Abstract
We have previously reported that the expression of antioxidative stress enzymes are upregulated by trans-hydroxytamoxifen (TOT) in breast epithelial cell lines providing protection against estrogen-induced DNA damage. This regulation involves Estrogen Receptor beta (ERβ) recruitment to the Electrophile Response Element (EpRE) and a novel protein, human homolog of Xenopus gene which Prevents Mitotic Catastrophe (hPMC2). We have also demonstrated that ERβ and hPMC2 are required for TOT-dependent recruitment of poly (ADP-ribose) polymerase 1 (PARP-1) and Topoisomerase IIβ (Topo IIβ) to the EpRE. Sequence analysis reveals that the C-terminus of hPMC2 encodes a putative exonuclease domain. Using in vitro kinetic assays, we found that hPMC2 is a 3'–5' non-processive exonuclease that degrades both single stranded and double stranded substrates. Mutation of two conserved carboxylate residues drastically reduced the exonuclease activity of hPMC2 indicating the relative importance of the catalytic residues. Western blot analysis of breast cancer cell lines for Quinone Reductase (QR) levels revealed that the intrinsic exonuclease activity of hPMC2 was required for TOT-induced QR upregulation. Chromatin immunoprecipitation assays (ChIP) also indicated that hPMC2 was involved in the formation of strand breaks observed with TOT-treatment and is specific for the EpRE-containing region of the QR gene. We also determined that the transcription factor NF-E2-related factor-2 (Nrf2) is involved in the specificity of hPMC2 for the EpRE. In addition, we determined that the catalytic activity of hPMC2 is required for repair of abasic sites that result from estrogen-induced DNA damage. Thus our study provides a mechanistic basis for transcriptional regulation by hPMC2 and provides novel insights into its role in cancer prevention.
Keywords: EpRE, hPMC2, Quninone Reductase, Tamoxifen
INTRODUCTION
Prolonged exposure to estrogen is strongly associated with an increased risk for developing breast cancer (Bolton and Thatcher, 2008). However, the precise role of estrogen in the initiation and progression of breast cancer has yet to be determined. Cytochrome P450 enzymes catalyze the oxidative metabolism of estrogens to hydroxy-catecholestrogens that is further oxidized to its semiquinone and quinone forms (Cavalieri et al., 2006). The estrogen quinones can form unstable adducts with DNA, leading to depurination and mutations. Further, redox cycling between the quinone and semiquinone produces reactive oxygen species that accounts for oxidative damage to DNA and lipids associated with estrogen. The sustained oxidative stress and the mutagenic potential of these catechol estrogen quinones may contribute to the initiation while upregulation of mitogenic genes through the Estrogen Receptor (ER) may facilitate progression of breast cancer (Liehr, 2000; Yager and Davidson, 2006).
The Selective ER Modulator (SERM) tamoxifen became the first drug to be approved for prophylactic use in the reduction of risk for breast cancer, showing a 50 % reduction in both noninvasive and invasive breast cancer (Fisher et al., 1998). Tamoxifen is believed to protect against breast cancer by blocking the ER-mediated transcription of mitogenic genes. However, we have previously determined that the antioxidative stress enzyme Quinone Reductase (QR) is upregulated by tamoxifen in breast epithelial cell lines (Montano et al., 1998; Montano and Katzenellenbogen, 1997). QR is known to catalyze the reduction of estradiol-3,4-quinone preventing the generation of reactive oxygen species (Gaikwad et al., 2007). QR expression is also increased in mammary glands of rats treated with tamoxifen and this correlates with a decrease in estrogen-induced DNA damage (Montano et al., 2007).
Analysis of the QR transcriptional regulation indicates Estrogen Receptor β (ERβ) recruitment to the Electrophile Response Element (EpRE) and involves a novel protein, human homolog of Xenopus gene which Prevents Mitotic Catastrophe (hPMC2), also known as RNA exonuclease 4 homolog (REXO4) (Montano et al., 1998; Montano et al., 2000). Our previous studies demonstrated a TOT-dependent, localized co-recruitment of transcription factors ERβ, hPMC2, poly (ADP-ribose) polymerase 1 (PARP-1) and Topoisomerase IIβ (Topo IIβ) to the EpRE of the QR gene (Sripathy et al., 2008). Lack of either ERβ or hPMC2 resulted in a complete loss of TOT-induced gene expression and loss of TOT-dependent decrease in levels of catechol estrogen quinones. The occupancy of NF-E2-related factor (Nrf2) was not dependent on TOT; however, its recruitment was enhanced by TOT-liganded ERβ and hPMC2. Nrf2 is a transcription factor that is a regulator of antioxidative response while both PARP-1 and Topo IIβ are involved in DNA damage/repair and allow for transcriptional activation by relaxing chromatin (Ju et al., 2006; Nioi et al., 2003). Our previous results suggested that ERβ and hPMC2 are capable of assembling an activation complex at the EpRE in response to TOT. ERβ and hPMC2 also regulate expression and transcription of other antioxidative enzymes, such as Glutathione S-Transferase Pi (GSTpi) and gamma-Glutamylcysteine Synthetase Heavy Subunit (GCSh) that are dependent on the EpRE (Montano et al., 2004).
Our previous work had provided a mechanistic basis for the protective effects of TOT against estrogen-induced DNA damage and show that it is primarily mediated by ERβ and hPMC2 (Sripathy et al., 2008). ERβ has been suggested to play a protective role against breast cancer and its elevated levels of expression has been correlated with a positive response for tamoxifen treatment (Hopp et al., 2004; Nakopoulou et al., 2004; Saji et al., 2005). Additionally, our studies imply a crucial role for hPMC2 in mediating chemoprevention against breast cancer. However, not much is known about the biological role of this novel protein. Interestingly, the C-terminus of hPMC2 encodes a putative exonuclease domain and we speculated that this domain could have a role in transcriptional upregulation of QR.
Proteins that possess exonuclease domains are involved in a wide variety of cellular functions and have important roles in repair of DNA breaks, recruitment of DNA damage/repair factors as well as in transcriptional regulation (Moser et al., 1997). Work by Ju et al. has shown that the enzyme Topo IIβ associates itself with gene promoter regions and is required for site-specific double strand break (DSB) formation during receptor-mediated transcriptional activation (Ju et al., 2006). The DSB formation creates a signal resulting in activation of PARP-1 and ultimately serves as a mechanism for initiation of gene transcription. This work thus mechanistically links the components of DNA damage and repair machinery to regulated gene transcription.
As a potential target for chemoprevention, it is important to understand the mechanism of action of hPMC2, in particular its exonuclease activity, subsequent to its recruitment at the EpRE. We provide supporting evidence that the exonuclease activity of hPMC2 is involved in transcriptional regulation of QR and in repair of estrogen-induced DNA damage.
RESULTS
hPMC2 has a putative exonuclease domain
We have previously shown that hPMC2 is important for TOT-mediated protection against estrogen-induced DNA damage (Sripathy et al., 2008). However, not much is known about the biological role of this protein apart from its ability to increase transcription from EpRE-containing promoters of antioxidative stress enzymes in response to tamoxifen. As the C-terminus of hPMC2 encodes a putative exonuclease domain, the enzyme was placed in an exonuclease superfamily that includes RNases, the proofreading domains of the Pol I family of DNA polymerases and DNases such as Exo I (Moser et al., 1997). Homology within the superfamily is concentrated at three conserved motifs termed ExoI, ExoII and ExoIII. Nguyen et al. have characterized one of these exonucleases, Isg20 (interferon-stimulated gene product of 20 kDa), that cleaves both single stranded RNA and DNA (Nguyen et al., 2001).
In order to compare Isg20 and hPMC2, we have performed an amino acid alignment of the two proteins by COBALT (Figure 1). The sequence alignment reveals that there is a significant homology between the two proteins. Work by Nguyen et al. has also shown that Isg20 is a 3'–5' exonuclease, and site specific mutations of D11 (in the ExoI motif), D94 (ExoII) and D154 (ExoIII) resulted in decreased exonuclease activities (Nguyen et al., 2001). Interestingly, these amino acid residues are also conserved in hPMC2. In order to characterize the exonuclease activity of hPMC2 and to understand the role of the conserved amino acid residues in its enzymatic activity, we mutated two of these residues (D247G/D386G) (Figure 1) and cloned it into a pET28 vector with an N-terminal His tag. The Wild Type (WT) and the Double Mutant (DM) hPMC2 were purified as described in “Supplementary Methods” (Supplementary Figure 1).
Figure 1. Sequence alignment of Isg20 and hPMC2.
The amino acid sequence of Isg20 was aligned with the C-terminus of hPMC2. Numbers indicate the amino acid positions, and gaps in the alignment are indicated by --. The two sequences were aligned using COBALT (Constraint-based Multiple Alignment Tool) from NCBI. The first line is the amino acid sequence of Isg20 while the next line indicates the sequence for hPMC2. Two conserved amino acids in hPMC2, (D247 and D386) enclosed by boxes are the residues that were mutated for the described studies.
Exonuclease Activity of hPMC2
In order to determine the active site concentration of WT hPMC2, we performed multiple-turnover experiments, i.e. several rounds of enzyme catalysis, in which the enzyme concentration is less than the substrate. Under these conditions, reaction of the single stranded EpRE core with WT hPMC2 was characterized by an exponential burst of product formation, followed by a slower steady-state phase (Supplementary Figure 2). In such cases, the amplitude of the burst phase can be used to determine the amount of active enzyme. The kinetic experiments revealed that the purified WT hPMC2 was either 50 % active or existed as a dimer (Hsieh et al., 1993).
In order to characterize the exonuclease activity of hPMC2, we performed single-turnover experiments, i.e. one round of enzyme catalysis, in which enzyme concentration is in excess of the DNA substrate. These experiments were conducted with WT and DM hPMC2 and with either a single or a double stranded substrate containing the EpRE sequence.
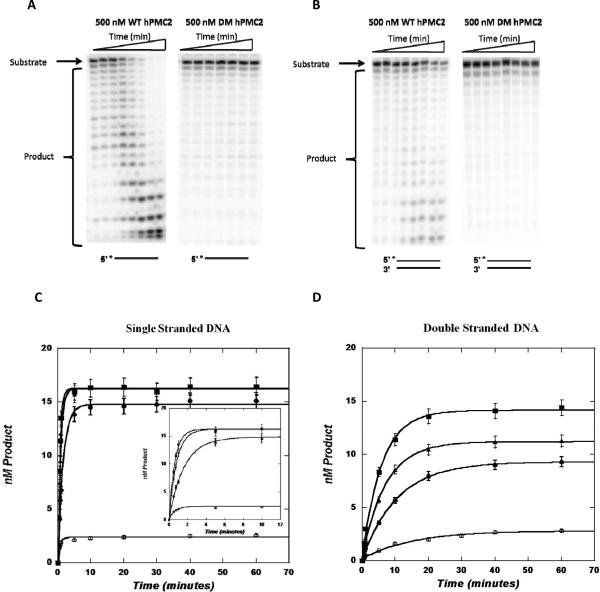
With the single stranded substrate, an increase in product formation was observed over the time course of the reaction (Figure 2A) indicating 3'–5' exonuclease activity. The appearance of successively shorter 20-mers, 19-mers, etc., is consistent with a distributive enzyme activity as opposed to a highly processive exonuclease (Skalski et al., 2000). In addition, increasing the concentration of WT hPMC2 led to increased product formation along with an increase in the observed rate constants (Figure 2C and Table 1). With an increase from 100 nM to 500 nM WT hPMC2, the observed rate constant (kobs) increased nearly 2.5-fold from 0.62 ± 0.03 min−1 to 1.62 ± 0.06 min−1. With DM hPMC2, there was less than 10 % product formation over a period of an hour and increasing concentrations of the enzyme did not lead to any increase in product formation (Figure 2A and 2C).
Figure 2. Representative autoradiograms and graphs of single and double stranded substrates with WT and DM hPMC2.
Single-turnover experiments, in which enzyme concentration is in excess of DNA, were performed with 20 nM single or double stranded EpRE substrate and increasing concentrations of either WT or DM hPMC2. The DNA was radioactively labeled on the 5'-end and increasing amounts of the enzyme was added and the reaction monitored for a period of one hour. Gel images were obtained with a Molecular Dynamics Storm 840 phosphorimager system and data analysis was performed using ImageQuaNT software. The increase in product formation observed over the time course of the reaction was then plotted on Kaleidagraph. A. Representative autoradiogram of three independent experiments of single stranded EpRE substrate with 500 nM WT or DM hPMC2. B. Representative autoradiogram of three independent experiments of double stranded EpRE substrate with 500 nM WT or DM hPMC2. C. Kaleidagraph of product formed over time with single stranded EpRE substrate at varying concentrations of WT or DM hPMC2: 100 (●), 300 (▲), 500 (■) nM WT hPMC2 and 500 nM (◯) DM hPMC2. The inset shows the same plot over a shorter time period (12 min.) to illustrate the difference in the rate constants at varying concentrations of the enzyme. D. Kaleidagraph of product formed over time with double stranded EpRE substrate at varying concentrations of WT or DM hPMC2: 100 (●), 300 (▲), 500 (■) nM WT hPMC2 and 500 nM (◯) DM hPMC2.
Table 1.
The observed rate constants (kobs) determined under single-turnover conditions for single and double stranded EpRE substrates with increasing concentrations of WT hPMC2.
| WT hPMC2 concentration | kobs(min−1) | |
|---|---|---|
| Single stranded | Double stranded | |
| 100 nM | 0.62 ± 0.03 | 0.093 ± 0.004 |
| 300 nM | 1.25 ± 0.06 | 0.141 ± 0.008 |
| 500 nM | 1.62 ± 0.06 | 0.171 ± .010 |
With the double stranded EpRE substrate, the trend observed was similar to the single strand. The WT enzyme degraded the double stranded substrate over the time course of the reaction, with increase in product formation observed with increased concentration of the enzyme (Figure 2B and 2D). The observed rate constant (kobs) increased nearly two-fold from 0.093 ± 0.004 min−1 to 0.171 ± 0.010 min−1 (Table 1) from 100–500 nM WT hPMC2. As expected, there was insignificant product formation observed with DM hPMC2. However, with increase in concentration, hPMC2 degraded the single stranded substrate more efficiently than the double stranded EpRE substrate (Table 1). In fact, at 500 nM WT hPMC2 concentration, the enzyme degraded single stranded DNA approximately 10-fold faster than the double-stranded DNA.
The catalytic activity of hPMC2 is required for TOT-induced QR expression
We have previously demonstrated a TOT-dependent recruitment of ERβ and hPMC2 to the EpRE, with subsequent recruitment of other factors to form a coactivator complex; resulting in transcriptional induction (Sripathy et al., 2008). This recruitment of a coactivator complex was observed in both the presence (MCF7 cell line) and absence of ERα (MCF10A-ERβ cell line, MCF10A cells stably transfected with ERβ). We also observed that recruitment of the complex to the QR promoter resulted in upregulation of QR expression (Sripathy et al., 2008). However, hPMC2 miRNA decreased expression of QR and other antioxidative stress enzymes, indicating that hPMC2 was crucial for the upregulation of antioxidative enzyme expression. From our kinetic studies reported above, we showed that the well conserved exonuclease domain of hPMC2 is functional. As a result, we determined if the exonuclease activity had a role in TOT-induced QR expression.
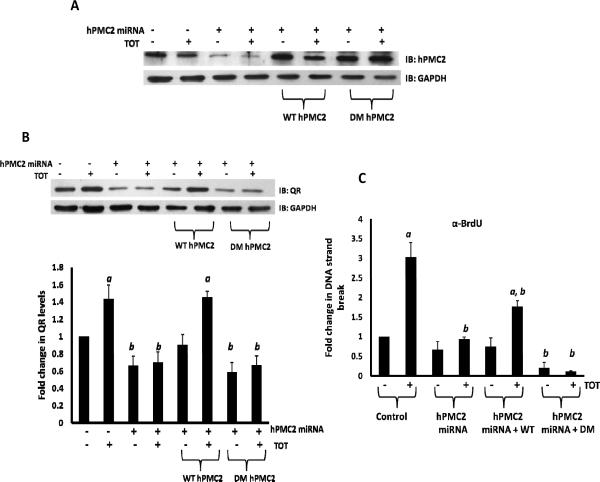
We used both MCF7 as well as the MCF10A-ERβ cell lines to study TOT-induced QR expression. Both of these cell lines were transfected with either hPMC2 miRNA that targets hPMC2 3'-UTR or hPMC2 miRNA along with plasmid expressing WT or DM hPMC2. The cell lines were either untreated or treated with 10−6 M TOT for 3 hours and analyzed via Western blot for expression levels of QR and hPMC2. We confirmed that hPMC2 protein expression was downregulated in hPMC2 miRNA cells when compared to control MCF7 cells. In addition, we also confirmed that transfection of a plasmid expressing WT or DM hPMC2 in hPMC2 miRNA cells increased hPMC2 protein expression levels (Figure 3A). As previously reported, TOT-treatment resulted in a significant increase in QR levels in control cells (Figure 3B). However, downregulation of hPMC2 using hPMC2 miRNA resulted in decreased basal QR expression and no induction of QR expression was evident after TOT-treatment. The expression of WT hPMC2 was able to rescue TOT-induced QR expression to a significant level, while the exonuclease double mutant (D247G/D386G) was unable to rescue TOT-induced QR expression (Figure 3B).
Figure 3. hPMC2 exonuclease activity is required for TOT-induced QR expression and DNA strand breaks.
MCF7 cells were transfected with either hPMC2 miRNA that targets hPMC2 3'-UTR or hPMC2 miRNA along with plasmid expressing WT or the DM hPMC2. Cells were either untreated or treated with 10−6 M TOT for 3 hours. A. Proteins were extracted from cells and processed for western blot analyses of hPMC2 as described in “Materials and Methods”. Image shown is representative of three independent experiments. B. Proteins were extracted from cells and processed for western blot analyses of QR as described in “Materials and Methods”. Levels of QR were quantitated and normalized to GAPDH. The gel image shown is representative of three independent experiments. Columns in the bar graph represent the fold change in QR levels in control or TOT-treated samples. Error bars indicate standard error of the mean of 3 independent experiments. a, significance (P < 0.05) vs. untreated cells; b, significance (P < 0.05) vs. control transfected cells with the same treatment. C. DNA strand breaks were detected by BrdUTP labeling by terminal deoxynucleotidyl transferase (TdT) and ChIP assays were performed using anti-BrdU antibodies as described in “Materials and Methods”. The EpRE containing region of the QR gene was then amplified and the bars represent the fold change in DNA strand breaks at the EpRE in control or TOT-treated samples. Error bars indicate standard error of the mean of 3 independent experiments. a, significance (P < 0.01) vs. untreated cells; b, significance (P < 0.01) vs. control transfected cells with the same treatment.
In MCF10A-ERβ cells, the trend observed was similar to that observed in MCF7 cells. There is a significant increase in QR levels after treatment of control cells with TOT (Supplementary Figure 3). However, there is a decrease in QR levels after downregulation of hPMC2. In addition, the expression of WT hPMC2 but not DM hPMC2, was able to rescue TOT-induced QR expression to a significant level.
The exonuclease activity of hPMC2 is required for DNA strand breaks
Previous work by Ju et al. has shown that Topo II-mediated DNA strand break is required for regulated gene transcription (Ju et al., 2006). However, this was observed only on the ERE containing region of the pS2 promoter in estrogen-treated MCF7 cells and not with TOT-treated cells. Previous work in our lab indicated a TOT-dependent recruitment of ERβ and hPMC2 to the EpRE, with subsequent recruitment of other factors including Topo IIβ to form a coactivator complex, resulting in transcriptional induction (Sripathy et al., 2008). Our experiments have proven that the exonuclease activity of hPMC2 is required for TOT-induced QR expression. As a result, we determined if hPMC2 had a role in the formation of DNA strand breaks during TOT-treatment at the EpRE region of the QR gene.
DNA strand breaks were detected via 5-Bromo-2′-Deoxyuridine-5′-Triphosphate (BrdUTP) labeling by terminal deoxynucleotidyl transferase (TdT). ChIP assays were performed using anti-BrdU antibodies and PCR was performed to amplify the EpRE-containing region of the QR gene. We compared DNA strand break formation in MCF7 cells expressing hPMC2 miRNA, hPMC2 miRNA/WT hPMC2 and hPMC2 miRNA/DM hPMC2. Our results revealed that there was a 3-fold increase in DNA strand breaks after TOT-treatment in control MCF7 cells (Figure 3C). However, when hPMC2 was downregulated with miRNA, there was a decrease in DNA strand breaks to the basal level. Addition of a plasmid expressing WT hPMC2 partially compensated for this decrease while addition of the DM did not result in significant increase in strand breaks. This experiment indicates that the exonuclease activity of hPMC2 is involved in strand break formation observed with TOT-treatment. In addition to the EpRE region of the QR gene, we also performed PCR amplification of the ERE-containing region of the ER target gene, pS2. The results indicated that TOT-induced strand breaks were specific for the EpRE region as strand breaks were not observed in the ERE region (Supplementary Figure 4).
As hPMC2 is involved in strand break formation, we determined if it has potential endonuclease activity. As a result, we performed endonuclease assays based on previous work by Ando et al. (Ando et al., 2008). The assay was performed with either APE1 or increasing concentrations of hPMC2 with supercoiled DNA containing the EpRE sequence as described in “Supplementary Methods”. Preliminary data indicates that while APE1 coverts the DNA into the nicked form, hPMC2 resolves it into nicked and linear forms suggesting endonuclease activity (Supplementary Figure 5).
Nrf2 is required for hPMC2 recruitment at the EpRE region of the QR gene
Transcription factor Nrf2 is essential for the antioxidant responsive element (ARE)-mediated induction of phase II detoxifying and oxidative stress enzyme genes that respond to agents that cause oxidative stress (Nioi et al., 2003). We have shown previously that while Nrf2 occupancy at the EpRE was not dependent on TOT, its recruitment was enhanced by TOT-liganded ERβ and hPMC2 (Sripathy et al., 2008). In order to evaluate if the recruitment to the EpRE is specific to TOT, we performed ChIP assays with raloxifene. Raloxifene, like TOT, can induce QR and protect against oxidative damage (Bianco et al., 2003). However, ChIP analysis in MCF7 cells reveals that raloxifene does not increase the occupancy of either hPMC2 or Nrf2 at the EpRE (Supplementary Figure 6A). It is possible that the structure of the ERβ-hPMC2 complex induced by the ligand is different due to difference in structures between TOT and raloxifene (Jordan, 1998; Osborne et al., 2004).
Previous work has shown that downregulation of hPMC2 with miRNA in presence of TOT abrogated the enhanced recruitment of Nrf2 (Sripathy et al., 2008). As this present work focuses on the role of the exonuclease activity of hPMC2, we were interested if it played a role in Nrf2 recruitment. MCF7 cells were transfected with hPMC2 miRNA that targets hPMC2 3'-UTR along with plasmid expressing WT or DM hPMC2. The cells were either untreated or treated with 10−6 M TOT for 3 hours and ChIP assays were performed for Nrf2 recruitment at the EpRE of the QR gene. Addition of WT hPMC2 increased Nrf2 recruitment, while the DM is incapable of inducing Nrf2 recruitment (Supplementary Figure 6B) at the EpRE. We have shown in this work that WT hPMC2 can cause DNA strand breaks at the EpRE while the DM is unable to do so (Figure 3C). It is possible to speculate that this inability to cause strand breaks by the DM prevents relaxation of chromatin thus preventing enhanced Nrf2 recruitment at the EpRE.
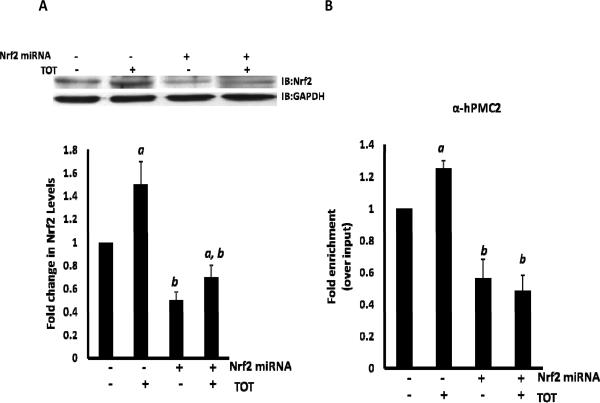
Another basis for TOT-induced recruitment of Nrf2 is upregulation of Nrf2 expression levels. Thus, a time-course experiment was performed in MCF7 cells to determine the expression levels of Nrf2 in presence of TOT. Cells were either untreated or treated with 10−6 M TOT for 45 min., 90 min. or 3 hr and Nrf2 levels were analyzed by Western blots. This revealed a significant increase of Nrf2 levels at the 3 hour time point (Supplementary Figure 7). We then downregulated Nrf2 with miRNA in MCF7 cells and the cells were either untreated or treated with 10−6 M TOT for 3 hours and analyzed via Western blot for expression levels of Nrf2. As expected, the Nrf2 levels increased with TOT-treatment in control MCF7 cells (Figure 4A). When treated with miRNA, there was a significant drop in Nrf2 expression levels. In order to ensure the specificity of the miRNA against Nrf2, we checked the expression levels of transcription factors such as hPMC2, ERβ, PARP-1, Topo IIβ and RNA Pol II via Western blot analysis. The results (Supplementary Figure 8) reveal that the levels are unaffected indicating the specificity of the miRNA against Nrf2.
Figure 4. Nrf2 is required for hPMC2 recruitment at the EpRE region of the QR gene.
A. Nrf2 miRNA or control MCF7 cells were treated with 10−6 M TOT for 3 hours. Proteins were extracted from cells and processed for western blot analyses of Nrf2 expression as described in “Materials and Methods”. Levels of Nrf2 were quantitated and normalized to GAPDH. The gel image shown is representative of three independent experiments. Columns in the bar graph represent the fold change in Nrf2 expression levels in control or TOT-treated samples. Error bars indicate standard error of the mean of 3 independent experiments. a, significance (P < 0.05) vs. untreated cells; b, significance (P < 0.01) vs. control transfected cells with the same treatment. B. Nrf2 miRNA or control MCF7 cells were treated with 10−6 M TOT for 3 hours and processed and analyzed using ChIP assays and hPMC2 antibody. The EpRE containing region of the QR gene was then amplified and the bars represent the mean of three replicate experiments for hPMC2 recruitment. Error bars indicate standard error of the mean of 3 independent experiments. a, significance (P < 0.01) vs. untreated cells; b, significance (P < 0.01) vs. control transfected cells with the same treatment.
Our ChIP assay results indicate that the TOT-induced DNA strand breaks were specific for the EpRE region of the QR gene. A possible factor influencing the recruitment of hPMC2 and thus DNA strand breaks in context of the EpRE but not the ERE is the presence of Nrf2 in the EpRE. Thus, we determined the effect of downregulation of Nrf2 on hPMC2 recruitment. The results revealed a significant increase in hPMC2 occupancy at the EpRE region on TOT-treatment when compared to the control cells (Figure 4B). However, when Nrf2 levels were downregulated with miRNA, hPMC2 recruitment at the EpRE region was attenuated both in the presence and absence of TOT. This revealed that Nrf2 is required for hPMC2 recruitment to the EpRE region of the QR gene.
The catalytic activity of hPMC2 is required for removal of estrogen-induced abasic sites
An increase in the number of apurinic/apyrimidinic (AP) sites is a marker for oxidative DNA damage (Nakamura et al., 2008). The AP sites resulting from oxidative damage to the deoxyribose moiety of DNA will lead to aldehydic forms of DNA lesions which, if not repaired, are promutagenic. Enzymes such as the human AP endonuclease (APE1) are known to catalyze the removal of abasic sites and avert potential mutagenic and cytotoxic effects of oxidative damage (Mol et al., 2000; Wilson et al., 1995).
Our previous work has shown that both ERβ and hPMC2 are required for TOT-mediated protection against oxidative DNA damage (Sripathy et al., 2008). In addition to its exonuclease activity, we determined if hPMC2 attenuates estrogen-induced DNA damage by playing a role in its repair. These experiments were carried out in ERα and ERβ negative-MCF10A cells thus allowing us to control for hPMC2-ERβ transcriptional regulation of antioxidative stress enzymes. Cells were transfected with either hPMC2 miRNA that targets hPMC2 3'-UTR or hPMC2 miRNA along with plasmid expressing WT or DM hPMC2. The cells were untreated or treated with E2 (17-beta estradiol) for 24 hours and a biotinylated hydroxylamine Aldehyde-Reactive Probe (ARP) was used to detect the abasic sites as explained in “Materials and Methods”.
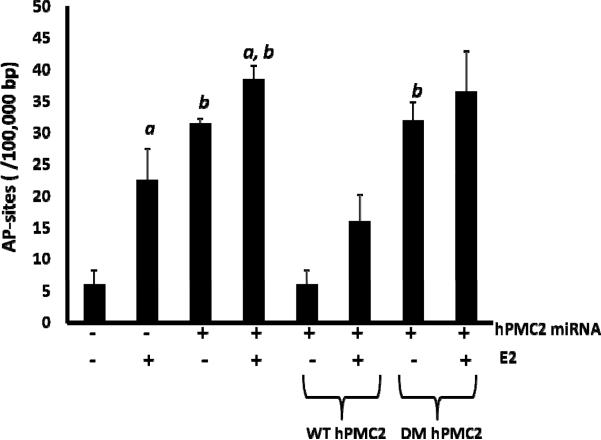
Our results revealed that in control MCF10A cells, treatment with E2 resulted in a 3-fold increase in the number of AP sites indicating that estrogen causes oxidative DNA damage (Figure 5). When hPMC2 was downregulated with miRNA, we observed an increase in number of AP sites not only in E2-treated but also in untreated cells. In order to ensure that this was not due to hPMC2 inhibiting APE1, the major AP endonuclease involved in repair of abasic sites in the DNA (Mol et al., 2000; Wilson et al., 1995), we tested the level of expression of APE1. Western blot analysis reveals that the level of expression of APE1 is similar in both control and hPMC2 downregulated cells (Supplementary Figure 9). We also observed that addition of a plasmid expressing WT hPMC2 resulted in a decrease in number of AP sites to the basal level, while addition of the DM did not. This experiment thus indicates that the exonuclease activity of hPMC2 is required for repair of estrogen-induced abasic sites in DNA.
Figure 5. hPMC2 exonuclease activity is required for repair of estrogen-induced abasic sites.
MCF10A cells were transfected with either hPMC2 miRNA that targets hPMC2 3'-UTR or hPMC2 miRNA along with plasmid expressing WT or the DM hPMC2. Cells were either untreated or treated with 50 nM E2 for 24 hours. Genomic DNA was isolated from cells and processed for Aldehyde-Reactive Probe (ARP) labeling as described in “Materials and Methods”. Columns represent the number of AP sites/105 base pair (bp) in control or E2-treated samples. Error bars indicate standard deviation of 2 independent experiments. a, significance (P < 0.05) vs. untreated cells; b, significance (P < 0.05) vs. control transfected cells with the same treatment.
DISCUSSION
Our studies provide evidence that hPMC2 has a 3'–5' exonuclease activity and this activity is required for TOT-dependent QR upregulation and TOT-induced DNA strand breaks. These DNA strand breaks have been shown to trigger the recruitment of coactivator complexes to promoter regions and serve as a general mechanism for regulated initiation of gene transcription upon ligand stimulation (Ju et al., 2006). Our studies also indicate that Nrf2 is required for hPMC2 recruitment to the EpRE region of the QR gene. In addition, our present work reveals for the first time that the catalytic activity of hPMC2 is required for removal of estrogen-induced abasic sites in DNA.
Previous work has shown that hPMC2 is important for TOT-mediated protection against estrogen-induced DNA damage (Sripathy et al., 2008). Sequence analysis of hPMC2 indicates that there is a putative exonuclease domain at amino acids 233–406 that may provide clues regarding the cellular function of hPMC2 (Moser et al., 1997). Analysis of WT hPMC2 exonuclease activity with an EpRE-containing DNA substrate revealed that hPMC2 degrades DNA in a non-processive fashion in the 3'–5' direction. In addition, WT hPMC2 degrades single stranded DNA nearly 10-fold faster than the double stranded DNA. Interestingly, other exonucleases such as TREX1 (DNAse III) and p53 also exhibit non-processive excision in the 3'–5' direction preferentially from single-stranded DNA (Hoss et al., 1999; Huang, 1998). These enzymes function as proof reading exonucleases for DNA polymerases in DNA repair processes (Skalski et al., 2000). It is thus possible to speculate that by virtue of its exonuclease activity, hPMC2 may also have a role in DNA repair.
In order to demonstrate the functional relevance of the exonuclease activity of hPMC2 in antioxidative gene expression, we downregulated hPMC2 protein expression and showed that decreased hPMC2 levels resulted in reduced QR levels. The expression of WT hPMC2 was able to rescue TOT-induced QR expression to a significant level unlike the double mutant (D247G/D386G). Therefore, our studies indicate a critical role for the exonuclease activity of hPMC2 in QR regulation.
Previous work by Ju et al. has shown that Topo II-mediated double strand break (DSB) is required for receptor-mediated gene transcription (Ju et al., 2006). We have already shown TOT-dependent recruitment of ERβ and hPMC2 to the EpRE, with subsequent recruitment of other factors including Topo IIβ to form a coactivator complex; resulting in transcriptional induction (Sripathy et al., 2008). In addition, our ChIP analysis of BrdUTP labeled cells indicates that hPMC2 exonuclease activity is required for DNA strand breaks resulting from TOT-treatment.
While hPMC2 is recruited to the ERE after E2 or TOT treatments; at the EpRE, we observe only TOT-dependent recruitment (Sripathy et al., 2008). One potential basis for the differential activation of EpRE vs. ERE could be the presence of Nrf2 at the EpRE region. Our ChIP analysis in MCF7 cells with Nrf2 miRNA revealed that downregulation of Nrf2 results in decreased recruitment of hPMC2 at the EpRE. This suggested that Nrf2 has a role in the recruitment of the ERβ-coactivator complex at the EpRE.
Proteins that possess exonuclease domains have important roles in the repair of DNA breaks, recruitment of DNA damage/repair factors as well as in transcriptional regulation (Moser et al., 1997). The well known AP Endonuclease I (APE1) possesses not only DNA repair activities of apurinic/apyrimidinic sites but also plays a role in transcription by redox regulation of Jun binding activity (Xanthoudakis et al., 1992). Similarly, p53 has sequence-specific DNA binding activity, which is required for transactivation functions and an exonuclease activity that may be involved in DNA repair (Marti and Fleck, 2004). However, the exonuclease activity of these proteins is separate from its ability to regulate transcription.
In this work, we have presented evidence that the exonuclease activity of hPMC2 is not only involved in transcriptional regulation but is also required for repair of estrogen-induced abasic sites in the DNA. While hPMC2-mediated regulation of QR and other antioxidative enzymes is crucial for TOT-ERβ protection, we have shown that hPMC2 also plays a role in protection against DNA damage by reducing the number of AP sites. Our study thus provides evidence for a crucial role for hPMC2 in transcription as well as in DNA repair. In turn, this will provide important insights in the role of estrogen metabolites in breast tumor initiation and cancer prevention by hPMC2.
MATERIALS AND METHODS
Exonuclease Assays
Single-turnover experiments, where [Enzyme] > [DNA], were performed using either the single stranded or the double stranded EpRE substrate. In each case, the final DNA concentration was 20 nM with increasing concentrations of either WT or DM hPMC2. All kinetic reactions were performed at 37 °C, monitored for an hour and quenched at various time points with 100 mM EDTA. The aliquots were treated with 5 μL of formamide denaturing dye (90 % formamide, 0.05 % xylene cyanol, 0.05 % bromophenol blue in TBE buffer) and were run on a 20 % denaturing polyacrylamide gel in 1× TBE at 1600 V for 4 hours. Gel images were obtained with a Molecular Dynamics Storm 840 phosphorimager system and data analysis was performed using ImageQuaNT software (version 4.2). The substrate and product bands on the gel were quantified and background in absence of enzyme was subtracted. The exonuclease products were plotted on KaleidaGraph version 3.0 (Synergy Software) and the observed rate constants were calculated (Berdis, 2001).
Active-site concentration experiments were performed in the same manner as the single-turnover assays using 200 nM single stranded EpRE DNA and 20–30 nM total protein. Appropriate fitting of the data using KaleidaGraph allowed for extraction of the active site concentration (Berdis, 2001).
Western blotting and quantitation
Whole cell lysates were processed and analyzed by western blotting as previously described (Sripathy et al., 2008).
Chromatin Immunoprecipitation (ChIP)
Cells were grown in 100-mm dishes and processed for ChIP analyses as previously described (Sripathy et al., 2008) with minor modifications.
DNA strand break labeling and ChIP
Detection of DNA strand break formation was performed as previously described with slight modifications (Ju et al., 2006) and ChIP was performed with an anti-BrdU antibody (BD Biosciences). The EpRE or the ERE-containing regions of the QR or the pS2 gene respectively were amplified by PCR (Sripathy et al., 2008).
Detection of AP sites
MCF10A cells were treated with either hPMC2 miRNA or hPMC2 miRNA along with a plasmid expressing WT or DM hPMC2. The cells were either untreated or treated with 50 nM E2 for 24 hours. DNA extraction was performed with a DNA isolation kit produced by Dojindo Molecular Technologies. Aldehyde reactive probe (ARP) labeling and quantification of abasic sites were performed with an AP sites assay kit (Dojindo Molecular Technologies) as previously described (Nakamura et al., 2008).
Supplementary Material
Acknowledgements
This work was supported by the Department of Defense Breast Cancer Postdoctoral award (BC087610) to N.K. and NIH grant (CA92240) to M.M.M.
Funding: Department of Defense Breast Cancer Postdoctoral award (BC087610) to N.K. and NIH grant (CA92240) to M.M.M.
Abbreviations
- E2
17-beta Estradiol
- EpRE
Electrophile Response Element
- ERE
Estrogen Response Element
- ERβ
Estrogen Receptor beta
- hPMC2
human homolog of Xenopus gene which Prevents Mitotic Catastrophe
- QR
Quinone Reductase
- TOT
trans-hydroxytamoxifen
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y, et al. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–36. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdis AJ. Dynamics of translesion DNA synthesis catalyzed by the bacteriophage T4 exonuclease-deficient DNA polymerase. Biochemistry. 2001;40:7180–91. doi: 10.1021/bi0101594. [DOI] [PubMed] [Google Scholar]
- Bianco NR, Perry G, Smith MA, Templeton DJ, Montano MM. Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Mol Endocrinol. 2003;17:1344–55. doi: 10.1210/me.2002-0382. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic Biol Med. 2007;43:1289–98. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res. 2004;10:7490–9. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- Hoss M, Robins P, Naven TJ, Pappin DJ, Sgouros J, Lindahl T. A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J. 1999;18:3868–75. doi: 10.1093/emboj/18.13.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Zinnen S, Modrich P. Kinetic mechanism of the DNA-dependent DNA polymerase activity of human immunodeficiency virus reverse transcriptase. J Biol Chem. 1993;268:24607–13. [PubMed] [Google Scholar]
- Huang P. Excision of mismatched nucleotides from DNA: a potential mechanism for enhancing DNA replication fidelity by the wild-type p53 protein. Oncogene. 1998;17:261–70. doi: 10.1038/sj.onc.1201946. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Antiestrogenic action of raloxifene and tamoxifen: today and tomorrow. J Natl Cancer Inst. 1998;90:967–71. doi: 10.1093/jnci/90.13.967. [DOI] [PubMed] [Google Scholar]
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- Marti TM, Fleck O. DNA repair nucleases. Cell Mol Life Sci. 2004;61:336–54. doi: 10.1007/s00018-003-3223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol CD, Izumi T, Mitra S, Tainer JA. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination [corrected] Nature. 2000;403:451–6. doi: 10.1038/35000249. [DOI] [PubMed] [Google Scholar]
- Montano MM, Chaplin LJ, Deng H, Mesia-Vela S, Gaikwad N, Zahid M, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–90. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- Montano MM, Deng H, Liu M, Sun X, Singal R. Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene. 2004;23:2442–53. doi: 10.1038/sj.onc.1207358. [DOI] [PubMed] [Google Scholar]
- Montano MM, Jaiswal AK, Katzenellenbogen BS. Transcriptional regulation of the human quinone reductase gene by antiestrogen-liganded estrogen receptor-alpha and estrogen receptor-beta. J Biol Chem. 1998;273:25443–9. doi: 10.1074/jbc.273.39.25443. [DOI] [PubMed] [Google Scholar]
- Montano MM, Katzenellenbogen BS. The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci U S A. 1997;94:2581–6. doi: 10.1073/pnas.94.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Wittmann BM, Bianco NR. Identification and characterization of a novel factor that regulates quinone reductase gene transcriptional activity. J Biol Chem. 2000;275:34306–13. doi: 10.1074/jbc.M003880200. [DOI] [PubMed] [Google Scholar]
- Moser MJ, Holley WR, Chatterjee A, Mian IS. The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res. 1997;25:5110–8. doi: 10.1093/nar/25.24.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, et al. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39:463–9. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, et al. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol. 2004;57:523–8. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Espert L, Mechti N, Wilson DM., 3rd The human interferon- and estrogen-regulated ISG20/HEM45 gene product degrades single-stranded RNA and DNA in vitro. Biochemistry. 2001;40:7174–9. doi: 10.1021/bi010141t. [DOI] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–48. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl 1):S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji S, Hirose M, Toi M. Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol. 2005;56(Suppl 1):21–6. doi: 10.1007/s00280-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Skalski V, Lin ZY, Choi BY, Brown KR. Substrate specificity of the p53-associated 3'–5' exonuclease. Oncogene. 2000;19:3321–9. doi: 10.1038/sj.onc.1203649. [DOI] [PubMed] [Google Scholar]
- Sripathy SP, Chaplin LJ, Gaikwad NW, Rogan EG, Montano MM. hPMC2 is required for recruiting an ERbeta coactivator complex to mediate transcriptional upregulation of NQO1 and protection against oxidative DNA damage by tamoxifen. Oncogene. 2008;27:6376–84. doi: 10.1038/onc.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Takeshita M, Grollman AP, Demple B. Incision activity of human apurinic endonuclease (Ape) at abasic site analogs in DNA. J Biol Chem. 1995;270:16002–7. doi: 10.1074/jbc.270.27.16002. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–35. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.