing angiogenesis for supply of nutrients, growth factors and oxygen consumption. Tea polyphenols have shown to affect epigenetic and various signaling pathways. → demonstrates activation, – demonstrates regulation, ⊥ demonstrates inhibition.
ACKNOWLEDGEMENT
We thank Kerry O. Grimberg, Ph.D., Case Western Reserve University, for her editorial assistance. The original work from author’s laboratory outlined in this review was supported by United States Public Health Service Grants RO1 CA115491 and R21 CA109424. We apologize to those investigators whose original work could not be cited owing to the space limitations.
Footnotes
Conflict of interest: The authors have no competing interest.
REFERENCES
- 1.Herceg Z, Hainaut P. Genetic and epigenetic alterations as biomarkers for cancer detection, diagnosis and prognosis. Mol. Oncol. 2007;1(1):26–41. doi: 10.1016/j.molonc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui IA, Adhami VM, Saleem M, Mukhtar H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol. Nutr. Food Res. 2006;50(2):130–143. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- 3.Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database of Systematic Reviews. 2009;3 doi: 10.1002/14651858.CD005004.pub2. CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M. Drug Resist. Updat. 2007;10(1–2):13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Clement Y. Can green tea do that? A literature review of the clinical evidence. Prev. Med. 2009;42(2–3):83–87. doi: 10.1016/j.ypmed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Kumar G, Pillare SP, Maru GB. Black tea polyphenols-mediated in vivo cellular responses during carcinogenesis. Med. Chem. 2010;10(6):492–505. doi: 10.2174/138955710791384063. [DOI] [PubMed] [Google Scholar]
- 7.Khan N, Adhani VM, Mukhtar H. Review: Green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61(6):836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007;18(7):427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol. Nutr. Food Res. 2009;53(9):1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 10.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6(5):389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi R, Sasaki K, Yoshida K. Identification of epigallocatechin-3-gallate in green tea polyphenols as a potent inducer of p53-dependent apoptosis in the human lung cancer cell line A549. Toxicol In Vitro. 2009;23(5):834–839. doi: 10.1016/j.tiv.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26(5):958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25(12):2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 15.Nie G, Jin C, Cao Y, Shen S, Zhao B. Distinct effects of tea catechins on 6 hydroxydopamine-induced apoptosis in PC12 cells. Arch Biochem Biophys. 2002;397(1):84–90. doi: 10.1006/abbi.2001.2636. [DOI] [PubMed] [Google Scholar]
- 16.Amin AR, Thakur VS, Paul RK, Feng GS, Qu CK, Mukhtar H, Agarwal ML. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc. Natl. Acad. Sci.U. S. A. 2007;104(13):5419–5424. doi: 10.1073/pnas.0700642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu A, Haldar S. Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int. J. Oncol. 2009;34(1):281–286. [PubMed] [Google Scholar]
- 18.Thakur VS, Ruhul Amin AR, Paul RK, Gupta K, Hastak K, Agarwal MK, Jackson MW, Wald DN, Mukhtar H, Agarwal ML. p53-Dependent p21-mediated growth arrest pre-empts and protects HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer Lett. 2010;296(2):225–232. doi: 10.1016/j.canlet.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo KL, Luo JHYP. (−)-Epigallocatechin-3-gallate induces DU145 prostate cancer cell death via downregulation of inhibitor of DNA binding 2, a dominant negative helix-loop-helix protein. Cancer Sci. 2010;103(3):707–712. doi: 10.1111/j.1349-7006.2009.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Q, Hu C, Chen Q, Xia Y, Feng J, Yang H. Development of a rat model by 3,4-benzopyrene intra-pulmonary injection and evaluation of the effect of green tea drinking on p53 and bcl-2 expression in lung carcinoma. Cancer Detect. Pev. 2009;32(5–6):444–451. doi: 10.1016/j.canep.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu PP, Kuo SC, Huang WW, Yang JS, Lai KC, Chen HJ, Lin KL, Chiu YJ, Huang LJ, Chung JG. (−)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res. 2009;29(4):1435–1442. [PubMed] [Google Scholar]
- 22.Kim MJ, Kim HI, Chung J, Jeong TS, Park HR. (−)-Epigallocatechin-3-gallate (EGCG) increases the viability of serum-starved A549 cells through its effect on Akt. Am. J. Chin. Med. 2009;37(4):723–734. doi: 10.1142/S0192415X09007193. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M, Shirakami Y, Moriwaki H. Targeting receptor tyrosine kinases for chemoprevention by green tee catechin EGCG. Int. J. Mol. Sci. 2008;9(6):1034–1049. doi: 10.3390/ijms9061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama S, Hirano H, Wakimaru N, Sarker KP, Kuratsu J. Inhibitory effect of epigallocatechin-gallate on brain tumor cell lines in vitro. Neuro. Oncol. 2001;3(1):22–28. doi: 10.1093/neuonc/3.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, Mukhtar H. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin. Cancer Res. 2009;15(6):1947–1953. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. (Phila. Pa) 2009;2(7):2673–2682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 27.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18(5):287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Adachi S, Shimizu M, Shirakami Y, Yamauchi J, Natsume H, Matsushima Nishiwaki R, To S, Weinstein IB, Moriwaki H, Kozawa O. (−)- Epigallocatechin gallate downregulates EGF receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon cancer cells. Carcinogenesis. 2009;30(9):1544–1552. doi: 10.1093/carcin/bgp166. [DOI] [PubMed] [Google Scholar]
- 29.Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J. Hepatol. 2004;40(1):52–59. doi: 10.1016/s0168-8278(03)00477-x. [DOI] [PubMed] [Google Scholar]
- 30.Tachibana H. Molecular Basis for Cancer Chemoprevention by Green Tea Polyphenol EGCG. Forum Nutr. 2009;61:156–169. doi: 10.1159/000212748. [DOI] [PubMed] [Google Scholar]
- 31.Patra SK, Rizzi F, Silva A, Rugina DO, Bettuzzi S. Molecular targets of (−)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J. Physiol Pharmacol. 2008;59 Suppl 9:217–235. [PubMed] [Google Scholar]
- 32.Khan N, Mukhtar H. Multitargted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269(2):269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li WG, Li QH, Tan Z. Epigallocatechin gallate induces telomere fragmentation in HeLa and 293 but not in MRC-5 cells. Life Sci. 2005;76(15):1735–1746. doi: 10.1016/j.lfs.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Slivova V, Zaloga G, DeMichele SJ, Mukerji P, Huang YS, Siddiqui R, Harvey K, Valachovicova T, Sliva D. Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr. Cancer. 2005;52(1):66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- 35.Maeda-Yamamoto M, Kawahara H, Tahara N, Tsuji K, Hara Y, Isemura M. Effects of tea polyphenols on the invasion and matrix metalloproteinases activities of human fibrosarcoma HT1080 cells. J. Agric. Food Chem. 1999;47(6):2350–2354. doi: 10.1021/jf9811525. [DOI] [PubMed] [Google Scholar]
- 36.Sen T, Moulik S, Dutta A, Roy Choudhury P, Banerji A, Das S, Roy M, Chatterjee A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84(7–8):194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Larsen CA, Dashwood RH. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: Minor role of hydrogen peroxide. Biochem. Biophys. Res. Commun. 2009;389(3):527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen CA, Bisson WH, Dashwood RH. Tea catechins inhibit hepatocyte growth factor receptor (MET Kinase) activity in human colon cancer cells: Kinetic and molecular docking studies. J. Med. Chem. 2009;52(21):6543–6545. doi: 10.1021/jm901330e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushima Y, Iida K, Nagaoka Y, Kawaratani Y, Shirahama T, Sakaguchi M, Baba K, Hara Y, Uesato S. Inhibitory effect of (−)-epigallocatechin and (−) epigallocatechin gallate against heregulin beta1-induced migration/invasion of the MCF-7 breast carcinoma cell line. Biol. Pharm. Bull. 2009;32(5):899–904. doi: 10.1248/bpb.32.899. [DOI] [PubMed] [Google Scholar]
- 40.Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 41.Liu JD, Chen SH, Lin CL, Tsai SH, Liang YC. Inhibition of melanoma growth and metastasis by combination with (−)-epigallocatechin-3-gallate and dacarbazine in mice. J. Cell Biochem. 2001;83(4):631–642. doi: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 42.Kim M, Murakami A, Kawabata K, Ohigashi H. (−)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis. 2005;26(9):1553–1562. doi: 10.1093/carcin/bgi104. [DOI] [PubMed] [Google Scholar]
- 43.Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetic. Biochem. Pharmacol. 2010 June 26; doi: 10.1016/j.bcp.2010.06.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia D, Jurkowska RZ, Zhang X, Jeltsch A, Cheng X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 2007;449(7159):248–251. doi: 10.1038/nature06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 46.Ehrlich M. DNA methylation in cancer: Too much, but also too little. Oncogene. 2002;21(35):5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 47.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma G, Mirza S, Yang YH, Parshad R, Hazrah P, Datta Gupta S, Ralhan R. Prognostic relevance of promoter hypermethylation of multiple genes in breast cancer patients. Cell. Oncol. 2009;31(6):487–500. doi: 10.3233/CLO-2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holloway AF, Oakford PC. Targeting epigenetic modifiers in cancer. Curr. Med. Chem. 2007;14(24):2540–2547. doi: 10.2174/092986707782023271. [DOI] [PubMed] [Google Scholar]
- 50.Myzak M, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr. Drug Targets. 2006;7(4):443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 51.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26(37):5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 52.Kuzuhara T, Tanabe A, Sei Y, Yamaguchi K, Suganuma M, Fujiki H. Synergistic effects of multiple treatments, and both DNA and RNA direct bindings on, green tea catechins. Mol Carcinog. 2007;46(8):640–645. doi: 10.1002/mc.20332. [DOI] [PubMed] [Google Scholar]
- 53.Ran ZH, Zou J, Xiao SD. Experimental study on anti-neoplastic activity of epigallocatechin-3-gallate to digestive tract carcinomas. Chin. Med. J. (Engl) 2005;118(16):1330–1337. [PubMed] [Google Scholar]
- 54.Xiao GS, Jin YS, Lu QY, Zhang ZF, Belldegrun A, Figlin R, Pantuck A, Yen Y, Li F, Rao J. Annexin-I as a potential target for green tea extract induced actin remodeling. Int. J. Cancer. 2007;120(1):111–120. doi: 10.1002/ijc.22164. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa K, Miyazawa T. Chemiluminescence-High-performance liquid chromatographic determination of tea catechin, (−)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal. Biochem. 1997;248(1):41–49. doi: 10.1006/abio.1997.2098. [DOI] [PubMed] [Google Scholar]
- 56.Lam WH, Kazi A, Kuhn DJ, Chow LMC, Chan ASC, Dou QP, Chan TH. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG] Bioorg. Med. Chem. 2004;12(21):5587–5593. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Landis-Piwowar KR, Milacic V, Dou QP. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J. Cell. Biochem. 2008;105(2):514–523. doi: 10.1002/jcb.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sánchez-del-Campo L, Tárraga A, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN. Melanoma activation of 3-o-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin to a potent irreversible inhibitor of dihydrofolate reductase. Mol. Pharm. 2009;6(3):883–894. doi: 10.1021/mp800259k. [DOI] [PubMed] [Google Scholar]
- 59.Henning SM, Choo JJ, Heber D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J. Nutr. 2008;138(8):1529S–1534S. doi: 10.1093/jn/138.8.1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bode AM, Dong Z. Epigallocatechin 3-Gallate and Green Tea Catechins: United they work, divided they fail. Cancer Prev. Res. 2009;2(6):514–517. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui H, Mousa SA, Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69(5):1712–1716. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amin AR, Khuri FR, Chen ZG, Shin DM. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and epigallocatechin-3-gallate: the role of p53-dependent inhibition of nuclear factor-kappaB. Cancer Prev. Res. (Phila Pa) 2009;2(6):538–545. doi: 10.1158/1940-6207.CAPR-09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu L, Miao W, Loignon M, Kandouz M, Batist G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother. Pharmacol. 2010;66(3):467–474. doi: 10.1007/s00280-009-1182-7. [DOI] [PubMed] [Google Scholar]
- 64.Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. 2001;479(1–2):197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 65.Jang EH, Choi JY, Park CS, Lee SK, Kim CE, Park HJ, Kang JS, Lee JW, Kang JH. Effects of green tea extract administration on the pharmacokinetics of clozapine in rats. J. Pharm. Pharmacol. 2005;57(3):311–316. doi: 10.1211/0022357055687. [DOI] [PubMed] [Google Scholar]
- 66.Dudka J, Jodynis-Liebert J, Korobowicz E, Burdan F, Korobowicz A, Szumilo J, Tokarska E, Klepacz R, Murias M. Activity of NADPH-cytochrome P-450 reductase of the human heart, liver and lungs in the presence of (−)-epigallocatechin gallate, quercetin and resveratrol: an in vitro study. Basic Clin. Pharmacol. Toxicol. 2005;97(2):74–79. doi: 10.1111/j.1742-7843.2005.pto_98.x. [DOI] [PubMed] [Google Scholar]
- 67.Kim TY, Park J, Oh B, Min HJ, Jeong TS, Lee JH, Suh C, Cheong JW, Kim HJ, Yoon SS, Park SB, Lee DS. Natural polyphenols antagonize the antimyeloma activity of proteasome inhibitor bortezomib by direct chemical interaction. Br. J. Haematol. 2009;146(3):270–281. doi: 10.1111/j.1365-2141.2009.07752.x. [DOI] [PubMed] [Google Scholar]
- 68.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schönthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113(23):5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]