Abstract
Objective
Shortened telomere length has been associated with mortality in patients with coronary heart disease (CHD) and is considered an emerging marker of biological age. Whether depression is associated with telomere length or trajectory has not been evaluated in patients with CHD.
Methods
In a prospective cohort study, we measured leukocyte telomere length in 952 participants with stable CHD at baseline and in 608 of these participants after 5 years of follow up. Presence of major depressive disorder (MDD) in the past month was assessed using the computerized Diagnostic Interview Schedule (CDIS-IV) at baseline. We used linear and logistic regression models to evaluate the association of depression with baseline and 5-year change in leukocyte telomere length.
Results
Of the 952 participants, 206 (22%) had major depression at baseline. After adjustment for age and sex, patients with current major depressive disorder had shorter baseline telomere length than those without depression (mean ± SE: 0.86±0.02 vs. 0.90±0.01, P= 0.02). This association was similar (but no longer statistically significant) after adjustment for body mass index, smoking, diabetes, left ventricular ejection fraction, statin use, antidepressant use, physical inactivity, and anxiety (0.85±0.02 vs. 0.89±0.01, P= 0.06). Depression was not predictive of 5-year change in telomere length after adjustment for the above covariates and baseline telomere length.
Conclusions
Depression is associated with reduced leukocyte telomere length in patients with coronary heart disease but does not predict 5-year change in telomere length. Future research is necessary to elucidate the potential mechanisms underlying the association between depression and telomere length.
Keywords: Depression, telomere length, stable CHD
Introduction
Telomeres are specialized tandem DNA repeat sequences (TTAGGG)n located at the ends of eukaryotic chromosomes, which protect somatic cells from genomic instability during mitotic cell proliferation (1). During mitosis the telomere is not fully replicated due to inherent properties of DNA polymerase, resulting in obligate telomere shortening with each cell division. Eventually, telomere shortening can result in cessation of mitosis (senescence) or programmed cell death (apoptosis) (2). Thus, telomere attrition has been proposed as the basis for a `biological clock' which integrates the cumulative effect of environmental stressors independently of chronological age (3).
Since the discovery of telomeres, there is a growing body of literature linking shortened telomeres with increased age-related morbidity and mortality. Previous studies have found that psychological distress is associated with short telomere length in otherwise healthy adults (4–6) and in older patients with heart failure (7). However, the association between depression and telomere length has not been evaluated in patients with coronary heart disease (CHD). Furthermore, the effect of depression on subsequent change in telomere length over time has not been examined in any patient population. Evaluating this effect is of importance for our understanding of human telomere biology over time in depressed patients.
Both depression and short telomere length predict mortality in patients with coronary heart disease (CHD) (8–11). Whether depression is associated with leukocyte telomere length or telomere trajectory among patients with stable CHD is unknown. We sought to investigate the association between depression, telomere length and telomere trajectory in a prospective cohort study of patients with stable coronary heart disease. In addition, we evaluated whether differences in leukocyte telomere length might contribute to the adverse cardiovascular outcomes associated with depressive symptoms.
Methods
Design and participants
The Heart and Soul study is a prospective cohort study focused on psychosocial factors and health outcomes in patients with stable coronary heart disease. Details regarding the study design have been described previously (12). Between September 2000 and December 2002, 1024 patients were recruited from 12 outpatient clinics in San Francisco Bay Area. Inclusion criteria were: history of myocardial infarction (MI) or coronary revascularization, angiographic evidence of at least 50% stenosis in at least one coronary vessel, or a diagnosis of CHD by an internist or cardiologist. Patients were excluded if they had a history of MI in the past 6 months, were unable to walk one block, or were planning to move out of the local area within 3 years. Patients underwent a baseline study examination that included a comprehensive health interview, blood samples, medical history, questionnaire, psychosocial questionnaire, and exercise treadmill test with stress echocardiography. Of the 1024 enrolled patients, 954 provided DNA samples for analysis at baseline, and 608 of these participants provided DNA samples again after 5 years of follow up (13). The study protocol was approved by the appropriate Institutional Review Boards, and all participants signed an informed consent.
Assessment of depression
We ascertained the presence of major depressive disorder (MDD) in the past month according to Diagnostic and Statistical Manual-IV (DSM-IV) criteria. We used the modified Computerized National Institute of Mental Health Diagnostic Interview Schedule (CDIS-IV), a highly structured interview designed to yield psychiatric diagnosis (14). The CDIS-IV is a validated computerized version of the health care professional-administered, structured, clinical interview for the diagnosis of psychiatric illness. Trained research assistants administered the interview during the daylong study appointment. We also assessed the presence and severity of depressive symptoms using the 9-item Patient Health Questionnaire (PHQ-9) (15). The PHQ-9 is a self-report checklist derived from the Primary Care Evaluation of Mental Disorders interview (16). The PHQ-9 measures the presence of depressive symptoms during the previous two week (0 = not at all, 1 = several days, 2 = more than half the days, 3 = nearly every day). We evaluated PHQ as a continuous variable (range 0–27).
Telomere length assay
Details regarding telomere length assay in the Heart and Soul study have been described previously (13). Telomere length measurements were performed in a blinded fashion without knowledge of depression status. According to standard procedures, genomic DNA was isolated from peripheral blood leukocytes that were stored at −70 degrees Celsius. Purified DNA samples were diluted in 96-well microtiter source plates to a fixed concentration of 3 ng/ul. A quantitative polymerase chain reaction (qPCR)-based assay was used to measure relative mean telomere length. This assay compares mean telomere repeat sequence copy number (T) to a reference single copy gene copy number (S) in each sample. Standard curves were derived from serially diluted reference DNA. The T/S ratio was calculated from the average quantity of reference DNA found to match with each experimental sample for the copy number of the targeted template (for T: the number of telomere repeats, and for S: the number of β-globin gene copies). The equation for conversion from T/S ratio to base pairs for this study was base pairs = 3274+2413*(T/S) (13). The inter-assay coefficient of variability for telomere length measurement was 3.7%, and the intra-assay coefficient of variability was 2.5%.
Other baseline characteristics
Age, gender, ethnicity, education, smoking status, and alcohol use were determined by questionnaire. Weight and height were measured and body mass index (BMI kg/m2) was calculated. Comorbid conditions were determined by self-report and included hypertension, myocardial infarction, congestive heart failure, and diabetes mellitus. Anxiety was assessed with the Hospital Anxiety and Depression Scale (HADS). We assessed left ventricular ejection fraction (LVEF) using resting echocardiography. Resting systolic and diastolic blood pressure were measured manually using a standard sphygmomanometer. To assess physical activity, we asked “which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15 to 20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants chose from 1 of the following 6 categories: not at all active, a little active (1–2 times per month), fairly active (3–4 times per month), quite active (1–2 times per week), very active (3–4 times per week), or extremely active (≥5 times per week). Self-report has been shown to be a reliable, valid, and accurate method of assessing physical activity (17;18). Participants who reported that they were not at all or a little active were considered physically inactive. Low- and high-density lipoprotein cholesterol levels were determined from fasting venous blood samples. Participants were instructed to bring their medication bottles to their appointment, and study personnel recorded all current medications, including dose and frequency use.
Mortality
To determine whether differences in leukocyte telomere length might contribute to the adverse cardiovascular outcomes associated with depressive symptoms, we evaluated the association of depressive symptoms with mortality before and after adjustment for baseline telomere length. Annual telephone interviews were conducted with participants or their proxies asking about emergency room visits, hospitalizations, or death. For any reported event, medical records, death certificates, and coroner's reports were reviewed by two independent blinded adjudicators. In the event of disagreement a third blinded adjudicator reviewed the event and determined the outcome variable. To be diagnosed with heart failure, patients had to be hospitalized for a clinical syndrome involving an acute change in at least two of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, cardiomegaly or pulmonary edema on chest radiography. Death was confirmed by review of death certificates.
Statistical analyses
For descriptive purposes, participants were grouped based on the presence or absence of current major depression (by CDIS-IV) and compared on clinical and demographic variables, using T tests and X2 tests. Telomere length was normally distributed. For primary analyses, the association between depression and mean telomere length at baseline was examined using generalized linear models (for telomere length as a continuous variable) and logistic regression for short telomere length, defined a priori as having leukocyte telomere length in Quartile I vs. IV.
Percent change in telomere length was calculated as = [(follow-up T/S minus baseline T/S) × 100] divided by baseline T/S. The association between depression and 5-year change in telomere length was assessed using generalized linear models (for percent change in telomere length as a continuous variable) and logistic regression models for predicting telomere shortening (defined a priori as a > 10% decrease in telomere length) versus maintained (± 10% change in telomere length) or lengthened (>10% increase in telomere length) (13). For multivariable models, the following covariates were chosen based on cross-sectional associations with telomere length and depression: age, sex, diabetes, BMI, smoking, LVEF, statin use, antidepressant use, physical inactivity, and anxiety (9). To determine whether the effect of depression on telomere trajectory differed in patients with shorter or longer baseline telomere length, we tested an interaction term (depression × baseline telomere length) as a predictor of shortening.
We have previously reported that depressive symptoms – but not MDD – predict subsequent heart failure and death in the Heart and Soul Study (19). To evaluate whether telomere length may be a mediator in this association, we estimated the association of depressive symptoms with heart failure or death using Cox proportional hazards models, with and without adjustment for baseline telomere length. Statistical analyses were performed using SAS software version 9.1 (SAS Institute inc).
Results
Of the 954 patients that provided DNA samples for analysis at baseline, 2 had no CDIS measurement, leaving 952 patients to be included in further analyses. The baseline characteristics of the study population categorized by current depression are presented in Table 1. Of the 952 patients, 206 (22%) participants had current (past month) depression. Compared with participants who did not have depression, those with depression were younger and less likely to be male. They were more likely to have higher LVEF, to smoke, to have diabetes mellitus, to have a higher anxiety score, to use antidepressants, and to be physically inactive, but less likely to use statins.
Table 1.
Baseline characteristics of 952 participants with baseline measurement of telomere length
| Current depression (n=206) | No current depression (n=746) | ||
|---|---|---|---|
| Variable | N (%) or Mean ± SD | P value | |
| Demographic characteristics | |||
| Age (years) | 61.7±10.8 | 68.1±10.6 | <0.001 |
| Male | 143(69%) | 632(85%) | <0.001 |
| White | 124(60%) | 449(60%) | 0.98 |
| High school graduate | 182(88%) | 646(87%) | 0.56 |
| Body mass index (kg/m2) | 29.01±5.66 | 28.31±5.31 | 0.10 |
| Regular alcohol use | 60(29%) | 216(29%) | 0.98 |
| Current smoking | 58(28%) | 131(18%) | <0.001 |
| Comorbid conditions | |||
| Hypertension | 146(71%) | 526(71%) | 0.96 |
| Myocardial infarction | 100(49%) | 408(55%) | 0.13 |
| Congestive heart failure | 37(18%) | 126(17%) | 0.72 |
| Diabetes mellitus | 66(32%) | 186(25%) | 0.04 |
| Anxiety score | 8.90±4.09 | 4.45±3.30 | <0.001 |
| PHQ score | 10.73±5.58 | 3.53±4.12 | <0.001 |
| Cardiac disease severity and risk factors | |||
| Resting left ventricular ejection fraction | 0.63±0.07 | 0.61±0.10 | 0.005 |
| Low-density lipoprotein cholesterol (mg/dl) | 107.54±36.74 | 103.31 ±32.84 | 0.12 |
| High-density lipoprotein cholesterol (mg/dl) | 45.35±14.84 | 45.63±13.93 | 0.80 |
| Systolic blood pressure | 132.84±21.90 | 133.06±20.93 | 0.90 |
| Diastolic blood pressure | 75.26±11.96 | 74.43±11.23 | 0.36 |
| Physical inactivity | 95(45%) | 250(34%) | 0.002 |
| Medication use | |||
| Aspirin | 158(77%) | 574(77%) | 0.94 |
| Beta blocker | 114(55%) | 433(58%) | 0.49 |
| Renin-angiotensin system inhibitor | 102(50%) | 386(52%) | 0.57 |
| Statin | 119(58%) | 492(66%) | 0.03 |
| Antidepressant use | 99(48%) | 78(10%) | <0.001 |
Depression and baseline telomere length
After adjustment for age and sex, patients with current major depressive disorder had shorter telomere length than patients without current depression (mean ± SE: 0.86±0.02 vs. 0.90±0.01, P= 0.02). This association was similar (but no longer statistically significant) after further adjustment for body mass index, smoking, diabetes, left ventricular ejection fraction, statin use, antidepressant use, physical inactivity, and anxiety (0.85±0.02 vs. 0.89±0.01, P= 0.06) (Table 2). The difference of 0.04 T/S units is comparable with 97 base pairs. Compared with nondepressed participants, those with major depression had a 71% greater odds of having short telomere length (adjusted OR 1.71, 95% CI, 0.98–2.98; p=0.06) (Table 3). When entered as a continuous variable, higher depressive symptom scores were also associated with shorter telomere length, adjusted for age, sex, diabetes, body mass index, smoking, LV ejection fraction and statin use (beta coefficient = −0.00297; p=0.03). Again, this association was no longer statistically significant after further adjustment for antidepressant use, physical inactivity and anxiety (beta coefficient = −0.00231; p=0.17).
Table 2.
Telomere length (analyzed as a continuous variable, mean +/− standard error) by presence of major depressive disorder among 952 participants at baseline.
| Adjusted for | Current depression N=206 | No current depression N=746 | P value |
|---|---|---|---|
| age, sex | 0.86±0.02 | 0.90±0.01 | .02 |
| age, sex, diabetes, body mass index, smoking | 0.86±0.02 | 0.89±0.01 | .04 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, and antidepressant use | 0.85±0.02 | 0.89±0.01 | .04 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, antidepressant use, and anxiety | 0.85±0.02 | 0.89±0.01 | 0.06 |
Table 3.
Association between major depressive disorder and short telomere length (analyzed as a dichotomous variable, Quartile I vs. IV).
| Adjusted for: | Odds Ratio (95% CI) | P value |
|---|---|---|
| age, sex | 1.73 (1.08–2.79) | 0.02 |
| age, sex, diabetes, body mass index, smoking | 1.65 (1.03–2.67) | 0.04 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, and antidepressant use | 1.72 (1.02–2.89) | 0.04 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, antidepressant use, and anxiety | 1.71 (0.98–2.98) | 0.06 |
Depression and 5-year change in telomere length
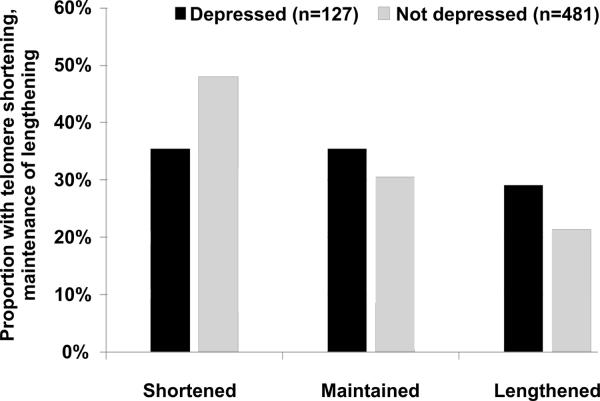
Of the 1024 original enrolees, 195 had died before the 5-year examination, and 667 (80%) of the eligible 829 participants completed the 5-year follow-up examination. Of the 667 participants who completed the 5-year examination, 59 were missing telomere length measurements at baseline and/or follow-up, leaving 608 participants for the analysis of 5-year change. Compared with the 221 participants who were alive at 5 years but not included in the analyses, these 608 participants had similar age and baseline telomere length. Overall, 276 participants (45%) experienced telomere shortening, 192 (32%) maintained their telomere length (±10%), and 140 experienced telomere lengthening (23%). Compared with the 481 nondepressed participants, the 127 participants with major depressive disorder at baseline were less likely to experience telomere shortening (35% vs. 48%) and more likely to experience telomere lengthening (26% vs. 21%) (Figure 1). After adjustment for age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, antidepressant use, physical inactivity, and anxiety, major depressive disorder was associated with a 32% decreased odds of shortening. However, this association was not significant after further adjustment for shorter baseline telomere length in the depressed participants (OR 0.76, 95% CI 0.40–1.44; p=0.40) (Table 4).
Figure 1.
Proportion of the participants who experienced telomere shortening, maintenance, or lengthening, during the 5 years of follow-up (p = .03 from overall χ2), unadjusted for age or baseline telomere length.
Table 4.
Association between major depressive disorder and subsequent shortening in leukocyte telomere length (>10% decrease)
| Adjusted for: | Odds ratio (95% CI) | P value |
|---|---|---|
| age, sex | 0.66 (0.43–1.00) | 0.05 |
| age, sex, diabetes, body mass index, smoking | 0.67 (0.44–1.01) | 0.06 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, and antidepressant use | 0.63 (0.40–0.99) | 0.04 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin us, physical inactivity, antidepressant use, and anxiety | 0.68 (0.42–1.12) | 0.13 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, antidepressant use, anxiety, and baseline telomere length | 0.76 (0.40–1.44) | 0.40 |
When 5-year percent change in telomere length was analyzed as a continuous variable, participants with major depressive disorder were also less likely to experience telomere shortening than those without depression (percent change: −0.9 ± 2.4% vs. −6.6 ± 1.9%; p=0.03), adjusted for age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, antidepressant use, physical inactivity, and anxiety. Again, this association was no longer significant after adjustment for shorter baseline telomere length in the depressed participants (percent change: −3.0 ± 1.7% vs. −5.6 ± 1.3%; p=0.13) (Table 5). We found no evidence that the effect of depression on change in telomere length differed in patients with shorter or longer baseline telomere length (p for interaction = 0.78).
Table 5.
Five-year change in telomere length (analyzed as a continuous variable, mean +/− standard error) by presence of major depressive disorder among 608 participants who provided follow-up DNA samples.
| Adjusted for | Current depression N=127 | No current depression N=481 | P value |
|---|---|---|---|
| age, sex | −0.6% ± 2.0% | −5.2% ± 1.3% | 0.03 |
| age, sex, diabetes, body mass index, smoking | −1.9% ± 2.2% | −6.1% ± 1.6% | 0.05 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, and antidepressant use | −1.1% ± 2.2% | −6.5% ± 1.9% | 0.02 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, antidepressant use, and anxiety | −0.9% ± 2.4% | −6.6% ± 1.9% | 0.03 |
| age, sex, diabetes, body mass index, smoking, left ventricular ejection fraction, statin use, physical inactivity, antidepressant use, anxiety, and baseline telomere length | −3.0% ± 1.7% | −5.6% ± 1.3% | 0.13 |
Depressive symptoms and cardiovascular outcomes
As of 18 December 2009, vital status was known for 949 (>99%) of the 954 study participants, and there were 277 deaths. Each standard deviation (5.5-point) increase in PHQ depressive symptom score was associated with a 16% increased rate of death (age-adjusted HR 1.16, 95% CI 1.04–1.31; p=0.01) and a 24% increased rate of heart failure (1.24, 95% CI, 1.07–1.45; p=0.006). Adjustment for shorter baseline telomere length in the depressed patients did not affect these associations (HR 1.14, 95% CI, 1.01 – 1.28; p=0.03 for death; HR 1.23, 95% CI 1.05–1.44; p=0.009 for heart failure).
Discussion
In a sample of 952 patients with stable CHD, we found that major depression was associated with a 71% greater odds of having short telomere length. Participants with major depression had an average telomere length that was 97 base pairs shorter than those without depression. Assuming an average rate of loss of around 42 base pairs per year (13), this indicates that their leukocytes had aged the equivalent of 2.3 additional years, compared with patients without depression.
Depression and baseline telomere length
Previous cross-sectional studies have found that psychosocial factors are associated with shorter telomere length, but the relation between depression and telomere length has not previously been evaluated in patients with CHD. Epel et al. demonstrated that the chronicity and perceived severity of psychosocial stress was directly associated with accelerated telomere shortening in middle-aged healthy women (n = 65) (4). Simon et al. measured leukocyte telomere length in 44 individuals with chronic mood disorders and 44 non-psychiatric ill age-matched control subjects and found that telomere length was significantly shorter in those with mood disorders (6). Lung et al found an association between depression and short telomere length among 253 depressed patients compared with 411 community controls (5). Another study found that poor perceived mental health – but not depressive symptoms – was associated with shorter telomere length in 890 patients with congestive heart failure (7). Our study adds to this growing literature by demonstrating that depression is associated with short telomere length in patients with CHD. In addition, our findings demonstrate that, although associated with shorter baseline telomere length, current depression does not predict subsequent shortening.
Underlying mechanisms
Further research is necessary to examine the mechanisms underlying the association between depression and reduced telomere length in CHD patients. Potential links between depression and shortened telomere length could be oxidative stress and inflammation (20). Previous studies have demonstrated an association between depression and oxidative stress. Depressed patients have increased levels of circulating oxidative stress markers and decreased levels of anti-oxidant enzymes (21–23). Additionally, some, but not all studies, have found that depression is associated with increased levels of pro-inflammatory cytokines (24;25). Both oxidative stress and pro-inflammatory cytokines have been found to influence telomere length. Oxidative stress has a negative effect on telomere length, through inhibition of telomerase activity (26) and direct erosion of GGG triplets in telomeric DNA (27). Pro-inflammatory cytokines may either decrease or increase telomerase activity (28–30) and are thought to lead to immune cell turnover, and thus decreased telomere length through greater replicative history.
Depression and 5-year change in telomere length
Little is known concerning the dynamic regulation of telomere length over time. Recently, it has become apparent that telomeres may lengthen as well as shorten (13;31). In our sample less than half of the participants experienced telomere shortening, and almost a quarter actually lengthened their telomeres during the 5 year follow up period. In this longitudinal study we observed that major depressive disorder was associated with a 32% decreased odds of shortening (i.e., greater odds of lengthening). However, short baseline telomere length is by far the strongest predictor of subsequent lengthening, and this association was not significant after further adjustment for shorter baseline telomere length in depressed participants. Therefore, depression does not seem to predict 5-year subsequent change in telomere length independently. These findings are in concordance with previous studies which found that telomere trajectory is powerfully influenced by baseline telomere length and that both healthy individuals and CHD patients with the longest telomeres experienced the greatest amount of shortening, while those with shorter telomeres either maintained or increased in their length (13;31;32).
An important regulator of this negative feedback is the enzyme telomerase, which is a reverse transcriptase enzyme that restores telomere length. Telomerase has been shown to act preferentially on short telomeres in mice models and cell culture systems (33–36). Moreover, chronically stressed caregivers, who are also high in depressive symptoms have increased levels of telomerase (37). Thus, it is possible that depression may have contributed to shorter baseline telomeres, but over a follow-up time of 5 years, the subsequent negative feedback from those short telomeres may overwhelm any independent effect on trajectory. Alternatively, the current findings are consistent with a model in which depression is a consequence of short telomeres or in which a shared (genetic) risk factor is responsible for both depression and short telomere length at baseline.
Depression and mortality
Currently, a large body of literature has confirmed that depressive symptoms are associated with greater mortality among patients with established CHD (8;11). A recent study showed that shorter telomere length was associated with all cause mortality and heart failure in patients with stable CHD (9). Because depression is associated with shorter telomere length, this raises the question of whether accelerated cellular aging is a mechanism that contributes to the excess morbidity and mortality associated with depression (6;38). To our knowledge, we are the first study that evaluates whether shortened telomere length may potentially underlie the relationship between depression and heart failure and mortality. Adjustment for baseline telomere length in the depressed patients did not affect the association between depression and prognosis.
Strengths and limitations
Our study has several strengths, including repeated measurements of telomere length, measurement of multiple potential confounding variables including BMI, LVEF, smoking, and physical inactivity, and detailed assessments of depression. However, some limitations of this study should be noted. First, this study included stable CHD patients, and mainly older men. Thus, the results may not generalize to women or to healthy or acute coronary syndrome populations. Second, we did not measure the impact of telomerase activity on the prognostic value of leukocyte telomere length. Third, telomere length measurements were restricted to circulating leukocytes and do not necessarily reflect telomere length in other cell compartments, and do not inform about accelerated aging of any particular immune cell subpopulation. Fourth, the association between depression and shortened telomere length may have been the result of greater cardiac disease severity in depressed patients. We attempted to address this possibility by carefully measuring and adjusting for cardiovascular disease severity. Fifth, the severity of depressive symptoms was relatively low with an average PHQ score of 10.7 among depressed participants. Finally, we did not assess the chronicity or duration of depression at the baseline examination nor did we account for continued depression or other psychiatric diagnoses at follow-up.
Conclusion
To our knowledge, this is the first study to examine and report an association between depression and telomere length in patients with stable CHD. In summary, we found that patients with current depression had a shorter telomere length at baseline. However, current depression did not predict subsequent change in telomere length. Future research is necessary to elucidate the mechanism underlying the association between depression and telomere length.
Acknowledgments
Financial support: Dr de Jonge was supported by a VIDI grant from the Dutch Medical Research Council (grant 016.086.397). The Heart and Soul study was supported by the Department of Veterans Affairs Epidemiology Merit Review Program; the Department of Veterans Affairs Health Services Research and Development service; the National Heart Lung and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); the Ischemia Research and Education Foundation; and the Nancy Kirwan Heart Research Fund. Dr. Farzaneh-Far is supported by an American Heart Association Fellow-to-Faculty Transition Award #0875014N. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Acronyms
- CHD
coronary heart disease
- MDD
major depressive disorder
- CDIS-IV
computerized diagnostic interview schedule
- MI
myocardial infarction
- DSM-IV
Diagnostic and Statistical Manual-IV
- PHQ
Patient Health Questionnaire
- qPCR
quantitative polymerase chain reaction
- BMI
body mass index
- LVEF
left ventricular ejection fraction
Footnotes
Conflict of Interest: Elisabeth Blackburn, Jue Lin and Elissa Epel will be co-founders of Telome Health, a diagnostics company related to telomere biology, and will own stock in the company. The other authors report no financial interests or potential conflicts of interest.
Relationship with industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- (2).Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–8. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- (3).Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996;31:443–8. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- (4).Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–9. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- (6).Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- (7).Huzen J, van der Harst P, de Boer RA, Lesman-Leegte I, Voors AA, van Gilst WH, Samani NJ, Jaarsma T, van Veldhuisen DJ. Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing. 2010 doi: 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- (8).Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- (9).Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28:1379–84. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–80. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- (11).Van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- (12).Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- (15).Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- (16).Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bowles HR, FitzGerald SJ, Morrow JR, Jr., Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–86. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- (18).Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand J Public Health. 2008;36:52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- (19).Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–38. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- (21).Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2'-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- (22).Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311:1014–8. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- (23).Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- (24).Leonard B. Stress, depression and the activation of the immune system. World J Biol Psychiatry. 2000;1:17–25. doi: 10.3109/15622970009150562. [DOI] [PubMed] [Google Scholar]
- (25).O'Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19:397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- (26).Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–26. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- (27).Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- (28).Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res. 2002;62:3876–82. [PubMed] [Google Scholar]
- (29).Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K, Eto Y, Yamada H, Anderson KC. TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochem Biophys Res Commun. 2004;316:528–32. doi: 10.1016/j.bbrc.2004.02.080. [DOI] [PubMed] [Google Scholar]
- (30).Xu D, Erickson S, Szeps M, Gruber A, Sangfelt O, Einhorn S, Pisa P, Grander D. Interferon alpha down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood. 2000;96:4313–8. [PubMed] [Google Scholar]
- (31).Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- (34).Samper E, Flores JM, Blasco MA. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep. 2001;2:800–7. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–35. doi: 10.1016/s0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- (36).Zhu L, Hathcock KS, Hande P, Lansdorp PM, Seldin MF, Hodes RJ. Telomere length regulation in mice is linked to a novel chromosome locus. Proc Natl Acad Sci U S A. 1998;95:8648–53. doi: 10.1073/pnas.95.15.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: do stress and depression accelerate cell aging? World J Biol Psychiatry. 2008;9:2–5. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]