Abstract
Leukocytes are rapidly recruited to the preovulatory ovary and play a crucial role as facilitators of ovulation and luteal formation. In this article, recent findings on leukocyte trafficking to the ovary, as well as the physiological role of leukocytes in the ovary, will be summarized and discussed. We then explore the novel hypothesis that the hypothalamus-pituitary-ovarian (HPO) axis might include the spleen as a reservoir of leukocytes by summarizing recent reports on this topic, both in the fields of immunology and reproductive biology.
Linking leukocytes with ovulation
Ovulation, a key step in the propagation of life, has always been a subject of human curiosity. This egg-releasing act of the ovary is still a mysterious event and much about the process has yet to be unveiled. Ovulation is a critical step in reproduction and has become a key therapeutic target for treating female infertility and various ovarian diseases. Ovulatory failure is associated with the development of ovarian disorders such as polycystic ovarian syndrome (PCOS), hemorrhagic cyst formation, and hormonal imbalance, all of which are major risk factors in women’s health [1–3]. Furthermore, controlling ovulation has become a hallmark for contraception, as blockage of ovulation ensures the absence of fertilizable eggs [4]. Understanding the mechanisms that govern this ovulatory process, however, is challenging because there is interplay between the reproductive system, the immune system, and possibly other systems. Recently, a comprehensive flow cytometry approach was applied to quantitatively measure inflammation during ovulation by determining the spatiotemporal patterns of leukocyte infiltration in the ovaries of immature and adult rats. This effort led to the finding of massive leukocyte infiltration into the ovary induced by the luteinizing hormone (LH) surge or human chorionic gonadotropins (hCG) injection during ovulation [5, 6]. Surprisingly, ovarian leukocyte infiltration was accompanied by the release of millions of leukocytes to the bloodstream from the spleen, indicating that this immune organ might be a source of leukocytes that infiltrate the preovulatory ovary. Supporting this idea, recent studies showed that splenic leukocytes are recruited to injured heart tissues following myocardial infarction [7]. Both of these studies demonstrate the importance of the spleen as an immediate source of leukocytes for inflammatory events. In this article we examine trafficking of leukocytes into the ovary, the requirement of leukocytes for ovulation, and consider in depth the spleen as a source of leukocytes.
Trafficking of leukocytes into the ovary
The migration of leukocytes in response to chemokines has been implicated in a plethora of normal and pathophysiological aspects of reproductive systems [8]. Multiple chemoattractants such as interleukin-8 (IL-8) and a variety of their target populations of leukocytes have been shown to play important roles in ovulation [9–11]. Here, we summarize ovarian leukocyte populations, their function and factors that affect their infiltration into the ovary.
(1) Leukocyte populations and their localization within the ovary
Traditionally, immunohistochemical techniques have been used to characterize ovarian leukocyte populations. Whilst these methods are effective for identifying the localization of leukocytes in ovarian tissues, determining the precise leukocyte subsets that are present in the tissue has been challenging. Modern techniques such as flow cytometry have made it possible to distinguish between CD4+ T-cells, CD8+ T-cells, B-cells, natural killer (NK) cells, regulatory T-cells or other cell types, each with different functions. Table 1 summarizes the leukocyte populations that have been identified in the ovary, their localization and possible functions. As shown in Table 1, most leukocyte subtypes are found in the ovary and are predominately localized in the periphery of the follicle, interstitium, and corpora lutea, but not inside follicles.
Table 1.
Ovarian leukocyte species, localization and functions
| Cell type | Location | Possible Function(s) | References |
|---|---|---|---|
| Monocyte/macrophage | periphery of follicles, tunica albugenia, corpora lutea, | IL-8 secretion, luteal regression | [5, 22, 99–111] |
| Neutrophils | theca layer, corpora lutea | production of proteolytic enzymes, ECM degradation, follicle maturation, ovulation, luteal formation | [5, 22, 110, 112, 113] |
| Lymphocytes | hilus, stroma, corpora lutea | selection of dominant follicle, luteal formation, luteal regression | [5, 101, 107, 110] |
| NK-cells | follicle, corpora lutea | angiogenesis | [114] |
| Mast cells | medulla, cortex, interstitium, corpora lutea | ECM degradation, ovulation | [109, 115, 116] |
| Eosinophils | theca layer, corpora lutea | ECM degradation, neovascularization | [111, 116, 117] |
(2) Mechanism of leukocyte infiltration into the ovary
The initiator of an inflammatory event is often a discrete signal that is rapidly amplified by chemical signals produced by responding tissues and infiltrating cells. Unlike during infection, where the inflammatory stimuli are obvious, the initiating factors for an inflammatory response that occurs during a normal physiological event, such as preovulatory inflammation are less clear. Infiltration and distribution of leukocytes in the ovary are correlated with hormonal changes associated with the estrous cycle [12, 13], indicating that reproductive hormones such as ovarian steroids and gonadotropins may elicit inflammatory responses in the ovary. The same adhesion molecules, chemokines and cytokines observed in immune responses to infectious agents are also found in the ovary during estrous [5, 14, 15], suggesting a similar mechanism of leukocyte infiltration in the preovulatory ovary. The cytokines (IL-1, IL-6 and IL-10) may contribute to increased cellular adhesion molecule (CAM) expression during ovulation [16]. Furthermore, our recent data show increased expression of ICAM-1 and E-selectin corresponds with increased infiltration of leukocytes into the ovary [5]. Accordingly, treatment with IL-1 receptor antagonist (IL-1Ra) inhibits hCG induced ovulation rates in rats by 40% [17].
The classical four step process of tethering and rolling, activation, firm adhesion and transmigration into the tissues is paramount to any event requiring infiltration of leukocytes [18]; we anticipate ovulatory inflammation to be the same. Once the leukocytes are tethered on the endothelial cell wall, specific populations of leukocytes, characterized by their chemokine receptor (CCR) expression, migrate towards the source of the corresponding chemokine, produced by theca cells (TC), granulosa cells (GC) or resident ovarian leukocytes (Table 2). For instance, neutrophils infiltrate the ovary in response to an increased concentration gradient of IL-8 [9]. Amounts of basal IL-8 in the ovary are low, but production increases in granulosa cells and theca cells upon LH stimulation [11]. IL-8 is also known to increase vascular permeability [19, 20], which may facilitate the infiltration process. In addition, the infiltrating leukocytes may interact with ovarian endothelial cells and other cell types via a multitude of chemokines during ovulation [21–23]. Treatment with neutralizing antibodies to either IL-8 [24] or neutrophils [1] significantly reduces ovulation rates in animal models.
Table 2.
List of chemokines, chemokine receptors and responsible cells present in the ovary
| Chemokine | Producing cells | Chemokine receptor | Responding cells | References |
|---|---|---|---|---|
| CCL-2 (MCP-1) | Mo/MΦ, cDC, BMDC, GC, GLC, stromal fibroblast | CCR-2a, b, CCR-8, CCR-11 | T, Mo, baso, DC, NK | [23, 31, 118–120] |
| CCL-3 (MIP-1α) | Mo/MΦ, cDC, BMDC, TE, TM, NK, B, GC, GLC | CCR-1, CCR-5 | Mo/MΦ, T, baso, eos, neut, DC, NK, GC, TC | [118–120] |
| CCL-4 (MIP-1β) | Mo/MΦ, cDC, BMDC, TE, TM, NK, B | CCR-5 | Mo/MΦ, cDC, BMDC, B, T, NK, baso, eos, B, GC, TC | [118–120] |
| CCL-5 (RANTES) | Mo/MΦ, cDC, BMDC, TE, GC, TM, NK | CCR-1, CCR-3, CCR-5 | TE, TM, NK, Mo/MΦ, DC, eos, baso, GC, TC | [118–120] |
| CCL-20(MIP-3α/LARC) | Epithelial, GC | CCR-6 | PBMC, TE, TM, NKT, DC, B | [118, 121] |
| CCL25 (TECK) | Mo/MΦ, cDC, BMDC, TC | CCR-9 | MΦ, thymocyte, DC, B | [37, 118] |
| CXCL-1 (Groα) | Mo/MΦ, DC, BMDC, GC | CXCR1, CXCR2 | neut, fibroblast | [20, 118, 120] |
| CXCL-5 (ENA-78) | BMDC | CXCR2 | neut, endo, BMDC | [120] |
| CXCL-8 (IL-8) | Mast, GC, GLC, TC, stromal fibroblast | CXCR1, CXCR2 | neut, baso, T, endo | [20] |
| CXCL-10 (IP-10) | Osteoblasts, BM endo, GC | CXCR3A CXCR3B | T, NK, B, endo, Mo/MΦ, DC, GC | [119, 120] |
| CXCL-12 (SDF-1) | BM reticular, GC, endo, stromal, fibroblast | CXCR4, CXCR7 | CD34+ BM, thymocytes, Mo/MΦ, TE, B, plasma, neut, cDC, BMDC | [122, 123] |
| CX3CL1 (Fractalkine) | Mast cells, GC, TC and stromal fibroblasts, endothelium | CX3CR1 | NK, Mo, neut, Mast, astrocytes, neurons, activated T cells | [118] |
NK, natural killer cell; neut, neutrophil; Mast, mast cell; Mo, monocyte; MΦ, macrophage; TE, effector T-cell; TM, memory T-cell; BMDC, bone marrow derived dendritic cell; cDC, conventional dendritic cell; GC, granulosa cell; TC, theca cell; GCL, granulosa-lutein cell; endo, endothelial cell; baso, basophil; PBMC, polymorphonuclear cell; DC, dendritic cell.
Two chemokines, monocyte chemotactic protein-1 (MCP-1/CCL2) and thymus-expressed cytokine (TECK/CCL25), are well characterized in ovarian leukocyte infiltration. MCP-1 is a potent chemoattractant for monocytes and is also effective in recruiting macrophages and T cells [25, 26]. The major producers of MCP-1 are monocytes and macrophages, although several other cell types including epithelial, endothelial, and smooth muscle cells have been shown to produce this protein [27]. In addition to the chemotactic properties, MCP-1 interacts with G-protein-coupled receptors to induce multiple intracellular responses including activation and degranulation (a comprehensive review of alternative functions for MCP-1 is given in [28]). Interestingly, studies have demonstrated in humans and rats that MCP-1 is involved in all aspects of ovarian function including follicular development [29, 30], ovulation and luteolysis [23, 31]. Several studies have demonstrated that inhibiting the production of monocytes/macrophages [32, 33] or the direct neutralization of ovarian macrophages [34] results in reduced or inhibited ovulation rates in mice, further supporting the role of monocytes/macrophages in ovulation. TECK was first described in the development of T-cells in the thymus, but now has a well-accepted role as a chemokine that recruits cells to sites of inflammation [35, 36]. Neutralization of TECK with specific antibodies inhibited leukocyte infiltration into the ovary by 85%, resulting in a lack of ovulation. Interestingly, ovulatory failure has been attributed to the lack of infiltration of a rare CD8α+ T cell population [37, 38]. Consistent with this finding are reports that ovarian TECK expression is tightly regulated by gonadotropins [39].
Two fascinating aspects of ovarian leukocyte infiltration are the speed and extent to which it occurs. In rats, this increased expression of CAMs and corresponding infiltration commences in as little as 1 to 3 hours after ovulatory gonadotropin stimulation. This is an extremely fast event, particularly in contrast to inflammation caused by infection in which infiltration of leukocytes occurs over several days [40]. In contrast, preovulatory leukocyte infiltration is not an occasional event but a frequent one as leukocyte infiltration takes place each time ovulation occurs, which is every four to five days in rodents and approximately once every month in women.
(3) Roles of ovarian leukocytes in ovulation
Leukocytes are involved in three main aspects of ovarian function: 1) loosening of the follicular wall to facilitate follicular growth and ovulation; 2) tissue repair following follicle rupture; and 3) luteal formation and regression. Ovulation, as well as the events that follow, requires major modifications in the extracellular matrix (ECM), and these rearrangements of the ECM involve tightly controlled production of tissue proteases. Matrix metalloproteinases (MMPs) are a family of soluble and membrane type (MT-MMPs) zinc dependent endopeptidases [41]. Both follicular cells (GC and TC) and leukocytes are known to produce MMPs [42–44]. However, further studies are needed for better characterization of the cells types responsible for production of each MMP subtype.
Leukocytes were recently described as major producers of MMP-9, the most abundant MMP found in the preovulatory ovary [45]. MMP-producing cells were identified as monocytes/macrophages and granulosa cells are the major producers of inhibitors of MMPs (TIMPs). In inflamed tissues, as monocytes move through tissue, they secrete MMPs that digest matrix proteins, facilitating easier migration through tissues to the sites of inflammation. It is feasible that ovarian monocytes produce one class of MMPs for migration purposes and, upon tissue specific differentiation, produce more potent MMPs that may further facilitate matrix breakdown. Interestingly, although the major role of MMPs in the ovary is related to their function in ECM breakdown, the substrates for MMPs are not restricted to matrix proteins. Many immune mediators, such as cytokines, chemokines, cell surface receptors and adhesions molecules also are substrates of MMP action [46]. In this regard, leukocyte-secreted MMPs might act both to breakdown the ECM, and to regulate the breakdown of chemotactic proteins thereby limiting leukocyte infiltration
Angiogenesis and neovascularization occur with great frequency within the ovary. As such, vascular endothelial growth factor (VEGF) expression closely correlates to the dynamic changes that take place in the ovary. In particular, a dramatic increase in angiogenesis occurs prior to ovulation and a fine network of capillaries develops and infiltrates the theca layer. An increase also occurs, immediately after ovulation forming a massive capillary network in the developing CL [47]. The source of VEGF that drives the active angiogenesis is not clearly determined. Whilst a body of literature suggests VEGF is expressed by TC, GC and the CL [48, 49], monocytes, macrophages and neutrophils also produce of VEGF in many scenarios [50, 51]. Macrophages isolated from human follicular aspirates upregulate VEGF production more than five-fold upon stimulation with hCG or LH [52]. Although the function of MMPs and VEGF appear mutually exclusive, several investigators have shown a mutual regulation between MMPs and VEGF [53]. It is expected that more detailed information on the leukocyte function in the ovary will emerge as new assay methods and animal models are developed.
HPO axis in regulating ovulation
The hypothalamus, pituitary and ovary have long been considered to constitute the axis of a regulatory loop that controls ovulation. These three key organs, the HPO axis, communicate between one another via hormonal signals. The result is that cyclic hormonal changes that result in the periodic expulsion of eggs in the process known as ovulation. Gonadotropin releasing hormone (GnRH) secreted from the hypothalamus stimulates pituitary gonadotrophs to synthesize and release the gonadotropins, follicle stimulating hormone (FSH) and LH. These two hormones then exert their effects on the ovary, leading to the growth and maturation of follicles and the expulsion of the oocyte [54–57]. The ovary is a complex organ, comprised of follicles that are at different stages of development including the quiescent primordial, primary, small pre-antral, antral, and large antral (or preovulatory) follicles. During follicular growth, granulosa cells (GCs) surround the oocyte, a basement membrane forms, and a theca cell (TC) layer develops and surrounds the follicle. The two cell layers act cooperatively in the production of steroid hormones. TC produce androgens that traverse the basement membrane to the GCs, where aromatase converts these androgens to estrogens that regulate FSH and LH release from the pituitary [54].
Inflammation and ovulation
Many hallmarks of inflammation are also observed in the ovary at the time of ovulation. Similar vascular changes include increased blood flow and vascular permeability, and cellular events such as increased leukocyte extravasation and activation occur at the site of inflammation and in the ovulating ovary. In addition, chemical mediators such as prostaglandins, vasoactive amines (histamine and serotonin), cytokines and chemokines are produced both in inflammatory responses and ovulation. The characteristic tissue damage, repair and remodeling that result from inflammation in non-ovarian tissues also occur in the ovary during ovulation [58–64]. Therefore, ovulation is now considered an outcome of acute inflammatory reactions in the ovary.
Leukocytes as the main mediators of ovarian inflammatory responses are a major target of investigation. Leukocytes circulate in the blood, become attracted by chemokines and adhesion molecules in inflamed tissues and traverse the blood vessel wall infiltrating interstitial tissues to reach their sites of action. Cytokines that are initially released by the inflamed tissue play a crucial role in increasing adhesion molecule expression on endothelial cells and in up-regulating the corresponding receptor expression on leukocytes, both of which greatly enhance leukocyte migration to the target tissues [65, 66]. At sites of inflammation, leukocytes and vascular endothelial cells release chemokines and cytokines that accelerate leukocyte recruitment and modulate leukocyte function. Eventually, however, the main function of leukocytes in the ovary is exerted through release of proteases [67, 68]. A number of molecules, including various cytokines, chemokines, and proteases that are commonly associated with immunological responses, are present in the preovulatory human ovary as well as ovaries in animal models [60–63]. Therefore, the last 30 years of research has clearly demonstrated the significance of infiltrating leukocytes in ovarian function. Much of this work implies that the infiltration of leukocytes in periovulatory period is a key event in ovulation [69]. However, the origin of these infiltrating leukocytes, their phenotype, and the mechanisms that govern their trafficking to the ovary are elusive.
Adding the spleen to the HPO axis
Leukocytes are hematopoietic in origin and are produced in the bone marrow. Upon release into the bloodstream, they circulate and infiltrate inflamed tissues or they are stored in lymphoid organs, such as the spleen, for future activation and release. The spleen releases leukocytes following induction of acute inflammation in the heart by ischemic myocardial injury [7, 70, 71]. Using a sophisticated approach that involved the transplantation of spleens from GFP mice into wild type mice, it was demonstrated that splenic leukocytes infiltrate heart tissues during acute inflammation. This finding indicates that at least one role of the spleen is to act as an immediate supplier of leukocytes for tissues that experience acute inflammation. This concept is supported by the fact that upon stimulation by an inflammatory signal, the bone marrow takes days to produce leukocytes whereas splenic leukocytes reach sites of inflammation within minutes to hours [7, 72, 73].
Upon ovulatory gonadotropin stimulation, the ovary experiences an acute inflammatory response. This inflammation is different from responses to infectious insults, as ovulatory inflammation is in response to a normal physiological event, LH stimulation. However, the nature and sequence of the inflammatory events occurring in the preovulatory ovary are essentially identical to those reactions taking place at the sites of infectious inflammation. In particular, the ovary utilizes the same molecular signals that attract leukocytes via the mechanism that governs their infiltration at the site of infections or injuries [11, 15, 74, 75]. Thus, does the spleen serve as a source of infiltrating leukocytes during this period of ovulatory inflammation? To answer this question, a study recently measured sequential changes of leukocyte content in the ovary and spleen after inducing superovulation, by injecting gonadotropins (a bolus injection with PMSG to stimulate follicular growth followed 48 hours later by hCG injection to induce ovulation) [5]. Flow cytometry was employed to count the actual numbers of leukocytes in these two distal organs using CD45 specific fluorescent antibodies. This approach revealed that as intraovarian leukocyte numbers increased, the leukocyte numbers in the spleen sharply decreased. The same inverse relationship was observed in adult rats during the period of proestrus to estrus, when ovulatory inflammation occurs. Lower numbers of leukocytes infiltrated the ovary upon superovulation induction in splenectomized rats [5]. Together, these findings strongly indicate that the spleen supplies leukocytes to the preovulatory ovary.
These findings raise the interesting question, should the spleen be considered a key component of the reproductive axis in regulating ovulation? Are splenic leukocytes under the regulation of LH, progesterone and/or prostaglandins whose ovarian functions are critical for successful ovulation? How does the HPO axis communicate with the spleen to trigger the preovulatory leukocyte release? Unfortunately, none of these questions can be clearly answered at present since very little research on the spleen has been done in relation to its reproductive function. However, a glimpse of the role the spleen might play in ovulation can be gathered from past and current literature.
(1) The impact of splenectomy on female fertility
The removal of the entire spleen, splenectomy, has been a successful surgical procedure for many hematological, immunological, and traumatic conditions. However, although the removal is advantageous in treating the specific disorders, it leaves the patient with a significant defect in both innate and adaptive immune responses. As a consequence, splenectomized patients have a 60- to 100-fold increased risk of sepsis [76]. The ovulatory consequences of splenectomy in humans, however, is difficult to assess as other treatment regimens such as chemotherapy or radiotherapy that often accompany the splenectomy procedure also result in severe damage to ovulatory function. However, there are several animal studies that indicate a role of the spleen in ovarian function. In particular, studies in rodents and rabbits show that splenectomy results in either a delay in ovulation [77], aberrant corpus luteal function [78] or an absence in leuteolysis [79, 80].
(2) Proposed method of communication between HPO axis and spleen
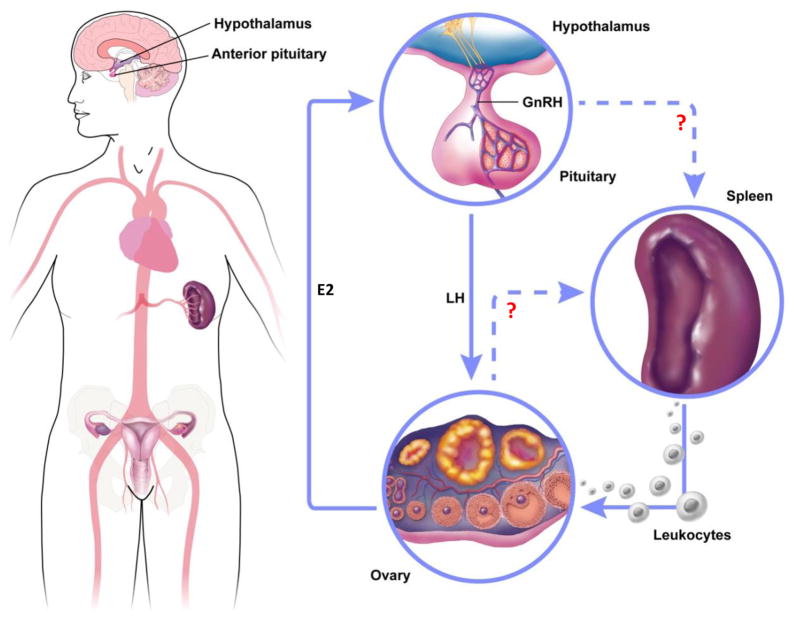
How would the HPO axis communicate with spleen to induce splenic leukocyte release? Since leukocytes are released as early as one hour after ovulatory LH surge, LH may directly stimulate spleen (Fig. 1). However, this is unlikely because the presence of LH receptors in the spleen is reported only in poultry [81], and the spleen does not express LH receptors in mammals [82]. Instead, an endocrine molecule(s) produced by the ovary in response to LH stimulation may travel to spleen via the circulation and stimulate leukocyte release (Fig. 1). In the case of the myocardial injury model, angiotensin II was shown to have such activity [7]. It will be interesting to determine if angiotensin II has this role in the periovulatory release of splenic leukocytes. In support of the potential role of angiotensin II, studies indicate that ovarian angiotensin II synthesis and secretion increases immediately after ovulatory LH/hCG stimulation and ovulation is inhibited if PD123319 or Saralasin, receptor antagonists, are injected into superovulation-induced rabbits and rats, respectively [83–85]. Other candidate molecules that may originate from the ovary and induce splenic leukocyte release include chemokines and cytokines that are produced by the ovary upon LH stimulation. It will be interesting to determine whether receptors for these ovary-borne molecules are present in the spleen and if the receptors are localized in leukocytes or splenic cells. It is however considered that the splenic leukocyte release could be a homeostatic response to the decrease in circulating leukocytes as many of them infiltrate the ovary. Taken together, we propose the following path of splenic leukocyte trafficking to the ovary (Fig. 2). The leukocytes reside in steady state equilibrium within the red pulp of the spleen where they are held via chemokine receptors (CCRs). The LH surge may alter CCR expression on the leukocytes and/or induce chemokine expression in the splenic endothelial cells stimulating mobilization of the leukocytes through the endothelial cell layer of the blood vessel and into the circulation. Leukocytes circulate through the periphery until reaching the ovary where an interaction with appropriate adhesion molecules on the endothelial cell wall occurs. Simultaneously, in the ovary, the LH surge decreases blood flow by dilating vessels and increases expressions of leukocyte receptors such as adhesion molecules so that leukocytes interact with receptors on ovarian endothelial cells.
Figure 1. Proposed interplay between HPO axis and spleen.
Preovulatory rise of E2 (estradiol) stimulates release of LH into the bloodstream. Then LH triggers leukocyte release from the spleen either by directly acting on leukocytes or through indirect effects on splenic tissues. Splenic leukocytes released into the bloodstream migrate to the ovary in response to cytokines and chemokines that act as leukocyte attractants, the cells enter the tissue through interactions between leukocyte receptors and adhesion molecules on the endothelial cells.
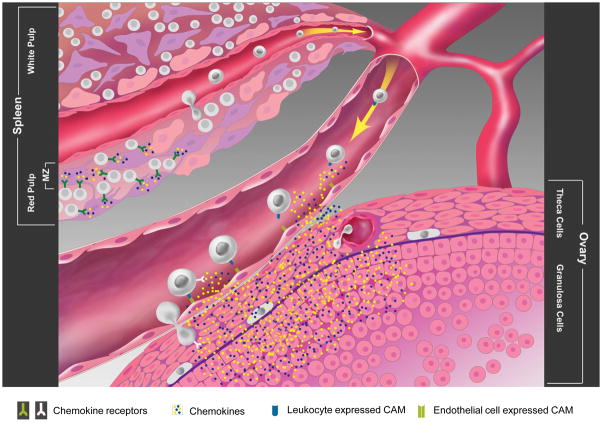
Figure 2. Splenic leukocyte trafficking to the ovary.
Splenic reservoir leukocytes are tethered in the open blood system of the red pulp through either specific chemokine-chemokine receptor or adhesion molecule interactions. Upon LH surge, changes in leukocyte CCR expression or the reduced chemokine production by reticular fibroblast within the red pulp results in the mobilization of splenic reservoir leukocytes into the bloodstream. Upon arriving at the ovary, LH mediated up-regulation of adhesion molecule expression on ovarian endothelial cells, together with LH mediated vasodilation, increases leukocyte adherence to the endothelial cells. Once tethered to the ovarian endothelial cells, leukocytes respond to follicular produced chemokines. The migration of leukocytes through the interstitial space towards the source (GC, TC or ovarian leukocytes) results in the production of MMPs to facilitate movement through the ECM. At the mature follicle, leukocytes become activated by locally produced cytokines and produce reactive oxygen species (ROS), MMPs and proteolytic granules that weaken the basement membrane of the follicle, enabling the release of the oocyte.
Leukocytes in other reproductive organs
Leukocytes also play critical roles in reproductive tissues other than the ovary, such as the uterus [86–88], pituitary [89, 90], oviduct [91–93], testis [94], and vagina [95–97]. Thus, implantation, pregnancy maintenance, embryonic development, menstrual tissue shedding and many other reproductive functions are regulated by leukocytes. With the recent finding that the spleen is a reservoir for leukocytes that rapidly respond to perturbation, it is likely that the spleen may also serve as leukocyte reservoir for these reproductive tissues. In fact, we found a discrepancy between the numbers of leukocytes that leave spleen upon LH stimulation and the combined increase of leukocyte numbers in the bloodstream and ovary [5], indicating that large numbers of splenic leukocytes migrate to other organs that may include uterus and oviduct where increased leukocyte infiltration have been documented [86, 87, 93, 98].
Concluding remarks
The immune system is not only important for battling foreign invaders but is also essential for female reproduction. Here, we propose that the spleen may bridge the immune and reproductive systems serving as a leukocyte reservoir required for the inflammatory events that regulate ovulation. For validation of this hypothesis and determination of the interaction between the reproductive organs and the spleen, rigorous collaborative studies between the fields of immunology and reproductive biology should follow.
Acknowledgments
We thank Dr. Thomas E. Curry for the critical comments and Mr. Tom Dolan for the artwork. This work was supported by National Institutes of Health Grants RO1HD052694 (to C.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urman B, Yakin K. Ovulatory disorders and infertility. J Reprod Med. 2006;51:267–282. [PubMed] [Google Scholar]
- 2.Gibson M. Reproductive health and polycystic ovary syndrome. Am J Med. 1995;98:67S–75S. doi: 10.1016/s0002-9343(99)80061-8. [DOI] [PubMed] [Google Scholar]
- 3.Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149–2160. doi: 10.2165/00003495-200666170-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cremer M, et al. Recent innovations in oral contraception. Semin Reprod Med. 2010;28:140–146. doi: 10.1055/s-0030-1248139. [DOI] [PubMed] [Google Scholar]
- 5.Oakley OR, et al. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology. 2010;151:4551–4559. doi: 10.1210/en.2009-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedin L. Invaders from the spleen: an unexpected origin of the leukocytes participating in ovulation. Endocrinology. 2010;151:4096–4099. doi: 10.1210/en.2010-0669. [DOI] [PubMed] [Google Scholar]
- 7.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitaya K, Yamada H. Pathophysiological Roles of Chemokines in Human Reproduction: An Overview. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00928.x. [DOI] [PubMed] [Google Scholar]
- 9.Runesson E, et al. The human preovulatory follicle is a source of the chemotactic cytokine interleukin-8. Mol Hum Reprod. 1996;2:245–250. doi: 10.1093/molehr/2.4.245. [DOI] [PubMed] [Google Scholar]
- 10.Arici A, et al. Interleukin-8 expression and modulation in human preovulatory follicles and ovarian cells. Endocrinology. 1996;137:3762–3769. doi: 10.1210/endo.137.9.8756544. [DOI] [PubMed] [Google Scholar]
- 11.Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update. 2000;6:1–15. doi: 10.1093/humupd/6.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Karaca T, et al. Distribution and heterogeneity of mast cells in female reproductive tract and ovary on different days of the oestrus cycle in Angora goats. Reprod Domest Anim. 2008;43:451–456. doi: 10.1111/j.1439-0531.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith MP, et al. Leukocyte origin and profile in follicular aspirates at oocyte retrieval. Hum Reprod. 2005;20:3526–3531. doi: 10.1093/humrep/dei240. [DOI] [PubMed] [Google Scholar]
- 14.Bonello N, et al. Periovulatory expression of intercellular adhesion molecule-1 in the rat ovary. Biol Reprod. 2004;71:1384–1390. doi: 10.1095/biolreprod.104.030650. [DOI] [PubMed] [Google Scholar]
- 15.Rohm F, et al. Correlation between expression of selectins and migration of eosinophils into the bovine ovary during the periovulatory period. Cell Tissue Res. 2002;309:313–322. doi: 10.1007/s00441-002-0602-3. [DOI] [PubMed] [Google Scholar]
- 16.Rebecca L, Robker LKA, JoAnne S, Richards C, Smith Wayne, Russell Darryl L. The Inflammatory Response at Ovulation Is Altered in Ovaries of Progesterone Receptor Null (PRKO) Mice. 43rd Annual Meeting of the Society for the Study of Reproduction; 2010. abstract #95. [Google Scholar]
- 17.Simon C, et al. Interleukin-1 receptor antagonist suppresses human chorionic gonadotropin-induced ovulation in the rat. Biol Reprod. 1994;51:662–667. doi: 10.1095/biolreprod51.4.662. [DOI] [PubMed] [Google Scholar]
- 18.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. Journal of cellular and molecular medicine. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murayama C, et al. Effect of VEGF (vascular endothelial growth factor) on expression of IL-8 (interleukin-8), IL-1beta and their receptors in bovine theca cells. Cell Biol Int. 2010;34:531–536. doi: 10.1042/CBI20090498. [DOI] [PubMed] [Google Scholar]
- 20.Kawano Y, et al. The effects of platelet-activating factor on the secretion of interleukin-8 and growth-regulated oncogene alpha in human immortalized granulosa cell line (GC1a) Am J Reprod Immunol. 2007;58:434–439. doi: 10.1111/j.1600-0897.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 21.Liptak AR, et al. Cooperative expression of monocyte chemoattractant protein 1 within the bovine corpus luteum: evidence of immune cell-endothelial cell interactions in a coculture system. Biol Reprod. 2005;72:1169–1176. doi: 10.1095/biolreprod.104.032953. [DOI] [PubMed] [Google Scholar]
- 22.Polec A, et al. Cellular interaction regulates interleukin-8 secretion by granulosa-lutein cells and monocytes/macrophages. Am J Reprod Immunol. 2009;61:85–94. doi: 10.1111/j.1600-0897.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 23.Dahm-Kahler P, et al. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil Steril. 2009;91:231–239. doi: 10.1016/j.fertnstert.2007.07.1330. [DOI] [PubMed] [Google Scholar]
- 24.Ujioka T, et al. Interleukin-8 as an essential factor in the human chorionic gonadotropin-induced rabbit ovulatory process: interleukin-8 induces neutrophil accumulation and activation in ovulation. Biol Reprod. 1998;58:526–530. doi: 10.1095/biolreprod58.2.526. [DOI] [PubMed] [Google Scholar]
- 25.Krensky AM, Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73:193–198. doi: 10.1111/j.1399-0039.2008.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcal JR, et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36:995–999. doi: 10.1016/j.joen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Riese J, et al. Expression of interleukin-6 and monocyte chemoattractant protein-1 by peritoneal sub-mesothelial cells during abdominal operations. J Pathol. 2004;202:34–40. doi: 10.1002/path.1455. [DOI] [PubMed] [Google Scholar]
- 28.Yadav A, et al. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Bornstein SR, et al. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–141. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Wong KH, et al. Expression, hormonal regulation, and cyclic variation of chemokines in the rat ovary: key determinants of the intraovarian residence of representatives of the white blood cell series. Endocrinology. 2002;143:784–791. doi: 10.1210/endo.143.3.8699. [DOI] [PubMed] [Google Scholar]
- 31.Dahm-Kahler P, et al. Monocyte chemotactic protein-1 in the follicle of the menstrual and IVF cycle. Mol Hum Reprod. 2006;12:1–6. doi: 10.1093/molehr/gah256. [DOI] [PubMed] [Google Scholar]
- 32.Cohen PE, et al. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrous cycles and ovulation. Biol Reprod. 1997;56:110–118. doi: 10.1095/biolreprod56.1.110. [DOI] [PubMed] [Google Scholar]
- 33.Cohen PE, et al. Macrophages: important accessory cells for reproductive function. J Leukoc Biol. 1999;66:765–772. doi: 10.1002/jlb.66.5.765. [DOI] [PubMed] [Google Scholar]
- 34.Van der Hoek KH, et al. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod. 2000;62:1059–1066. doi: 10.1095/biolreprod62.4.1059. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel EJ, et al. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 36.Youn BS, et al. Role of the CC chemokine receptor 9/TECK interaction in apoptosis. Apoptosis. 2002;7:271–276. doi: 10.1023/a:1015320321511. [DOI] [PubMed] [Google Scholar]
- 37.Zhou C, et al. Transient expression of CC chemokine TECK in the ovary during ovulation: its potential role in ovulation. Am J Reprod Immunol. 2005;53:238–248. doi: 10.1111/j.1600-0897.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, et al. Potential roles of a special CD8 alpha alpha+ cell population and CC chemokine thymus-expressed chemokine in ovulation related inflammation. J Immunol. 2009;182:596–603. [PMC free article] [PubMed] [Google Scholar]
- 39.Foster R, et al. A differential cytokine expression profile is induced by highly purified human menopausal gonadotropin and recombinant follicle-stimulating hormone in a pre- and postovulatory mouse follicle culture model. Fertil Steril. 2010;93:1464–1476. doi: 10.1016/j.fertnstert.2009.01.136. [DOI] [PubMed] [Google Scholar]
- 40.Buchweitz JP, et al. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicologic pathology. 2007;35:424–435. doi: 10.1080/01926230701302558. [DOI] [PubMed] [Google Scholar]
- 41.Szklarczyk A, Conant K. Matrix metalloproteinases, synaptic injury, and multiple sclerosis. Frontiers in Psychiatry. 2010;1:12. doi: 10.3389/fpsyt.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jo M, Curry TE., Jr Regulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biol Reprod. 2004;71:1796–1806. doi: 10.1095/biolreprod.104.031823. [DOI] [PubMed] [Google Scholar]
- 43.Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 44.Duffy DM, Stouffer RL. Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: modulation of luteinizing hormone effects by prostaglandins. Endocrine. 2003;22:249–256. doi: 10.1385/ENDO:22:3:249. [DOI] [PubMed] [Google Scholar]
- 45.Fedorcsak P, et al. Differential release of matrix metalloproteinases and tissue inhibitors of metalloproteinases by human granulosa-lutein cells and ovarian leukocytes. Endocrinology. 2010;151:1290–1298. doi: 10.1210/en.2009-0605. [DOI] [PubMed] [Google Scholar]
- 46.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 47.Reisinger K, et al. The gonadotropins: tissue-specific angiogenic factors? Mol Cell Endocrinol. 2007;269:65–80. doi: 10.1016/j.mce.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury MW, et al. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology. 2010;73:856–872. doi: 10.1016/j.theriogenology.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Stouffer RL, et al. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. doi: 10.2741/2065. [DOI] [PubMed] [Google Scholar]
- 50.Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121:181–186. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- 51.Heissig B, et al. Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration. Histol Histopathol. 2010;25:765–770. doi: 10.14670/HH-25.765. [DOI] [PubMed] [Google Scholar]
- 52.Guimera M, et al. LH/HCG stimulation of VEGF and adrenomedullin production by follicular fluid macrophages and luteinized granulosa cells. Reprod Biomed Online. 2009;18:743–749. doi: 10.1016/s1472-6483(10)60021-1. [DOI] [PubMed] [Google Scholar]
- 53.Belotti D, et al. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol Cancer Res. 2008;6:525–534. doi: 10.1158/1541-7786.MCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 54.Channing CP, et al. Ovarian follicular and luteal physiology. Int Rev Physiol. 1980;22:117–201. [PubMed] [Google Scholar]
- 55.Bliss SP, et al. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31:322–340. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- 57.Doufas AG, Mastorakos G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann N Y Acad Sci. 2000;900:65–76. doi: 10.1111/j.1749-6632.2000.tb06217.x. [DOI] [PubMed] [Google Scholar]
- 58.Zeineh K, et al. Possible modulators of IL-8 and GRO-alpha production by granulosa cells. American journal of reproductive immunology. 2003;50:98–103. doi: 10.1034/j.1600-0897.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 59.Jokubkiene L, et al. Assessment of changes in volume and vascularity of the ovaries during the normal menstrual cycle using three-dimensional power Doppler ultrasound. Human reproduction. 2006;21:2661–2668. doi: 10.1093/humrep/del211. [DOI] [PubMed] [Google Scholar]
- 60.Shimada M, et al. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–2502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- 61.Son DS, Roby KF. Interleukin-1alpha-induced chemokines in mouse granulosa cells: impact on keratinocyte chemoattractant chemokine, a CXC subfamily. Mol Endocrinol. 2006;20:2999–3013. doi: 10.1210/me.2006-0001. [DOI] [PubMed] [Google Scholar]
- 62.Szlosarek PW, et al. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 63.Karstrom-Encrantz L, et al. Selective presence of the chemokine growth-regulated oncogene alpha (GROalpha) in the human follicle and secretion from cultured granulosa-lutein cells at ovulation. Mol Hum Reprod. 1998;4:1077–1083. doi: 10.1093/molehr/4.11.1077. [DOI] [PubMed] [Google Scholar]
- 64.Jasper MJ, et al. Characterization of ovarian function in granulocyte-macrophage colony-stimulating factor-deficient mice. Biology of reproduction. 2000;62:704–713. doi: 10.1095/biolreprod62.3.704. [DOI] [PubMed] [Google Scholar]
- 65.Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Adv Dermatol. 2004;20:163–201. [PubMed] [Google Scholar]
- 66.Radi ZA, et al. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med. 2001;15:516–529. doi: 10.1892/0891-6640(2001)015<0516:camlta>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 67.Korpos E, et al. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15:1349–1357. doi: 10.2174/138161209787846685. [DOI] [PubMed] [Google Scholar]
- 68.Weber C, Koenen RR. Fine-tuning leukocyte responses: towards a chemokine ‘interactome’. Trends Immunol. 2006;27:268–273. doi: 10.1016/j.it.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Pate JL, et al. The interface of the immune and reproductive systems in the ovary: lessons learned from the corpus luteum of domestic animal models. Am J Reprod Immunol. 2010;64:275–286. doi: 10.1111/j.1600-0897.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 70.Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 2010;67:2685–2693. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leuschner F, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popovich PG, Hickey WF. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J Neuropathol Exp Neurol. 2001;60:676–685. doi: 10.1093/jnen/60.7.676. [DOI] [PubMed] [Google Scholar]
- 73.Jovcic G, et al. Acute sterile inflammation--correlation between cellular changes and extramedullary-produced regulators in vivo. Ann Hematol. 1993;66:195–201. doi: 10.1007/BF01703235. [DOI] [PubMed] [Google Scholar]
- 74.Brannstrom M, et al. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod. 1993;48:277–286. doi: 10.1095/biolreprod48.2.277. [DOI] [PubMed] [Google Scholar]
- 75.Godiska R, et al. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tracy ET, Rice HE. Partial splenectomy for hereditary spherocytosis. Pediatr Clin North Am. 2008;55:503–519. x. doi: 10.1016/j.pcl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Matsuyama S, et al. The critical period in which splenectomy causes functional disorder of the ovary in adult rats. Endocrinol Jpn. 1987;34:849–855. doi: 10.1507/endocrj1954.34.849. [DOI] [PubMed] [Google Scholar]
- 78.Saito S, et al. Involvement of splenocytes in the control of corpus luteum function in the rat. Endocrinol Jpn. 1988;35:891–898. doi: 10.1507/endocrj1954.35.891. [DOI] [PubMed] [Google Scholar]
- 79.Endo T, Kanayama K. Effects of splenectomy on luteal function in pseudopregnant rabbits. J Int Med Res. 1998;26:93–97. doi: 10.1177/030006059802600206. [DOI] [PubMed] [Google Scholar]
- 80.Nariai K, et al. Effects of splenectomy on luteolysis in pseudopregnant rabbits. J Vet Med Sci. 1995;57:503–505. doi: 10.1292/jvms.57.503. [DOI] [PubMed] [Google Scholar]
- 81.You S, et al. Three different turkey luteinizing hormone receptor (tLH-R) isoforms I: characterization of alternatively spliced tLH-R isoforms and their regulated expression in diverse tissues. Biol Reprod. 2000;62:108–116. doi: 10.1095/biolreprod62.1.108. [DOI] [PubMed] [Google Scholar]
- 82.Leuschner C, Hansel W. Targeting breast and prostate cancers through their hormone receptors. Biol Reprod. 2005;73:860–865. doi: 10.1095/biolreprod.105.043471. [DOI] [PubMed] [Google Scholar]
- 83.Yoshimura Y, et al. Gonadotropin stimulates ovarian renin-angiotensin system in the rabbit. J Clin Invest. 1994;93:180–187. doi: 10.1172/JCI116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pellicer A, et al. Blockage of ovulation by an angiotensin antagonist. Science. 1988;240:1660–1661. doi: 10.1126/science.3381087. [DOI] [PubMed] [Google Scholar]
- 85.Kuji N, et al. Involvement of angiotensin II in the process of gonadotropin-induced ovulation in rabbits. Biol Reprod. 1996;55:984–991. doi: 10.1095/biolreprod55.5.984. [DOI] [PubMed] [Google Scholar]
- 86.Gomez-Lopez N, et al. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 87.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 88.Christiaens I, et al. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Barbieri F, et al. Role of stromal cell-derived factor 1 (SDF1/CXCL12) in regulating anterior pituitary function. J Mol Endocrinol. 2007;38:383–389. doi: 10.1677/JME-06-0014. [DOI] [PubMed] [Google Scholar]
- 90.Callewaere C, et al. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol. 2007;38:355–363. doi: 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Martinez H, et al. Boar spermatozoa in the oviduct. Theriogenology. 2005;63:514–535. doi: 10.1016/j.theriogenology.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 92.Matsuda H, et al. Tissue concentrations of eosinophilia in the bovine oviduct and uterus of different stages of the oestrous cycle. Res Vet Sci. 1983;34:369–370. [PubMed] [Google Scholar]
- 93.van Bogaert LJ, et al. The percentage of granulocyte-like cells in human oviduct epithelium. Br J Obstet Gynaecol. 1978;85:373–375. doi: 10.1111/j.1471-0528.1978.tb14897.x. [DOI] [PubMed] [Google Scholar]
- 94.Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod. 1997;2:38–47. doi: 10.1530/ror.0.0020038. [DOI] [PubMed] [Google Scholar]
- 95.Chen B, et al. Elastin metabolism in pelvic tissues: is it modulated by reproductive hormones? Am J Obstet Gynecol. 2005;192:1605–1613. doi: 10.1016/j.ajog.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 96.Givan AL, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 97.Hill JA, Anderson DJ. Human vaginal leukocytes and the effects of vaginal fluid on lymphocyte and macrophage defense functions. Am J Obstet Gynecol. 1992;166:720–726. doi: 10.1016/0002-9378(92)91703-d. [DOI] [PubMed] [Google Scholar]
- 98.Li TC, et al. Histological and clinical features of menstruation induced by the antiprogestin mifepristone (RU486) compared to menstruation occurring spontaneously. J Obstet Gynaecol (Lahore) 1990;10:411–414. doi: 10.3109/01443619009151233. [DOI] [PubMed] [Google Scholar]
- 99.Bowen JM, Keyes PL. The proestrous prolactin surge is not the sole initiator of regressive changes in corpora lutea of normally cycling rats. Biol Reprod. 1999;61:1208–1215. doi: 10.1095/biolreprod61.5.1208. [DOI] [PubMed] [Google Scholar]
- 100.Bowen JM, et al. Luteal regression in the normally cycling rat: apoptosis, monocyte chemoattractant protein-1, and inflammatory cell involvement. Biol Reprod. 1999;60:740–746. doi: 10.1095/biolreprod60.3.740. [DOI] [PubMed] [Google Scholar]
- 101.Hameed A, et al. Perforin expression in human cell-mediated luteolysis. Int J Gynecol Pathol. 1995;14:151–157. doi: 10.1097/00004347-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 102.Olson KK, Townson DH. Prolactin-induced expression of intercellular adhesion molecule-1 and the accumulation of monocytes/macrophages during regression of the rat corpus luteum. Biol Reprod. 2000;62:1571–1578. doi: 10.1095/biolreprod62.6.1571. [DOI] [PubMed] [Google Scholar]
- 103.Olson KK, et al. Actions of prostaglandin F2alpha and prolactin on intercellular adhesion molecule-1 expression and monocyte/macrophage accumulation in the rat corpus luteum. Biol Reprod. 2001;64:890–897. doi: 10.1095/biolreprod64.3.890. [DOI] [PubMed] [Google Scholar]
- 104.Nagaosa K, et al. Determination of cell type specificity and estrous cycle dependency of monocyte chemoattractant protein-1 expression in corpora lutea of normally cycling rats in relation to apoptosis and monocyte/macrophage accumulation. Biol Reprod. 2002;67:1502–1508. doi: 10.1095/biolreprod.102.005009. [DOI] [PubMed] [Google Scholar]
- 105.Bowen JM, et al. Prolactin-induced regression of the rat corpus luteum: expression of monocyte chemoattractant protein-1 and invasion of macrophages. Biol Reprod. 1996;54:1120–1127. doi: 10.1095/biolreprod54.5.1120. [DOI] [PubMed] [Google Scholar]
- 106.Kuranaga E, et al. Fas/Fas ligand system in prolactin-induced apoptosis in rat corpus luteum: possible role of luteal immune cells. Biochem Biophys Res Commun. 1999;260:167–173. doi: 10.1006/bbrc.1999.0858. [DOI] [PubMed] [Google Scholar]
- 107.Wu R, et al. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22:527–535. doi: 10.1093/humrep/del371. [DOI] [PubMed] [Google Scholar]
- 108.Gaytan F, et al. Role of prolactin in the regulation of macrophages and in the proliferative activity of vascular cells in newly formed and regressing rat corpora lutea. Biol Reprod. 1997;57:478–486. doi: 10.1095/biolreprod57.2.478. [DOI] [PubMed] [Google Scholar]
- 109.Brannstrom M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol. 2002;57:47–60. doi: 10.1016/s0165-0378(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 110.Kucharski J, Jana B. Immuno-endocrine mechanisms connected with the creation of corpora lutea persistent in animal ovaries. Pol J Vet Sci. 2005;8:255–259. [PubMed] [Google Scholar]
- 111.Gaytan F, et al. Effects of indomethacin on ovarian leukocytes during the periovulatory period in the rat. Reprod Biol Endocrinol. 2003;1:26. doi: 10.1186/1477-7827-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Das S, et al. Follicular fluid expression of alpha-defensins and their role in ovulation. J Assist Reprod Genet. 2008;25:83–87. doi: 10.1007/s10815-007-9197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Nefiawy N, et al. Role of prostaglandin E2 receptor subtypes in ovarian follicle growth in the rat in vivo. Correlation with interleukin-8 and neutrophils. Histol Histopathol. 2005;20:825–831. doi: 10.14670/HH-20.825. [DOI] [PubMed] [Google Scholar]
- 114.Fainaru O, et al. CD56brightCD16- natural killer cells accumulate in the ovarian follicular fluid of patients undergoing in vitro fertilization. Fertil Steril. 2010;94:1918–1921. doi: 10.1016/j.fertnstert.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 115.Karaca T, Simsek N. Effects of spirulina on the number of ovary mast cells in lead-induced toxicity in rats. Phytother Res. 2007;21:44–46. doi: 10.1002/ptr.2015. [DOI] [PubMed] [Google Scholar]
- 116.Gaytan F, et al. Estrous cycle-related changes in mast cell numbers in several ovarian compartments in the rat. Biol Reprod. 1991;45:27–33. doi: 10.1095/biolreprod45.1.27. [DOI] [PubMed] [Google Scholar]
- 117.Vogel B, et al. Bovine eotaxin (CCL11)--an unusual member of the eotaxin group--attracts eosinophils in vitro but is not responsible for eosinophilia in the ovary. Vet Immunol Immunopathol. 2005;107:67–77. doi: 10.1016/j.vetimm.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 118.Yuan GH, et al. Role of chemokines/chemokine receptor systems in cartilage degradation. Drug News Perspect. 2001;14:591–600. [PubMed] [Google Scholar]
- 119.Skinner MK, et al. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–1472. doi: 10.1002/mrd.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eberlein J, et al. Comprehensive assessment of chemokine expression profiles by flow cytometry. J Clin Invest. 2010;120:907–923. doi: 10.1172/JCI40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 122.Dewan MZ, et al. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Kalinkovich A, et al. Blood-forming stem cells are nervous: direct and indirect regulation of immature human CD34+ cells by the nervous system. Brain Behav Immun. 2009;23:1059–1065. doi: 10.1016/j.bbi.2009.03.008. [DOI] [PubMed] [Google Scholar]