Abstract
BACKGROUND
Decreases below target temperature were noted among neonates undergoing cooling in the NICHD Neonatal Research Network Trial of whole body hypothermia for neonatal hypoxic-ischemic encephalopathy.
OBJECTIVE
To examine the temperature profile and impact on outcome among ≥ 36 week gestation neonates randomized at ≤ 6 hours of age targeting esophageal temperature of 33.5°C for 72 hours.
DESIGN/SETTING/PATIENTS
Infants with intermittent temperatures recorded < 32.0°C during induction and maintenance of cooling were compared to all other cooled infants and relationship with outcome at 18 months was evaluated.
RESULTS
There were no differences in stage of encephalopathy, acidosis, or 10 minute Apgar scores between infants with temperatures < 32.0°C during induction (n=33) or maintenance (n=10) and all other infants who were cooled (n=58); however birth weight was lower and need for blood pressure support higher among infants with temperatures < 32.0 °C compared to all other cooled infants. No increase in acute adverse events were noted among infants with temperatures < 32.0 °C and hours spent < 32°C were not associated with the primary outcome of death or moderate/severe disability or the Bayley II Mental Developmental Index at 18 months.
CONCLUSION
Term infants with a lower birth weight are at risk for decreasing temperatures < 32.0°C while undergoing body cooling using a servo controlled system. This information suggests extra caution during the application of hypothermia as these lower birth weight infants are at risk for overcooling. Our findings may assist in planning additional trials of lower target temperature for neonatal hypoxic-ischemic encephalopathy.
Keywords: temperature, hypothermia, newborn, hypoxia-ischemia, encephalopathy, whole-body cooling
INTRODUCTION
Therapeutic hypothermia has been shown to reduce death and disabilities among neonates with hypoxic-ischemic encephalopathy (HIE) (1–3) and increase the number of survivors without neurodevelopmental disabilities (4). Therapeutic hypothermia is currently characterized in the adult literature as consisting of three phases: induction, maintenance and rewarming (5). The temperature profile of term neonates with encephalopathy undergoing therapeutic hypothermia has not been evaluated in the context of the phases of hypothermia. In the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network trial of whole body hypothermia to a target esophageal temperature of 33.5°C for 72 hours for neonatal HIE, decreases below target temperature were noted with intermittent recording of temperatures recorded among some neonates undergoing cooling using with a servo-controlled system (6). One or more temperatures < 32.0°C were noted during the induction phase of cooling in 33 infants, 2 during both induction and maintenance and 8 additional infants during the maintenance phase of cooling. Since the rate of death and disability following cooling to a target of 33.5°C continues to be high, we wished to explore whether cooling to a greater depth would be beneficial. Therefore we undertook an analysis to examine whether temperatures < 32.0°C noted in the original RCT were associated with increased adverse effects or a suggestion of increased efficacy. The objective of this secondary analysis of the NICHD randomized controlled trial (RCT) data was to assess safety and outcome of the infants with esophageal temperatures recorded <32.0°C during induction and maintenance of cooling compared to those who did not have these decreases in temperature during therapeutic whole body hypothermia for neonatal HIE. We chose 32.0°C as this value is greater than three standard deviations below the target mean temperature of 33.5°C and thus clearly represented an outlier that needed closer scrutiny.
DESIGN and METHODS
The study included data from all infants in the centers of NICHD Neonatal Research Network that participated in the hypothermia trial. Infants who were ≥ 36 weeks gestation with severe acidosis and/or birth resuscitation at < 6 hours of age were evaluated by physiologic criteria as described previously (3). All eligible infants underwent the modified Sarnat neurologic examination performed by a certified physician examiner and were eligible for the study when moderate or severe encephalopathy or seizures were present. After informed consent was obtained, infants were randomized to cooling or control groups with assignments stratified by center. All infants received intensive care per usual care at each participating center; the RCT protocol did not mandate specific use or dosage of medications or ventilator support among the Level III academic Neonatal Intensive Care Units in the NICHD Neonatal Research Network. Infants in the hypothermia group were placed on an infant blanket that was pre-cooled to 5°C (Blanketrol II Hyper/hypothermia System, Cincinnati SubZero). An esophageal probe was inserted and the esophageal temperature was set at 33.5°C by the servo mechanism. Based on the pilot study, a second adult blanket was attached to the cooling system with water circulating through both blankets to diminish fluctuations in esophageal temperature (7). No external heat sources were used during the cooling period. Esophageal, skin and blanket temperatures were monitored continuously and were recorded on predesigned study data forms every 15 minutes for the first 4 hours, every hour for the next 8 hours and every 4 hours during the remaining period of cooling. After 72 hours of hypothermia, the set-point of the automatic control on the cooling system was increased by 0.5°C per hour. Six hours after onset of rewarming the esophageal probe was removed and skin temperatures were controlled by the radiant warmer. The temperature of the warmer was set 0.5°C higher than the skin temperature and was increased 0.5°C every hour until the set-point of the warmer reached 36.5°C. The induction phase of cooling (onset of cooling to achievement of equilibration defined as within 0.1°C of target temperature after maximum decrease below target temperature) and maintenance phase (once equilibration was achieved until initiation of rewarming) were evaluated for the present study.
The outcome of the primary study was designated as death or moderate/severe disability at 18 months of age. Neurologic and developmental evaluations were performed by certified examiners who were trained to reliability and were unaware of cooling status. Moderate disability was defined as a Mental Developmental Index (MDI, Bayley II Scales of Infant Development) score of 70–84 and either a Gross Motor Function Classification System (GMFCS) level of 2, hearing impairment with no amplification or persistent seizure disorder at 18 months. Severe disability was defined as any of the following: MDI < 70, GMFCS level 3–5 (corresponding to moderate or severe cerebral palsy), hearing impairment requiring hearing aids or blindness. The original trial was approved by the Institutional Review Board of each of the participating centers and informed consent was obtained from parents for participation in the trial. An External Data Safety Monitoring Board evaluated safety of the study at periodic intervals.
STASTICAL ANALYSIS
This is a cohort study identifying cases based on recorded temperature achieved during the intervention of whole body hypothermia in an RCT. Antecedent characteristics of the two groups of infants (those with temperatures <32.0°C compared to all other cooled infants) were evaluated. The data were analyzed to examine whether the maternal and infant characteristics at baseline defined as prior to initiating of cooling were similar between the two groups of infants. Acute adverse events were compared between the two groups during the cooling intervention period. The use of medications, including those required for blood pressure support, was evaluated between the two groups. Comparisons among groups were performed using the Fisher’s Exact Test for the categorical variables and Kruskal-Wallis tests for continuous variables. Among infants with temperatures < 32.0°C, the relationship between hours spent below this temperature and the primary outcome and components of the primary outcome were evaluated by the Wilcoxon Two-Sample Test. Logistic regression analyses for the primary outcome of death or disability at 18 months of age were performed, adjusting for the following covariates: center, temperature groups, level of encephalopathy (moderate or severe) and birth weight.
RESULTS
102 infants were randomized to the Hypothermia group, and of these, one infant did not receive cooling; thus data from 101 infants were available for evaluation of the temperature profile during induced hypothermia (Figure 1). Six infants had discontinuation of hypothermia during the 72 hour intervention period; 1) one infant at 68 hours of intervention due to diagnosis of persistent pulmonary hypertension and need for extra corporeal membrane oxygenation, 2) 3 infants at 5, 44 and 60 hours of intervention, respectively, due to withdrawal of support, 3) one infant had a one hour temperature recording missing at 9 hours of intervention and four hours missing at 52 hours of intervention due to equipment malfunction, and 4) one infant had missing temperature data for 4 hours at 40 hours of intervention due to infant instability. The protocol deviations noted for these seven infants regarding the hypothermia therapy were not localized to certain sites. During the induction phase, time to initially surpass target esophageal temperature of 33.5°C among the 101 infants was 0.9 ± 0.5 hours (mean ± SD, range 0.25 to 3.5 hours). The maximum deviation below the target temperature was −1.4 ± 0.6°C (range − 0.0 to − 4.1°C). One infant, with a temperature at baseline of 37.5 °C did not have any deviation below target esophageal temperature, while another infant had a decrease from the baseline of 36.4 °C to 29.4 °C. The time to maximum deviation below the target temperature was 1.3 ± 1.0 hours (range 0.25 to 8.0 hours). The time to equilibration was 3.2 ± 4.2 hours following initiation of cooling (range 1 to 28 hours). One infant never achieved equilibration.
Figure 1.
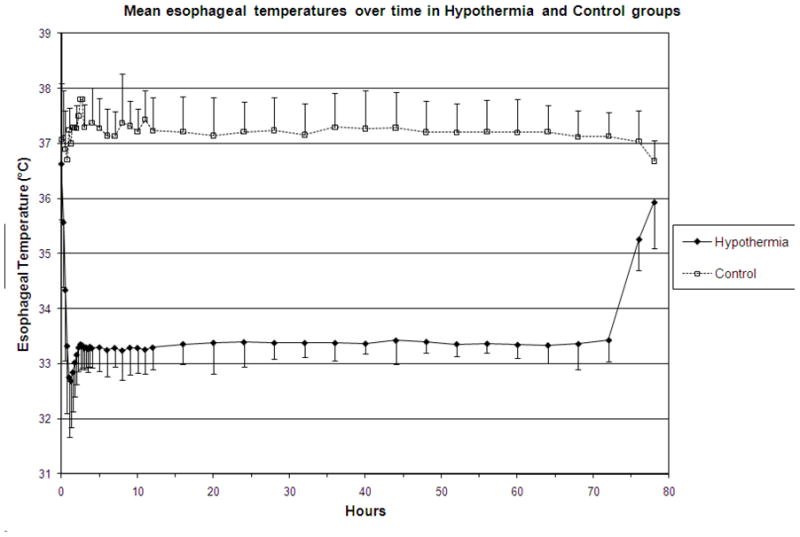
Temperature profile of infants in the hypothermia group (circles) and control group (squares). Values represent mean ± SD temperature readings
Among the 101 infants, 43 had temperatures recorded below 32°C for a duration of 1.48 ± 2.51 hours (mean ± SD, range 0.25 to 12.25). During the induction phase of cooling, 33 infants had esophageal temperatures < 32.0°C for 0.95 ± 2.1 hours (mean ± SD range 0.25 to 12.25). One isolated recording < 32.0°C was noted in 9 infants and two non-consecutive recordings were noted in one infant. Two consecutive recordings below 32.0°C were noted in 16 infants, three consecutive recordings in three infants and > 4 consecutive recordings in four other infants. During the maintenance phase, 10 infants had esophageal temperatures recorded <32.0°C for 3.23 ± 3.05 hours (mean ± SD, range 0.25 to 8.0). Six infants had only one isolated recording, two infants had 2 consecutive recordings below 32.0 °C and the remaining two infants had recordings below 32.0 °C during both induction and a single low recording during the maintenance phase. Blanket temperatures were higher among infants with temperatures < 32°C compared to all other cooled infants (Figure 2).
Figure 2.
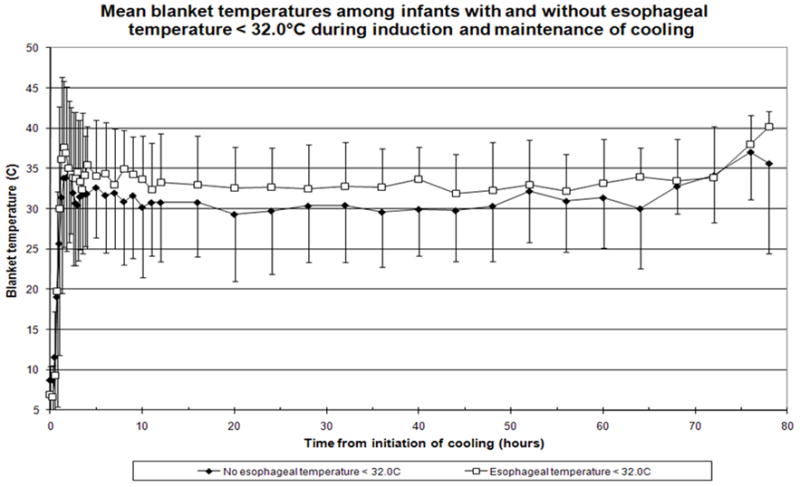
Blanket Temperatures (mean ± SD) during induction and maintenance of cooling among infants with decreases < 32.0°C (open squares) compared to all other cooled infants (solid circles)
The baseline characteristics of the 58 infants with no temperatures recorded < 32.0°C, the 33 infants with temperatures < 32.0°C during the induction phase and the 10 infants with temperatures < 32.0°C during the maintenance phase are shown in Table 1. There was a higher percentage of infants with birth weight < 25th percentile in the groups with temperatures <32.0°C during induction and maintenance as compared to the group of infants with no temperature decreases < 32.0°. During the induction of cooling, infants with a birth weight <25th percentile comprised 48% of those with a minimum esophageal temperature < 32.0°C. A greater need for blood pressure support at any time during the maintenance period of cooling was noted between the infants with temperatures < 32.0°C compared to all other cooled infants (Table 2). Other medications (that may influence temperatures) administered to the infants in the 3 groups over time are also noted in Table 2.
Table 1.
Baseline characteristics of infants with no esophageal temperatures < 32.0°C compared to those with decreases < 32°C during induction or maintenance phase of cooling
| No temp <32 °C (N=58) | Temp. < 32°C during induction (N=33) | Temp. < 32°C during maintenance (N=10) | Any differences between groups (P value) | |
|---|---|---|---|---|
| Outborn, n (%) | 27 (47) | 16 (48) | 4 (40) | 0.92 |
| Male gender, n (%) | 27 (47) | 21(64) | 3 (30) | 0.12 |
| 10 minute Apgar ≤ 5 *, n (%) | 47 (85) | 23 (79) | 9 (90) | 0.70 |
| Seizures at randomization, n (%) | 23 (40) | 16 (48) | 4 (40) | 0.69 |
| Severe level of initial HIE †, n (%) Moderate level of initial HIE, n (%) |
16 (28) 41 (72) |
12 (36) 21 (64) |
3 (30) 7 (70) |
0.70 |
| Inotropic support at randomization, n (%) | 16 (28) | 11 (33) | 6 (60) | 0.12 |
| Below 25th percentile for weight at birth, n (%) | 15 (26) | 16 (48) | 6 (60) | 0.03 |
| Anti-convulsants, n (%) ‡ | 39 (70) | 24 (73) | 8 (80) | 0.85 |
| Analgesics, n (%) ‡ | 20 (37) | 15 (45) | 5 (56) | 0.53 |
| Neuromuscular blocking agents, n (%) ‡ | 8 (15) | 7 (21) | 1 (11) | 0.71 |
| Age at randomization, hrs (mean ± SD) | 4.2 ± 1.3 | 4.5 ± 1.2 | 4.1 ± 1.3 | 0.48 |
| Cord pH (mean ± SD) | 6.87 ± 0.21 | 6.86 ± 0.15 | 6.87 ± 0.15 | 0.89 |
| Cord base deficit mmol/liter (mean ± SD) | 18.5 ± 8.0 | 18.2 ± 4.6 | 19.3 ± 4.8 | 0.87 |
| Birth weight g (mean ± SD) | 3555 ± 673 | 3172 ± 450 | 3109 ± 500 | 0.005 |
7 infants are missing this data (3 in the first group and 4 in the second group).
1 infant is missing initial HIE in the first group.
At any time within 72 hours study period. Infants missing this data: 2 for anti-convulsants (both in first group); 5 for analgesics (4 in the first and 1 in the third group); 7 for neuromuscular blocking agents (6 in the first and 1 in the third group).
Table 2.
Medications over time by temperature group
| No drops < 32°C (N=58) | Temp. < 32°C during induction or maintenance (N=43) | All infants (N=101) | |
|---|---|---|---|
| Phenobarbital only | |||
| Prior to baseline | 19 (36%) | 15 (37%) | 34 (36%) |
| At baseline | 17 (33%) | 15 (36%) | 32 (34%) |
| At 24H | 21 (41%) | 16 (37%) | 37 (39%) |
| At 48H | 21 (43%) | 17 (43%) | 38 (43%) |
| At 72H | 20 (42%) | 14 (36%) | 34 (39%) |
| Other anticonvulsants1 | |||
| Prior to baseline | 3 (6%) | 2 (5%) | 5 (5%) |
| At baseline | 1 (2%) | 3 (7%) | 4 (4%) |
| At 24H | 2 (4%) | 6 (14%) | 8 (9%) |
| At 48H | 0 (0%) | 0 (0%) | 0 (0%) |
| At 72H | 1 (2%) | 1 (3%) | 2 (2%) |
| Phenobarbital and other anticonvulsants | |||
| Prior to baseline | 6 (11%) | 4 (10%) | 10 (11%) |
| At baseline | 2 (4%) | 3 (7%) | 5 (5%) |
| At 24H | 5 (10%) | 5 (12%) | 10 (11%) |
| At 48H | 3 (6%) | 4 (10%) | 7 (8%) |
| At 72H | 1 (2%) | 2 (5%) | 3 (3%) |
| Neuromuscular blockage agents 2 | |||
| Prior to baseline | 3 (6%) | 1 (2%) | 4 (4%) |
| At baseline | 1 (2%) | 1 (2%) | 2 (2%) |
| At 24H | 3 (6%) | 5 (12%) | 8 (9%) |
| At 48H | 5 (11%) | 2 (5%) | 7 (8%) |
| At 72H | 3 (7%) | 3 (8%) | 6 (7%) |
| Pressor medications 3 | |||
| Prior to baseline | 17 (33%) | 16 (38%) | 33 (35%) |
| At baseline | 17 (31%) | 19 (44%) | 36 (37%) |
| At 24H ** | 20 (38%) | 29 (67%) | 49 (52%) |
| At 48H ** | 16 (32%) | 26 (63%) | 42 (46%) |
| At 72H ** | 12 (26%) | 24 (60%) | 36 (41%) |
| Multiple pressor medications | |||
| Prior to baseline | 2 (4%) | 4 (10%) | 6 (6%) |
| At baseline | 2 (4%) | 4 (9%) | 6 (6%) |
| At 24H ** | 3 (6%) | 12 (28%) | 15 (16%) |
| At 48H ** | 3 (6%) | 11 (27%) | 14 (15%) |
| At 72H * | 4 (9%) | 10 (25%) | 14 (16%) |
| Analgesics/sedatives 4 | |||
| Prior to baseline | 5 (10%) | 7 (17%) | 12 (13%) |
| At baseline | 8 (15%) | 6 (14%) | 14 (15%) |
| At 24H | 15 (29%) | 14 (33%) | 29 (31%) |
| At 48H | 13 (27%) | 11 (28%) | 24 (27%) |
| At 72H | 9 (20%) | 8 (21%) | 17 (20%) |
Significant at p<0.05.
Significant at p<0.01.
Other anticonvulsants include Lorazepam (Ativan), Phenytoin (Dilantin), Paraldehyde, and Other.
Neuromuscular blockage agents include Pancuronium, Vecuronium, and Other.
Pressor medications include Dopamine, Isoproterenol, Dobutamine, Epinephrine, Steroids, and Other.
Analgesics/sedatives include Morphine, Chloral hydrate, Fentanyl, Midazolam (Versed), and Other.
Adverse events between infants with temperatures < 32.0°C compared to all other cooled infants were similar during the study intervention period (Table 3). Time spent < 32.0°C during induction was 0.95 ± 2.1 hours (n = 33 infants) and during maintenance was 3.23 ± 3.05 hours (n = 10 infants). There was no association between hours spent with a temperature below 32.0°C and the primary outcome or components of the primary outcome (Table 4). Logistic regression analysis for the primary outcome of death and disability at 18 months revealed that level of encephalopathy was significant while center, birth weight and temperature group were not (Table 5a and b).
Table 3.
Acute adverse events during the 72 hours of cooling among infants with and without esophageal temperatures < 32°C
| Adverse Events during 72 hours of treatment | No drops < 32°C (N=58) | Temp. < 32°C during induction or maintenance (N=43) | p-value (Fisher’s Exact Test) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Cardiac arrhythmia | 2 | 3 % | 0 | 0 % | 0.51 |
| Metabolic acidosis | 1 | 2 % | 1 | 2 % | 1.00 |
| Thrombosis | 0 | 0 % | 0 | 0 % | N/A |
| Bleeding | 3 | 5 % | 0 | 0 % | 0.26 |
| Alteration of skin integrity | 3 | 5 % | 1 | 2 % | 0.63 |
| Death | 7 | 12 % | 6 | 14 % | 0.77 |
| Any SAE | 11 | 19 % | 7 | 16 % | 0.80 |
Table 4.
Hours spent with esophageal temperature < 32°C and primary outcome of death/disability
| N | Mean | SD | p-value† | |
|---|---|---|---|---|
| All infants | 101* | 0.63 | 1.78 | - |
| Death or mod-severe disability | 44 | 0.61 | 1.58 | 0.66 |
| Mild/no disability | 57 | 0.64 | 1.94 | |
| Death | 23 | 1.03 | 2.11 | 0.65 |
| Survival | 78 | 0.51 | 1.67 | |
| Mod-severe disability | 21 | 0.15 | 0.23 | 0.34 |
| Mild/no disability | 57 | 0.64 | 1.94 | |
| MDI < 70 | 19 | 0.13 | 0.19 | 0.24 |
| MDI ≥ 70 | 56 | 0.65 | 1.95 |
One infant had missing esophageal temperatures.
P-values are from Wilcoxon Two-Sample Test, t approximation.
Table 5a.
Adjusted logistic regression for primary outcome (death or moderate-severe disability)
| Variable | p-value |
|---|---|
| Temperature group (2 categories) (“No drops below 32C” (N=58) vs. “Any drops below 32C” (N=43) |
0.40 |
| Level of HIE | 0.0015 |
| Birth weight | 0.34 |
Model also includes center as a random effect.
Table 5b.
Adjusted logistic regression for primary outcome (death or moderate-severe disability)
| Variable | p-value |
|---|---|
| Temperature group (3 categories) “No drops below 32C” (N=58) vs. “Drops during induction” (N=33) vs. “Drops during maintenance” (N=10) |
0.61 |
| Level of HIE | 0.0014 |
| Birth weight | 0.35 |
Model also includes center as a random effect.
DISCUSSION
In this study we have demonstrated that term infants with lower birth weight are at risk for decreasing temperatures < 32°C while undergoing whole body cooling in a controlled environment to a target temperature of 33.5°C for 72 hours for neonatal HIE. This information suggests extra caution during the application of therapeutic hypothermia as these lower birth weight infants are at risk for “overcooling”. In the pre-clinical model of newborn piglets undergoing head cooling, those with lower weight had lower superficial brain temperature (8). The authors speculate that this is probably due to heat loss being proportional to head surface area and heat generation proportional to brain volume, resulting in smaller piglets having more efficient heat dissipation and lower cerebral temperatures due to the head surface-to-volume ratio being larger for smaller spheres (9). Hey and Katz have noted that heat production increases with low environmental temperature in all infants, but does so to a lesser extent in infants of lower birth weight (10).
The use of servo control methods of cooling in adults does allow greater control of fluctuation and variability than the manual control methods (5). In the NICHD Neonatal Research Network trial we needed an adult blanket to dampen fluctuations of temperatures during whole body cooling (7). In 2 recent observational studies with fewer infants in each study comparing manually adjusted systems with a servo-controlled system, less temperature variability was noted with the servo-controlled method (11, 12). Most devices and probes that are currently used to monitor core temperatures in critically ill patients are not designed to detect rapid changes in temperature; rather, they were designed to reflect small temperature changes over prolonged periods of time. In adults, the probes have been shown to take time to equilibrate, and a “time lag” between registered temperature and measured core temperature occurs unless blood temperature is measured directly. In the induction phase, this time lag can lead to significant decreases of core temperature below the desired target temperature, as the cooling device continues cooling, based on the measured temperature while the target temperature has in fact already been reached (5). In the cooling method used in this study, the servo-mechanism is an on/off device; heating does not begin until the esophageal temperature goes below 33.5°C and then it takes some time for the blanket to warm up. In the interval of time that the blanket is heating up, the body temperature continues to fall.
In this study we noted that medications for blood pressor support at any time during study intervention was greater among infant with temperatures recorded <32.0°C compared to all cooled infants. The sample size of the study is too small and precludes evaluating the interaction between exposure to lower temperature and exposure to blood pressure medications. Indications for medications for blood pressure support were per usual care at each site. We did not find any difference in the use of medications that may affect temperature such as anti-convulsants, analgesics/sedatives or neuromuscular agents between infants with temperatures recorded <32.0°C compared to all cooled infants. We have previously demonstrated that both systolic and diastolic blood pressure were similar between infants in the hypothermia and controls groups throughout the study intervention period(3) and that medications for blood pressure support was similar between infants in the hypothermia group and those in the control group(6). The need for blood pressure support may reflect the severity of the clinical status of the infants.
CONCLUSIONS
This report is a descriptive study of a secondary analysis of a prospective trial. The study limitations include small numbers of infants evaluated; hence our ability to detect adverse effects due to temperatures <32.0°C may have been limited. We acknowledge that although core esophageal, blanket and skin temperatures were monitored continuously throughout the study intervention period, temperatures were recorded at specific intervals and thus these intermittent recordings do not allow an accurate assessment of duration of time at a specific temperature. We are also aware that lack of a significant difference in acute adverse events or outcome at 18 months between infants with temperatures recorded <32.0°C and all other cooled infants does not suggest or prove equivalency between groups due to the small sample size. The relatively short interval of time spent <32.0°C may also limit the ability to detect adverse effects of temperature <32.0°C between groups. However, these observations are important and may be helpful as the practice of therapeutic hypothermia becomes more widely disseminated. In this study, an increase in acute adverse events among infants with a temperature < 32.0°C was not observed nor was an association noted between hours spent < 32.0°C and the primary outcome or the components of the primary outcome. Term infants with lower birth weight were observed to have better outcomes in the Cool Cap study (13). In the preclinical model, it appears that the depth of cooling improves the neuroprotection pattern after hypoxia-ischemia (8). Further studies will be needed to determine whether cooling to a lower target temperature in a controlled environment provides greater neuroprotection without any increase in adverse events in neonates with HIE. In the NICHD Neonatal Research Network we are currently evaluating the role of deeper (32.0°C) and longer duration of cooling (120 hours) as compared to usual care (33.5°C for 72 hours) in a randomized controlled trial with a factorial design. As a result of the findings of this observational study, infants randomized to 32.0°C undergo incremental cooling with the target temperature set initially at 33.5°C before being reset to 32.0°C(14) and data analyses will include examination of the temperature profile of neonates in the study.
Acknowledgments
Supported in part by grants:
U10 HD21364 (Case)
U10 HD21373 (Houston)
U10 HD21385 (Wayne)
U10 HD21397 (Miami)
U10 HD27851 (Emory)
U10 HD27853 (Cincinnati)
U10 HD27856 (Indiana)
U10 HD27871 (Yale)
U10 HD27880 (Stanford)
U10 HD27904 (Brown)
U10 HD34216 (Alabama)
U10 HD36790 (RTI)
U10 HD40461 (UCSD)
U10 HD40492 (Duke)
U10 HD40498 (Wake)
Abbreviations
- HIE
hypoxic-ischemic encephalopathy
- NICHD
National Institute of Child Health and Human Development
- RCT
randomized controlled trial
- MDI
Mental Developmental Index
- GMFCS
Gross motor function classification system
The Hypothermia Study Group
Case Western Reserve University Rainbow Children’s Hospital Principal Investigator: Avroy A. Fanaroff, MD; Co-PI: Michele C. Walsh, MD; Study Coordinator: Nancy Newman, BA; RN; Follow Up Principal Investigator: DeeAnne Wilson-Costello, MD; Follow Up Coordinator: Bonnie Siner, RN. Brown University Women & Infant’s Hospital Principal Investigator: William Oh, MD; Study Coordinator: Angelita Hensman, BSN, RNC; Follow Up Principal Investigator: Betty Vohr, MD; Follow Up Coordinator: Lucy Noel, RN. Duke University Principal Investigator: C. Michael Cotten, MD; Study Coordinator: Kathy Auten, BS; Follow Up Principal Investigator: Ricki Goldstein, MD; Follow Up Coordinator: Melody Lohmeyer, RN. Emory University Grady Memorial Hospital and Crawford Long Hospital Principal Investigator: Barbara J. Stoll, MD; Co-PI: Lucky Jain, MD; Study Coordinator: Ellen Hale, RN, BS. Indiana University Riley Hospital for Children and Methodist Hospital Principal Investigator: James A. Lemons, MD; Study Coordinators: Diana Dawn Appel, RN BSN, Lucy Miller, RN, BSN; Follow Up Principal Investigator: Anna Dusick, MD; Follow Up Coordinator: Leslie Richard, RN. Stanford University Principal Investigator: David K. Stevenson, MD; Co-PI: Krisa VanMeurs, MD; Study Coordinator: M. Bethany Ball, BS, CCRC; Follow Up Principal Investigator: Susan R. Hintz, MD. University of Alabama at Birmingham University Hospital-UAB Principal Investigator: Waldemar A. Carlo, MD; Study Coordinator: Monica Collins, RN, BSN, Shirley Cosby, RN, BSN; Follow Up Principal Investigator: Myriam Peralta-Carcelen, MD; Follow Up Coordinator: Vivien Phillips, RN, BSN. University of Cincinnati The University Hospital, Cincinnati Children’s Hospital Medical Center; Principal Investigator: Edward F. Donovan, MD; Study Coordinators: Cathy Grisby, BSN, Barb Alexander, RN, Jody Shively, RN, Holly Mincey, RN; Follow Up Principal Investigator: Jean Steichen, MD; Follow Up Coordinator: Teresa Gratton, PA. University of California-San Diego UCSD Medical Center and Sharp Mary Birch Hospital for Women Principal Investigators: Neil N. Finer, MD; Co-PI: David Kaegi, MD; Study Coordinators: Chris Henderson, CRTT, Wade Rich, RRT-NPS, Kathy Arnell, RN; Follow Up Principal Investigator: Yvonne E. Vaucher, MD, MPH; Follow Up Coordinator: Martha Fuller, RN, MSN. University of Miami Principal Investigator: Shahnaz Duara, MD; Study Coordinator: Ruth Everett, BSN; Follow Up Principal Investigator: Charles R. Bauer, MD. University of Rochester Golisano Children’s Hospital at Strong Principal Investigator: Ronnie Guillet, MD; PhD; Study Coordinator: Linda Reubens, RN; Follow Up Principal Investigator: Gary Myers, MD; Follow Up Coordinator: Diane Hust, RN. The University of Texas Southwestern Medical Center at Dallas: Parkland Hospital Principal Investigator: Abbot R. Laptook, MD; Study Coordinators: Susie Madison, RN, Gay Hensley, RN, Nancy Miller, RN; Follow Up Principal Investigator: Roy Heyne, MD, Sue Broyles, MD; Follow Up Coordinator: Jackie Hickman, RN. University of Texas Health Science Center at Houston Medical School: Children’s Memorial Hermann Hospital and Lyndon Baines Johnson General Hospital/Harris County Hospital District Principal Investigator: Jon E. Tyson, MD, MPH; Study Coordinator: Georgia McDavid, RN, Esther G. Akpa, RN, BSN, Claudia Y. Franco, RN, BNS, MSN, NNP, Patty A. Cluff, RN, Anna E. Lis, RN, BSN; Follow-Up Principal Investigators: Brenda H. Morris, MD, Pamela J. Bradt, MD, MPH. Wayne State University Hutzel Women’s Hospital & Children’s Hospital of Michigan Principal Investigator: Seetha Shankaran, MD; Study Coordinators: Rebecca Bara, RN, BSN, Geraldine Muran, RN, BSN; Follow Up Principal Investigator: Yvette Johnson, MD; Follow Up Coordinator: Debbie Kennedy, RN. Yale University New Haven Children’s Hospital Principal Investigator: Richard A. Ehrenkranz, M.D. Study Coordinator: Patricia Gettner, RN; Follow Up Coordinator: Elaine Romano, RN.
NICHD Neonatal Research Steering Committee
Brown University William Oh, MD; Case Western University Avroy A. Fanaroff, MD; Duke University Ronald N. Goldberg, MD; Emory University Barbara J. Stoll, MD; Indiana University James A. Lemons, MD; Stanford University David K. Stevenson, M.D.; University of Alabama at Birmingham Waldemar A. Carlo, MD; University of Cincinnati Edward F. Donovan, MD; University of California-San Diego Neil N. Finer, MD; University of Miami Shahnaz Duara, MD; University of Rochester Dale L. Phelps, MD; University of Texas – Dallas Abbot R. Laptook, MD; University of Texas – Houston Jon E. Tyson, MD, MPH; Wake Forest University T. Michael O’Shea, MD, MPH; Wayne State University Seetha Shankaran, MD; Yale University Richard A. Ehrenkranz, MD, Chair, Alan Jobe, University of Cincinnati
Data Coordinating Center: RTI International
Principal Investigator: W. Kenneth Poole, PhD; Coordinators: Betty Hastings and Carolyn M. Petrie, MS
National Institute of Child Health and Human Development
Program Scientist: Rosemary D. Higgins, MD, Linda L. Wright, MD; Coordinator: Elizabeth McClure, MEd
Data Safety and Monitoring Committee
Children’s National Medical Center Gordon Avery, MD; Columbia University Mary D’Alton, MD; RTI International W. Kenneth Poole, PhD (ex officio); University of Virginia John C. Fletcher, Ph.D. (deceased); University of Washington Christine A. Gleason, MD; University of Pittsburgh Carol Redmond, Ph.D.
Footnotes
Financial Disclosures: None
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19–25;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: Efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body Hypothermia for Neonates with Hypoxic-Ischemic Encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran S, Pappas A, Laptook AR, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122(4):e791–8. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110:377–85. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]
- 8.Iwata S, Iwata O, Thornton JS, et al. Superficial brain is cooler in small piglets: neonatal hypothermia implications. Ann Neurol. 2006;60:575–585. doi: 10.1002/ana.20978. [DOI] [PubMed] [Google Scholar]
- 9.Robertson NJ, Iwata O. Bench to bedside strategies for optimizing neuroprotection following perinatal hypoxia-ischaemia in high and low resource settings. Early Hum Dev. 2007 Dec;83(12):801–11. doi: 10.1016/j.earlhumdev.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Hey EN, Katz G. The optimum thermal environment for naked babies. Arch Dis Child. 1970;45(241):328–34. doi: 10.1136/adc.45.241.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzopardi D, Strohm B. Temperature control during therapeutic moderate whole body hypothermia for neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/adc.2009.163816. published online 13 Aug 2009. [DOI] [PubMed] [Google Scholar]
- 12.Hoque N, Chakkarapani E, Xiu L, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia>. Pediatrics. 2010;126:e124–130. doi: 10.1542/peds.2009-2995. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119(5):912–21. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 14.Optimizing cooling strategies at < 6 hours of age for neonatal hypoxic-ischemic encephalopathy. Clinical trials.gov NCT 01192776


