SUMMARY
Preclinical and clinical evidence suggests that docosahexaenoic acid (DHA), an omega-3 fatty acid derived from diet or synthesized in the liver, decreases the risk of developing Alzheimer's disease (AD). DHA levels are reduced in the brain of subjects with AD, but it is still unclear whether human dementias are associated with dysregulations of DHA metabolism. A systems biological view of omega-3 fatty acid metabolism offered unexpected insights on the regulation of DHA homeostasis in AD1. Results of multi-organ lipidomic analyses were integrated with clinical and gene-expression data sets to develop testable hypotheses on the functional significance of lipid abnormalities observed and on their possible mechanistic bases. One surprising outcome of this integrative approach was the discovery that the liver of AD patients has a limited capacity to convert shorter chain omega-3 fatty acids into DHA due to a deficit in the peroxisomal D-bifunctional protein. This deficit may contribute to the decrease in brain DHA levels and contribute to cognitive impairment.
Alzheimer's disease
Alzheimer's disease (AD) is the most common cause of adult dementia, affecting an estimated 35 million of elderly people worldwide2. As life span increases, this number is expected to climb to over 80 million by 2040. This neurodegenerative disorder is characterized clinically by progressive memory impairment, deterioration of language, and visuospatial deficits3. Age is the most important factor that predisposes to the non-familial or sporadic form of the disease. How aging might interact with other pathogenic factors for AD, such as abnormal accumulation of beta-amyloid (Aβ) peptides and hyperphosphorylated tau protein in the brain4, is still unknown. It appears, however, that obesity, diabetes, and atherosclerosis – age-related pathologies that are closely associated with systemic dysfunctions in lipid metabolism – may be involved3, 5.
DHA in subjects with AD
Epidemiological studies and animal experiments have provided evidence that increased docosahexaenoic acid (DHA) consumption decreases the risk of developing AD6–17. It is still unclear, however, how the metabolism of DHA is affected in AD. A comprehensive description of the DHA metabolism in AD requires placing DHA in the context of the interconnected network of its substrates and products. Thus, the development of a lipidomic approach that considers the entire omega-3 fatty acids system, rather than its individual components in isolation, is essential to elucidate a role for DHA in AD. Such an approach cannot be limited to the brain. Indeed, in conditions of low dietary omega-3 fatty acids intake (e.g. Western diets), DHA brain levels depend on the liver's capacity to metabolize diet-derived omega-3 fatty acids18–20. Therefore, a systems biological approach that includes the liver-brain axis is required to investigate DHA regulation in AD.
Biological roles of DHA
DHA is an omega-3 essential fatty acid that is highly enriched in the brain and the eyes, where it accumulates during late fetal and early neonatal life. Because of its high degree of unsaturation, the accumulation of DHA-containing phospholipids affects membrane biophysical properties such as fluidity, permeability and compressibility, and alters the function of many integral and membrane associated proteins21–23. High levels of DHA have been found in growth cones24, synaptic plasma membranes and synaptic vesicles25; however, the functional significance of this localization is still unclear. For example, it has been suggested that the increased membrane fluidity may regulate the speed of signal transduction26, neurotransmission27, and formation of lipid rafts28, mediating essential processes for the neurodevelopment and functional synaptic plasticity of the brain. In addition to altering the structural functionality of neural cell membranes, DHA can be released from phospholipids due to PLA2 activation29, 30, acting as a signaling molecule. Non-esterified DHA may bind Retinoid X Receptor, a ligand-activated transcription factor31, or it may be oxygenated to produce various bioactive lipids. DHA oxygenation is thought to proceed through two main pathways: i) a lipoxygenase-mediated pathway converts DHA to resolvins and neuroprotectins such as neuroprotectin D132, two families of lipid signals with marked antiinflammatory and neuroprotective effects33–35; and ii) free radical-mediated peroxidation of DHA produces neuroprostanes, which are involved in oxidative stress36. Both mechanisms may be relevant to the alterations in DHA levels observed in aging and AD33–35, 37–40. It is likely, however, that the positive effects excited by DHA are the result of multiple signaling events, many of which remain to be discovered.
For example, DHA is known to play important roles in the cardiovascular system, which in turn may contribute to the clinical manifestation and the pathology of AD in the brain5, 41. The roles of DHA on the vascular component and the cerebral parenchyma itself, however, have not been systematically explored42. Therefore, the contribution of liver-derived DHA in vascular dementia and, more generally in cardiovascular diseases, should be object of further investigation.
DHA in brains of subjects with AD
Numerous studies indicates that DHA serves important neurotrophic functions during the last trimester of fetal life and the first two years of childhood43, but it is still unclear whether this fatty acid plays similar roles in aged subjects. Several, albeit not all, epidemiological and clinical studies suggest that higher intake of DHA decreases the risk of cognitive decline and dementia in elderly adults44. Animal experiments support this conclusion7, 8, 13 and further indicate that DHA might exert these effects by promoting, directly or through biologically active metabolites, the survival and repair of neuronal cells45, 46.
The cognate question of whether changes in brain DHA levels might accompany cognitive decline has been addressed using post mortem brain tissue from AD patients and age-matched control subjects34, 38, 47–52. With some inconsistency, deficits in DHA-containing phospholipids have been reported in AD brains, but only as localized to selected brain regions38, 47, 48, 50, 51, 53. Discrepancies may be due to both 1) very small number of subjects in each series (<10 per cohort); and 2) variations in sampling and methodology. Furthermore, only one reports described the levels of non-esterified DHA, which was reported to be lower in hippocampus of AD patients than control subjects34.
In conclusion, despite some disparities, the results of these investigations generally support the possibility that AD is associated with lower than normal levels of DHA in the brain6. In the following sections, we describe how we reexamined this possibility and searched for supporting correlative evidence that a disruption in brain DHA integrity might result from defective omega-3 fatty acid metabolism in liver.
DHA biosynthesis in human liver
Like other mammals, humans obtain DHA directly from dietary sources, especially fish, but can also produce it in liver from omega-3 fatty acid precursors present in green plant leaves20, 54, 55. When food does not provide a sufficient supply of these nutrients, the liver's capacity to generate DHA may become critical to keep brain levels of this fatty acid within a normal range19, 55.
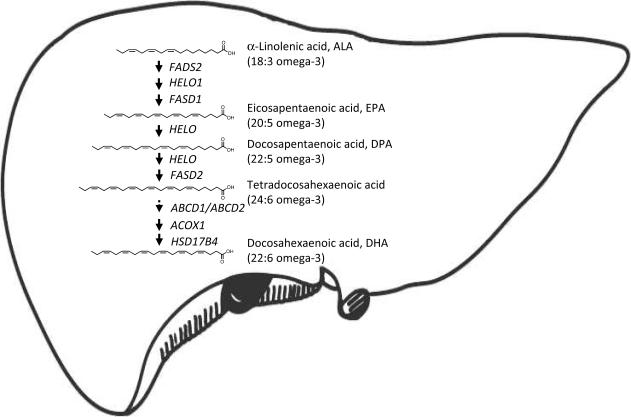
A properly functioning liver synthesizes DHA from shorter-chain omega-3 precursors, such as α-linolenic acid (C18:3 omega-3) and eicosapentaenoic acid (C20:5 omega-3). A cascade of elongase and desaturase enzymes localized in the endoplasmic reticulum of the hepatocyte progressively add carbon units and double bonds to shorter-chain omega-3 fatty acids, producing the very-long-chain tetracosahexaenoic acid (C24:6 omega-3). This faty acid is transported into peroxisomes and then converted to DHA by the sequential action of acyl-coenzyme A oxidases, D-bifunctional protein (DBP) and peroxisomal thiolases56–59. Liver-derived DHA reaches the brain through the general circulation, most likely as a complex with lipid-binding proteins (e.g., albumin) that are also synthesized in the hepatocyte20. Notably, whole-body β-oxidation of a single dose of 13C-DHA in healthy, young adults is <5% in one-month period follow-up60, suggesting that the human body does not rapidly catabolize DHA. Recent evidence also indicates that the half-life of DHA in the human brain approximates 2.5 years18, with a consumption rate of 3.8 mg/day18. These observations indicate that the need for DHA in humans might be covered by dietary α-linolenic acid when liver metabolic conversion machinery is intact and the diet has a high α-linolenic acid content19. For example, it has been calculated that assuming an average ingestion of 1,400 mg/day of α-linolenic acid61, 62, and that 0.5–10% of ingested α-linolenic acid is converted to DHA63–67, the liver is able to synthesize DHA at rates of 7–140 mg/day, 1.8–36-fold, respectively, the human brain requirement18. This evidence suggests that liver-mediated DHA biosynthesis may be sufficient (and indeed essential) for normal brain activity.
A lipidomic approach for the study of AD
Acquiring a broad view of lipid metabolic pathways in AD might offer unexpected insights on the regulation of DHA homeostasis, which may contribute the pathogenesis of this disease. Technical progress in lipid analysis has opened unprecedented opportunities for the field of lipidomics – the branch of metabolomics that studies large-scale lipid profiles in healthy and diseased tissues68. AD is an especially promising area of application for lipidomics. Risk factors for AD – such as aging and genetic vulnerability – alter specific lipid pathways in brain and peripheral tissues, and these alterations may influence in turn AD progression (Fig. 1). In our studies, we used a functional lipidomic approach that has two key features. First, biological specimens from clinically characterized AD patients and closely matched controls were analyzed by liquid chromatography/mass spectrometry (LC/MS). Second, the obtained information was integrated with clinical and molecular data to generate testable hypotheses on the functional significance of newly described lipid abnormalities, and on the possible mechanistic bases for their development. The application of this approach allowed us to uncover multiple lipid alterations in post mortem brain and liver tissue from AD patients, some of which strongly correlate with AD clinical symptoms.
Figure 1.
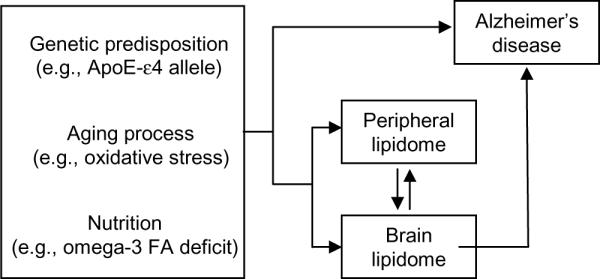
Scheme illustrating the core hypothesis of our study. Risk factors for AD (which include genetic-predisposition, age and, possibly, nutritional deficits) influence interacting lipid pathways throughout the body. Over time, accumulating lipid changes compound with those factors to increase the risk for AD.
Functional lipidomic analyses in subjects with AD
A lipidomic analysis of DHA metabolism requires (i) to analyze structurally diverse classes of lipids, and (ii) to achieve a high sensitivity of detection for low-abundance lipids. The lipid work-up procedure utilized in our studies is illustrated schematically in Figure 269. Organic solvent extraction and open-bed silica-gel chromatography were used to divide the lipidome into 4 fractions: fraction 1, which contained water-extractable polyanionic phospholipids (e.g., phosphatidylinositol-4,5-bisphosphate [PIP2]); fraction 2, which included large cationic phospholipids (e.g., phosphatidylcholine [PC]); fraction 3a, which comprised small amphipatic and non-polar lipids (e.g., fatty acids [FA] and diacylglycerols [DAG]); and fraction 3b, which included large anionic phospholipids (e.g., phosphatidylethanolamine [PE]). Each lipid fraction was subjected to LC separation on appropriate C18-based columns. Following these initial steps, lipid classes and individual compounds of interest were characterized by LC/MSn and then quantified using non-endogenous lipid standards. Finally, lipidomic results were integrated with clinical and gene expression profile data sets.
Figure 2.
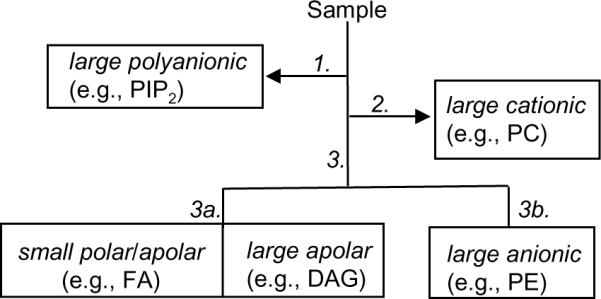
Schematic flow chart of our lipid work-up procedure. 1, extraction in acidic solvent; 2, extraction in water; 3, fractionation by open-bed silica-gel chromatography; elution with 3a, chloroform/methanol (9:1); 3b, chloroform/methanol (1:1). See text for details and abbreviations.
Lipidomics of brain tissues from subjects with AD
Our lipidomic analyses have uncovered multiple lipid alterations in the brain of AD patients1. Some of these changes have already been documented in the scientific literature. For example, corroborating the work of Bazan and others45, 70, we found that levels of non-esterified (free) DHA are reduced in brain tissues from AD patients compared to control subjects1. Analyses of lipid fraction 3b also confirmed that levels of DHA-containing phospholipids are decreased in AD (for review, see6). Our lipidome-wide search revealed, however, two aspects of AD-associated DHA deficiency, which have not been previously recognized. The first is that the positive statistical correlation between DHA content and clinical measures of dementia, which was measured by the Mini-Mental Status Examination (MMSE). Though these results do not clarify the putative role of DHA in the pathogenesis of AD, they are consistent with epidemiological surveys and animal studies corroborating the hypothesis of a link between dietary DHA intake and cognitive function (reviewed in6).
The second new element uncovered by our experiments is that the deficiency in DHA occurs throughout the brain, including regions such as cerebellum, which are not generally regarded as being directly involved in AD pathology. This led us to investigate a possible systemic cause for the decline in DHA levels. Previous evidence indicates a peripheral decrease of omega-3 fatty acids with AD, and suggests that increasing the levels of peripheral levels of omega-3 fatty acids may have substantial benefits in reducing their risk of cognitive decline71,15, 72–76.
Functional lipidomics of liver tissues in AD
Although AD is conceptualized as a neurodegenerative disease of the brain, there is increasing awareness that it may involve abnormalities in multiple peripheral tissues34, 77, 78,79–83. In this regard, several reports indicate that the liver may play an important role in peripheral Aβ clearance from the central nervous system84–86. To identify potential mechanisms responsible for the observed DHA deficit in AD brain, we focused our attention on the liver because of the essential contribution of this organ in supplying DHA to the brain1 (Fig. 3). Our analyses showed that liver tissue from AD patients contains reduced levels of DHA, but elevated levels of shorter chain omega-3 fatty acids precursors – from α-linolenic to tetracosahexaenoic acid (24:6 omega-3). This profile cannot be caused by a nutritional deficit in omega-3 fatty acids. Rather, the profile suggests a defect in the last step of DHA biosynthesis – the β-oxidative conversion of tetracosahexaenoic acid into DHA, which is catalyzed by DBP in liver peroxisomes. Two additional findings support this interpretation. First, expression of the hydroxysteroid (17-β) dehydrogenase 4 (HSD17B4) gene, which encodes for DBP58, 59, 87, is lower in AD. Second, pristanic acid and phytanic acid, two substrates for liver DBP activity, accumulate in the liver of AD patients. Notably, no other gene included in our panel was significantly altered in liver tissue from AD patients, including those encoding for proteins involved in peroxisome biogenesis, such as PEX13, PEX14 and PEX19. These results are consistent with those of previous studies, which have shown that genetic mutations that selectively disrupt DBP activity reduce DHA levels in human plasma and brain88, 89. The pathological changes that trigger the down-regulation of liver DBP expression in AD are still unknown. One possible candidate is oxidative stress, which is known to accelerate age-dependent damage to peroxisomes90, 91. Additional studies should also evaluate the existence of other possible links between liver peroxisomal function and cognition. Moreover, other aspects of DHA metabolism – such as transport and ApoE genotype14, 92–94 – might contribute to the observed changes and await further investigation.
Figure 3.
Overview of DHA biosynthesis in liver. Liver transforms diet-derived α-linolenic acid (18:3 omega-3) into DHA (22:6 omega-3). In the endoplasmatic reticulum, the serial activities of Δ6 and Δ5 desaturases (encoded by the FADS2 and FADS1 genes, respectively) and elongases (such as that encoded by the HELO1 gene) convert alinolenic acid into tetracosahexaenoic acid (24:6 omega-3). Proteins encoded by the ABCD1 or ABCD2 genes transport tetracosahexaenoic acid into peroxisomes. The sequential action of acyl coenzyme-A oxidase (encoded by the ACOX1 gene), D-bifunctional protein (encoded by the HSD17B4 gene), and various peroxisomal thiolases (not shown) convert tetracosahexaenoic acid into DHA.
Importantly, the functional significance of the peroxysomal liver dysfunction is underscored by the identification of a strong positive correlation between liver DHA content and cognitive status, indicating a previously unrecognized association between hepatic DHA homeostasis and global cognition1. Although it is well established that patients with advanced liver diseases (e.g., hepatitis C, non-alcoholic liver steatosis and end-stage liver disease) show a decline in cognitive abilities (e.g., hepatic encephalophaty)95–97, our novel findings reveal that, even in the absence of overt liver pathology, subtle molecular dysfunctions in the liver can be associated with dementia and AD pathology.
It appears, however, that an overall healthy liver is required for optimal DHA biosynthesis. It has been reported that during conditions of hepatic stress such as in chronic alcohol intake98, 99, and liver steatosis and injury100, 101, the levels of hepatic DHA are compromised. Supplementation of DHA has been suggested as a new therapeutic approach in the treatment of these conditions102, 103. Further research will be required to determine the contribution of a dysfunctional hepatic DHA biosynthesis to cognition in relation to liver injury or impaired functioning.
Role of peroxisomal metabolism in DHA deficiency
Peroxisomes are essential for the last steps of the biosynthesis of DHA (Fig.3) and have been involved in neurodevelopment104, and mental and visual health. These organelles are particularly enriched in liver and kidneys, which are also the organs deputed to the DHA biosynthesis105, 106. DHA levels are extremely low in brain of patients with severe peroxisomal disorders, such as Zellweger syndrome and X-adrenoleukodystrophy, where some clinical symptoms can be improved following the administration of DHA107. In particular, previous reports suggest that DHA levels were reduced in plasma and brain tissue of patients carrying DBP deficiency88, 89. In light of this evidence, we should consider dietary interventions to include preformed DHA, rather than its precursors, to normalize brain content of DHA in patients with AD. Moreover, it has been shown that administration of shorter-chain omega-3 precursors of DHA, such as EPA (C20:5, omega-3), for 12 weeks was ineffective in increasing the levels of DHA in AD patients, while increasing DPA (C22:5 omega-3)108.
These observations add to the accumulating evidence that only a small percent of the dietary shorter-chain omega-3 fatty acids (0.5–10%) is fully converted into DHA63–67. Overall, it appears that the peroxisomal step for the biosynthesis of DHA may work as a checkpoint for the control of DHA homeostasis and it could be subject to fine regulatory mechanisms. In addition, the general functionality of peroxisomes could affect DHA metabolism. Thus, further investigation should focus on determining how the integrity and number of these organelles is affected in the livers of AD patients.
Finally, because kidneys are rich in peroxisomes and together with liver contribute to DHA biosynthesis, their contribution to the DHA biosynthesis in AD patients should be object of further investigation.
A role for lived-derived DHA in cognitive aging?
Peroxisomal function is known to decline with age90 and this may explain the decrease in the synthesis of plasmalogens37, 89, 109 and DHA54, 110, 111 observed in elderly people. Indeed, it has been observed that DHA composition progressively declines in human and rat frontal cortex with increasing age112, despite the availability of dietary short chain omega-3 PUFA37, 113, 114. In a recent clinical trial, supplementation of DHA as been shown to provide a net benefit roughly equivalent to having the learning and memory skills of someone 3 years younger115. Overall, this evidence supports the hypothesis that peroxisomal DHA biosynthesis may have a significant role in aging and that AD is an acceleration of the peroxisomal aging process.
A role for liver-derived DHA in neuropsychiatric disorders?
In addition to AD, DHA deficits may also occur in brain and peripheral tissues from patients with neuropsychiatric illnesses, including bipolar disorder, major depressive disorder, schizophrenia112, 116–121, attention deficit (hyperactivity) disorder, suicide122, dyslexia, autism123, neuroticism124, stress disorders, and chronic fatigue (for review see125). It has been suggested that deficits in peroxisomal metabolism may contribute to the DHA deficit observed in some of these patients112. In particular, analogous to the AD treatment108, shorter chain omega-3 precursors appear to be less efficacious than DHA in the treatment of mood symptoms in bipolar disorder patients126. Furthermore, the symptoms of depression are sometimes indistinguishable from early AD127 and it has been reported that depression early in life may be a risk factor for the later development of AD128, 129. Similar to AD, it has been suggested that the deficits in DHA observed in neuropsychiatric diseases may contribute to (i) cognitive impairment and (ii) structural brain changes, such as reduced cerebral volume, enlarged ventricles, cerebral atrophy, and frontotemporal-sulcal widening130–135. In this context, peripheral DHA supplementation has been shown effective in increasing cortical gray matter volume135,136, which may explain some of the benefits of this lipid on mood. In light of this evidence, the role for liver biosynthesis of DHA in the development and progression of neuropsychiatric disorders that may accompany AD requires further examination.
Conclusions and future perspective
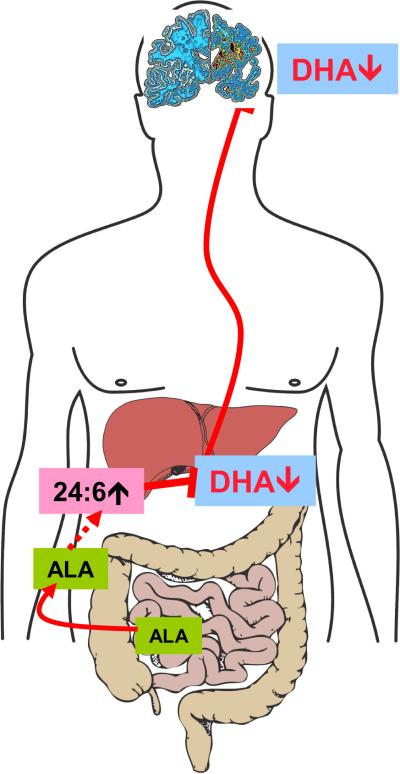
The use of a multi-organ lipidomic approach allowed us to identify a dysfunction in the liver's ability to synthesize DHA in subjects with AD, which possibly lessens the flux of this neuroprotective fatty acid to the brain (Fig. 4). This systemic deficiency in DHA correlates with the cognitive impairment observed in AD patients and has implications in two main areas. First, interventions with omega-3 fatty acids – both dietary and supplement-based9, 10, 14, 93 – should take into consideration the partial inability of AD patients to complete DHA biosynthesis. For example, future clinical studies might consider using appropriate forms of purified DHA, DHA-delivering prodrugs, or routes of administration that bypass the liver.. A similar approach was shown to improve clinical symptoms in patients with severe peroxisomal disorders107, 137, and it has been previously suggested in older individuals111 or individuals with end-stage liver disease138. Second, the altered pattern in lipid metabolism in liver produced by DBP dysfunction might be exploited to develop peripheral biomarker strategies for AD.
Figure 4.
Hepatic DHA biosynthesis is linked to cognition. Diet-derived ALA (α-linolenic acid, 18:3 omega-3) is absorbed by the intestine and delivered to the liver where it serves as precursor for DHA. A peroxisomal dysfunction impairs the conversion of tetracosahexaenoic acid (24:6 omega-3) into DHA in the livers of subjects with AD. This systemic deficiency in DHA possibly lessens the flux of this neuroprotective fatty acid to the brain leading to cognitive impairment.
Acknowledgments
This work was supported by grants from the National Institutes on Drug Abuse (ARRA) (to D.P.). We are indebted to Carl Cotman, Paul Coleman, Thomas Beach, the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona, the Alzheimer's Disease Research Center and the Institute for Brain Aging and Dementia of the University of California, Irvine for the provision of human biological materials. We thank Teresa Orazio for providing graphic illustrations. The contribution of the Agilent Technologies/UCI Analytical Discovery Facility, Center for Drug Discovery is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Astarita G, Jung K, Berchtold N, et al. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoSOne. 2010 doi: 10.1371/journal.pone.0012538. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Jackson J. A.s.D. International, ed. London: 2009. [Google Scholar]
- 3.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 4.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 5.Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113(1):13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 6.Cunnane SC, Plourde M, Pifferi F, Begin M, Feart C, Barberger-Gateau P. Fish, Docosahexaenoic Acid and Alzheimer's Disease. Prog Lipid Res. 2009 doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25(12):3032–40. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calon F, Lim GP, Yang F, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43(5):633–45. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H, Morikawa Y, Takahashi H. Effect of DHA oil supplementation on intelligence and visual acuity in the elderly. World Rev Nutr Diet. 2001;88:68–71. doi: 10.1159/000059767. [DOI] [PubMed] [Google Scholar]
- 10.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60(7):940–6. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 11.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 12.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70(4):361–72. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Green KN, Martinez-Coria H, Khashwji H, et al. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27(16):4385–95. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–30. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 15.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305–12. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 16.Tully AM, Roche HM, Doyle R, et al. Low serum cholesteryl esterdocosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89(4):483–9. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 17.Young G, Conquer J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod Nutr Dev. 2005;45(1):1–28. doi: 10.1051/rnd:2005001. [DOI] [PubMed] [Google Scholar]
- 18.Umhau JC, Zhou W, Carson RE, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50(7):1259–68. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):119–23. doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott B, Bazan N. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86(8):2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36(9):945–59. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim HY. Novel Metabolism of Docosahexaenoic Acid in Neural Cells. J Biol Chem. 2007;282(26):18661. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 23.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin RE, Bazan NG. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem. 1992;59(1):318–25. doi: 10.1111/j.1471-4159.1992.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 25.Breckenridge WC, Gombos G, Morgan IG. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta. 1972;266(3):695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- 26.Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006;103(13):4888–93. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):259–69. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45(5):559–79. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 29.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12(3):245–60. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 30.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–57. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 31.de Urquiza AM, Liu S, Sjoberg M, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290(5499):2140–4. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 32.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel Docosatrienes and 17 S-Resolvins Generated from Docosahexaenoic Acid in Murine Brain, Human Blood, and Glial Cells. J Biol Chem. 2003;278(17):14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 33.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101(22):8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fam SS, Murphey LJ, Terry ES, et al. Formation of Highly Reactive A-ring and J-ring Isoprostane-like Compounds (A4/J4-neuroprostanes) in Vivo from Docosahexaenoic Acid. J Biol Chem. 2002;277(39):36076–84. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- 37.Favrelere S, Stadelmann-Ingrand S, Huguet F, et al. Age-related changes in ethanolamine glycerophospholipid fatty acid levels in rat frontal cortex and hippocampus. Neurobiol Aging. 2000;21(5):653–60. doi: 10.1016/s0197-4580(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 38.Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 1998;23(1):81–8. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 39.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10(2):136–41. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 40.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 41.Milionis HJ, Florentin M, Giannopoulos S. Metabolic syndrome and Alzheimer's disease: a link to a vascular hypothesis? CNS Spectr. 2008;13(7):606–13. doi: 10.1017/s1092852900016886. [DOI] [PubMed] [Google Scholar]
- 42.de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3(3):184–90. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 43.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 44.Crawford MA, Bazinet RP, Sinclair AJ. Fat intake and CNS functioning: ageing and disease. Ann Nutr Metab. 2009;55(1–3):202–28. doi: 10.1159/000229003. [DOI] [PubMed] [Google Scholar]
- 45.Lukiw W, Bazan N. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138(12):2510. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florent S, Malaplate-Armand C, Youssef I, et al. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96(2):385–95. doi: 10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 47.Brooksbank BW, Martinez M. Lipid abnormalities in the brain in adult Down's syndrome and Alzheimer's disease. Mol Chem Neuropathol. 1989;11(3):157–85. doi: 10.1007/BF03160049. [DOI] [PubMed] [Google Scholar]
- 48.Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26(6):421–5. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 49.Guan Z, Wang Y, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(7):740–7. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Skinner ER, Watt C, Besson JA, Best PV. Differences in the fatty acid composition of the grey and white matter of different regions of the brains of patients with Alzheimer's disease and control subjects. Brain. 1993;116(Pt 3):717–25. doi: 10.1093/brain/116.3.717. [DOI] [PubMed] [Google Scholar]
- 51.Corrigan FM, Horrobin DF, Skinner ER, Besson JA, Cooper MB. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer's disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30(2):197–207. doi: 10.1016/s1357-2725(97)00125-8. [DOI] [PubMed] [Google Scholar]
- 52.Fraser T, Tayler H, Love S. Fatty Acid Composition of Frontal, Temporal and Parietal Neocortex in the Normal Human Brain and in Alzheimer's Disease. Neurochem Res. 2009;35(3):503–13. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 53.Kwee IL, Nakada T, Ellis WG. Elevation in relative levels of brain membrane unsaturated fatty acids in Alzheimer's disease: high resolution proton spectroscopic studies of membrane lipid extracts. Magn Reson Med. 1991;21(1):49–54. doi: 10.1002/mrm.1910210108. [DOI] [PubMed] [Google Scholar]
- 54.Burdge G, Calder P. Conversion of/ alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction, Nutrition, Development. 2005;45(5):581–97. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 55.Rapoport S, Rao J, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–6):251–61. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991;266(30):19995–20000. [PubMed] [Google Scholar]
- 57.Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res. 1995;36(12):2471–7. [PubMed] [Google Scholar]
- 58.Moore SA, Hurt E, Yoder E, Sprecher H, Spector AA. Docosahexaenoic acid synthesis in human skin fibroblasts involves peroxisomal retroconversion of tetracosahexaenoic acid. J Lipid Res. 1995;36(11):2433–43. [PubMed] [Google Scholar]
- 59.Su HM, Moser AB, Moser HW, Watkins PA. Peroxisomal straight-chain Acyl-CoA oxidase and D-bifunctional protein are essential for the retroconversion step in docosahexaenoic acid synthesis. J Biol Chem. 2001;276(41):38115–20. doi: 10.1074/jbc.M106326200. [DOI] [PubMed] [Google Scholar]
- 60.Freemantle E, Vandal M, Tremblay-Mercier J, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):213–20. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Albert CM, Oh K, Whang W, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112(21):3232–8. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 62.Denomme J, Stark KD, Holub BJ. Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations. J Nutr. 2005;135(2):206–11. doi: 10.1093/jn/135.2.206. [DOI] [PubMed] [Google Scholar]
- 63.Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5(2):127–32. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88(4):411–20. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 65.Sinclair AJ, Attar-Bashi NM, Li D. What is the role of alpha-linolenic acid for mammals? Lipids. 2002;37(12):1113–23. doi: 10.1007/s11745-002-1008-x. [DOI] [PubMed] [Google Scholar]
- 66.Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46(2):269–80. doi: 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32(4):619–34. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 68.Astarita G, Ahmed F, Piomelli D. Lipidomic analysis of biological samples by liquid chromatography coupled to mass spectrometry. Methods Mol Biol. 2009;579:201–19. doi: 10.1007/978-1-60761-322-0_10. [DOI] [PubMed] [Google Scholar]
- 69.Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009 doi: 10.1016/j.jchromb.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Muldoon MF, Ryan CM, Sheu L, Yao JK, Conklin SM, Manuck SB. Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J Nutr. 140(4):848–53. doi: 10.3945/jn.109.119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrigan FM, Van Rhijn AG, Ijomah G, et al. Tin and fatty acids in dementia. Prostaglandins Leukot Essent Fatty Acids. 1991;43(4):229–38. doi: 10.1016/0952-3278(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 73.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85(4):1103–11. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 74.Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63(11):1545–50. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 75.Cherubini A, Andres-Lacueva C, Martin A, et al. Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(10):1120–6. doi: 10.1093/gerona/62.10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodenowe DB, Cook LL, Liu J, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer's disease and dementia. J Lipid Res. 2007;48(11):2485–98. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Hooijmans C, Kiliaan A. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008;585(1):176–96. doi: 10.1016/j.ejphar.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez-Mejia R, Newman J, Toh S, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008;11(11):1311–8. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Britschgi M, Wyss-Coray T. Systemic and acquired immune responses in Alzheimer's disease. Int Rev Neurobiol. 2007;82:205–33. doi: 10.1016/S0074-7742(07)82011-3. [DOI] [PubMed] [Google Scholar]
- 80.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12(9):1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 81.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 82.SCOTT R. Extraneuronal manifestations of Alzheimer's disease. J Am Geriatr Soc. 1993;41(3):268–76. doi: 10.1111/j.1532-5415.1993.tb06704.x. [DOI] [PubMed] [Google Scholar]
- 83.Blass J, Zemcov A. Alzheimer's disease. A metabolic systems degeneration? Neurochem Pathol. 1984;2(2):103–14. doi: 10.1007/BF02834249. [DOI] [PubMed] [Google Scholar]
- 84.Ghiso J, Shayo M, Calero M, et al. Systemic catabolism of Alzheimer's Abeta40 and Abeta42. J Biol Chem. 2004;279(44):45897–908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 85.Marques MA, Kulstad JJ, Savard CE, et al. Peripheral amyloid-beta levels regulate amyloid-beta clearance from the central nervous system. J Alzheimers Dis. 2009;16(2):325–9. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roher AE, Esh CL, Kokjohn TA, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5(1):18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang LL, Kurosawa T, Sato M, Suzuki Y, Hashimoto T. Physiological role of D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem. 1997;121(3):506–13. doi: 10.1093/oxfordjournals.jbchem.a021615. [DOI] [PubMed] [Google Scholar]
- 88.Martinez M. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res. 1992;583(1–2):171–82. doi: 10.1016/s0006-8993(10)80021-6. [DOI] [PubMed] [Google Scholar]
- 89.Martinez M. Severe deficiency of docosahexaenoic acid in peroxisomal disorders: a defect of delta 4 desaturation? Neurology. 1990;40(8):1292–8. doi: 10.1212/wnl.40.8.1292. [DOI] [PubMed] [Google Scholar]
- 90.Youssef J, Badr M. Biology of senescent liver peroxisomes: role in hepatocellular aging and disease. Environ Health Perspect. 1999;107(10):791–7. doi: 10.1289/ehp.99107791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haining JL, Legan JS. Catalase turnover in rat liver and kidney as a function of age. Exp Gerontol. 1973;8(2):85–91. doi: 10.1016/0531-5565(73)90018-1. [DOI] [PubMed] [Google Scholar]
- 92.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65(9):1409–14. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 93.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–8. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 94.Plourde M, Vohl MC, Vandal M, Couture P, Lemieux S, Cunnane SC. Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by apoE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. Br J Nutr. 2009;102(8):1121–4. doi: 10.1017/S000711450938215X. [DOI] [PubMed] [Google Scholar]
- 95.Lewis M, Howdle PD. The neurology of liver failure. QJM. 2003;96(9):623–33. doi: 10.1093/qjmed/hcg110. [DOI] [PubMed] [Google Scholar]
- 96.Collie A. Cognition in liver disease. Liver Int. 2005;25(1):1–8. doi: 10.1111/j.1478-3231.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- 97.Yilmaz Y, Ozdogan O. Liver disease as a risk factor for cognitive decline and dementia: an under-recognized issue. Hepatology. 49(2):698. doi: 10.1002/hep.22752. author reply 2009. [DOI] [PubMed] [Google Scholar]
- 98.Pawlosky RJ, Salem N., Jr. Alcohol consumption in rhesus monkeys depletes tissues of polyunsaturated fatty acids and alters essential fatty acid metabolism. Alcohol Clin Exp Res. 1999;23(2):311–7. [PubMed] [Google Scholar]
- 99.Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord. 2006;8(6):677–85. doi: 10.1111/j.1399-5618.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 100.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106(6):635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 101.Allard JP, Aghdassi E, Mohammed S, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2008;48(2):300–7. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Spadaro L, Magliocco O, Spampinato D, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40(3):194–9. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Capanni M, Calella F, Biagini MR, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23(8):1143–51. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 104.Janssen A, Gressens P, Grabenbauer M, et al. Neuronal migration depends on intact peroxisomal function in brain and in extraneuronal tissues. J Neurosci. 2003;23(30):9732–41. doi: 10.1523/JNEUROSCI.23-30-09732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perichon R, Bourre JM. Peroxisomal beta-oxidation activity and catalase activity during development and aging in mouse liver. Biochimie. 1995;77(4):288–93. doi: 10.1016/0300-9084(96)88138-7. [DOI] [PubMed] [Google Scholar]
- 106.Masters CJ, Crane DI. On the role of the peroxisome in ontogeny, ageing and degenerative disease. Mech Ageing Dev. 1995;80(2):69–83. doi: 10.1016/0047-6374(94)01563-2. [DOI] [PubMed] [Google Scholar]
- 107.Martinez M. Docosahexaenoic acid therapy in docosahexaenoic acid-deficient patients with disorders of peroxisomal biogenesis. Lipids. 1996;31(Suppl):S145–52. doi: 10.1007/BF02637067. [DOI] [PubMed] [Google Scholar]
- 108.Boston PF, Bennett A, Horrobin DF, Bennett CN. Ethyl-EPA in Alzheimer's disease--a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;71(5):341–6. doi: 10.1016/j.plefa.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Zoeller RA, Raetz CR. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci USA. 1986;83(14):5170–4. doi: 10.1073/pnas.83.14.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br J Nutr. 2003;90(2):311–21. doi: 10.1079/bjn2003901. [DOI] [PubMed] [Google Scholar]
- 111.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br J Nutr. 2002;88(4):355–63. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 112.McNamara RK, Liu Y, Jandacek R, Rider T, Tso P. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids. 2008;78(4–5):293–304. doi: 10.1016/j.plefa.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saiz JL, Lopez-Zumel C, Monterroso B, et al. Characterization of Ejl, the cell-wall amidase coded by the pneumococcal bacteriophage Ej-1. Protein Sci. 2002;11(7):1788–99. doi: 10.1110/ps.4680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ulmann L, Mimouni V, Roux S, Porsolt R, Poisson JP. Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot Essent Fatty Acids. 2001;64(3):189–95. doi: 10.1054/plef.2001.0260. [DOI] [PubMed] [Google Scholar]
- 115.Yurko-Mauro K, McCarthy D, Rom D, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. doi: 10.1016/j.jalz.2010.01.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160(3):285–99. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNamara RK, Jandacek R, Rider T, et al. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91(1–3):37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62(1):17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 119.Sarri KO, Linardakis M, Tzanakis N, Kafatos AG. Adipose DHA inversely associated with depression as measured by the Beck Depression Inventory. Prostaglandins Leukot Essent Fatty Acids. 2008;78(2):117–22. doi: 10.1016/j.plefa.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 120.Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol Psychiatry. 1991;30(8):795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- 121.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42(1):7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 122.Hibbeln JR. Depression, suicide and deficiencies of omega-3 essential fatty acids in modern diets. World Rev Nutr Diet. 2009;99:17–30. doi: 10.1159/000192992. [DOI] [PubMed] [Google Scholar]
- 123.Amminger GP, Berger GE, Schafer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry. 2007;61(4):551–3. doi: 10.1016/j.biopsych.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 124.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69(9):932–4. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 125.Bourre JM. Dietary omega-3 Fatty acids and psychiatry: mood, behaviour, stress, depression, dementia and aging. J Nutr Health Aging. 2005;9(1):31–8. [PubMed] [Google Scholar]
- 126.Keck PE, Jr., Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60(9):1020–2. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 127.VanItallie TB. Subsyndromal depression in the elderly: underdiagnosed and undertreated. Metabolism. 2005;54(5 Suppl 1):39–44. doi: 10.1016/j.metabol.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 128.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–64. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 129.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65(4):439–45. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 130.Nasrallah HA. Neurodevelopmental aspects of bipolar affective disorder. Biol Psychiatry. 1991;29(1):1–2. doi: 10.1016/0006-3223(91)90205-z. [DOI] [PubMed] [Google Scholar]
- 131.Taylor MA. Are schizophrenia and affective disorder related? A selective literature review. Am J Psychiatry. 1992;149(1):22–32. doi: 10.1176/ajp.149.1.22. [DOI] [PubMed] [Google Scholar]
- 132.Elkis H, Friedman L, Wise A, Meltzer HY. Meta-analyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry. 1995;52(9):735–46. doi: 10.1001/archpsyc.1995.03950210029008. [DOI] [PubMed] [Google Scholar]
- 133.Najt P, Nicoletti M, Chen HH, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413(3):183–6. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51(4):273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 135.Conklin SM, Gianaros PJ, Brown SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421(3):209–12. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 136.Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002;26(3):210–8. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 137.Noguer MT, Martinez M. Visual Follow-Up In Peroxisomal-Disorder Patients Treated With Docosahexaenoic Acid Ethyl Ester. Invest Ophthalmol Vis Sci. 2009;51(4):2277–85. doi: 10.1167/iovs.09-4020. [DOI] [PubMed] [Google Scholar]
- 138.Burke PA, Ling PR, Forse RA, Lewis DW, Jenkins R, Bistrian BR. Sites of conditional essential fatty acid deficiency in end stage liver disease. J Parenter Enteral Nutr. 2001;25(4):188–93. doi: 10.1177/0148607101025004188. [DOI] [PubMed] [Google Scholar]