Abstract
Background
Signal transducer and activator of transcription (Stat)-3 and nuclear factor-kappa B (NF-κB) are important signaling pathways constitutively activated during inflammation. We previously reported that high-fat diet (HFD) intake induces oxidative stress in the prostate through elevated expression of NADPH oxidase subunits causing NF-κB activation. We sought to determine whether Stat-3 is involved in the activation of NF-κB in the prostate as a result of HFD feeding, leading to inflammation.
Methods
C57BL/6 mice were either fed with regular diet (RD) or HFD for 4 and 8 weeks. Plasma cytokine levels were determined by multiplex analysis. Western blotting was performed to determine the expression of NF-κB, Stat-3, Akt, PDK1, PKCε and their phosphorylated forms along with pathologic evaluation of the prostate. Immunoprecipitation and electrophoretic mobility shift assay were conducted to study the association between Stat-3 and NF-κB.
Results
C57BL/6 mice fed with HFD showed a significant increase in the plasma levels of IL-1β, IL-6, IL-17 and TNFα after 4 and 8 weeks of feeding, compared with RD controls. HFD feeding elevated the intraprostatic expression of IL-6 and caused activation of PKCε and Akt, the upstream kinase regulating Stat-3 and NF-κB. Nuclear extracts from the prostates of mice fed with HFD exhibited constitutively activated levels of Stat-3 and NF-κB/p65. Increased association between the activated forms of Stat-3 and NF-κB/p65 was observed in the nucleus as a result of HFD feeding, a finding that was accompanied by morphologic evidence of increased intraprostatic inflammation.
Conclusions
Our findings suggest that HFD activates Stat-3 and NF-κB/p65 in the prostate, and their interaction is associated with increased inflammation in the prostate.
Keywords: inflammation, high-fat diet, prostate, Stat-3, NF-κB
INTRODUCTION
Consumption of nutrients with high caloric density can result in a metabolic syndrome with symptoms associated with insulin resistance, hyperinsulinemia, dyslipidemia and some degree of obesity [1–3]. The incidence of this metabolic syndrome is high in Western nations, probably due to high intake of total fat, saturated fat and cholesterol-rich diets associated with sedentary lifestyles [2, 3]. Consequently, this metabolic syndrome is thought to be a major contributor to the development of diabetes, cardiovascular complications, and a variety of urologic disorders, including urinary incontinence, erectile dysfunction, benign prostatic hyperplasia and cancer [1–5].
In our earlier studies, we demonstrated that prolonged consumption of high-fat diet (HFD) induces oxidative stress and inflammation in the prostate of NF-κB-Luc reporter mice [6]. There is a general consensus that metabolic diseases are commonly linked to the inflammatory process, characterized by a low-grade and chronic activation of the inflammatory response, a phenomenon recently denoted ‘metabolically triggered inflammation’ or meta-inflammation [1, 7]. The physiological mechanisms linking HFD to inflammation include production of various adipocytokines viz. adiponectin, resistin or leptin and pro-inflammatory cytokines such as IL-1, IL-6 and TNFα by the expanded adipose tissue [1, 7]. These pro-inflammatory cytokines are thought to affect certain target tissues, causing inflammation via activation of transcription factors that result in the recruitment and activation of macrophages and lymphocyte infiltration [8].
Signal transducer and activator of transcription (Stat)-3 and nuclear factor-kappa B (NF-κB) belong to a family of transcription factors that are activated in response to inflammatory mediators [9, 10]. They also have a role as core transcription factors in various immune responses [11]. The similarity in these pathways is that they are essential for cytosolic signals from external stimuli and function as transcription factors required for regulating genes involved in proliferation, survival, angiogenesis, invasion and inflammation [12]. Pro-inflammatory cytokines have been shown to activate both Stat-3 and NF-κB and are also their transcriptional targets, establishing a feedback loop [11, 12]. Upregulation of NF-κB during inflammation results in the recruitment of inflammatory mediators leading to the production of various pro-inflammatory cytokines, such as IL-1, IL-6 and IL-8 at the site of inflammation, whereas Stat-3 initiates the activation of IL-17 and IL-23 [9, 13]. IL-6 generated by NF-κB persistently activates Stat-3 in epithelial and immune cells [11]. In fact, in the pro-inflammatory microenvironment, IL-1 and IL-6 stimulation causes association between Stat-3 and NF-κB in the nucleus. During this association Stat-3 maintains persistent activation of NF-κB/p65, which prevents its expulsion from the nucleus [14].
In our previous studies we demonstrated that IL-1β-induced NF-κB activity plays a role in chemoattraction, stimulating the initial phase of prostatic inflammation, and may also play a role in the development and maintenance of chronic inflammation and proliferative inflammatory atrophy (PIA) in the prostate [15]. Chronic inflammation and proliferative inflammatory atrophy are regarded by some investigators as putative precursor lesions in the development of prostrate cancer [16]. Recently, it has been shown that HFD is a stimulator for NF-κB responses in mice with diet-induced obesity [17]. We have shown that HFD caused oxidative stress in the prostate, activating NF-κB and possibly contributing to intraprostatic inflammation [6]. In this study our objective was to investigate the mechanism involved in the activation of NF-κB as a result of HFD and elucidate the role played by Stat-3, another possible contributor to prostatic inflammation.
MATERIALS AND METHODS
Animals
Forty non-transgenic C57BL/6 males raised from the colony of NF-κB-Luciferase transgenic mice (purchased from Jackson Laboratory, Bar Harbor, Maine) were used for these experiments. The animals were bred and maintained at the AAA-LAC accredited Animal Resource Facility of Case Western Reserve University. All animal experiments were performed according to institutional guidelines for animal care. The mice were matched according to age and divided into two groups. One group was fed with regular diet (RD) and the other was subjected to high-fat diet (HFD) typical of ‘Western style’ high-fat diet. The HFD formula consisted of rodent chow containing of 23% fat, 43% carbohydrate, and 20% of protein. In contrast, RD consisted of standard rodent chow containing 4.5% fat, 56% complex carbohydrate, and 23% protein (all from Harlan Labs Inc., Madison, WI). The animals were kept on these diets for 4 and 8 weeks respectively, sacrificed and used for in vivo and ex vivo experiments.
Materials
Antibodies for anti-Akt, anti-phospho-Akt (Ser473), anti-phospho-NF-κB/p65 (Ser536), anti-Stat-3, anti-phospho-Stat-3 (Tyr705 and Ser727), and anti-PDK1 were purchased from Cell Signaling Technology (Danvers, MA). Anti-PKCε, anti-NF-κB/p65, anti-Oct-1, anti-β-actin and secondary antibodies for mouse and rabbit (horseradish peroxidase conjugates) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced Chemiluminescence Kit (ECL) for chemiluminescence was purchased from GE Healthcare Biosciences (Piscataway, NJ).
Cytokine quantitation by Luminex™ assay
Cytokine levels were determined by the Beadlyte™ mouse multi-cytokine detection (Bio-Rad, Hercules, CA). Briefly, filter bottom ELISA plates were rinsed with 100µL of Bio-plex assay buffer and liquid was removed using a Millipore™ Multiscreen Separation Vacuum Manifold System set at 5mm Hg. Analyte beads in assay buffer were added to the wells followed by 50µL of plasma or standard solution. The plates were incubated for 30 min at room temperature with continuous shaking using a Lab-Line™ Instrument Titer Plate Shaker. The filter bottom plates were washed, as before, and centrifuged at 300 × g for 30 seconds. Subsequently, 50 µL of anti-mouse IL-1β, IL-6, IL-17, TNFα antibody-biotin reporter solution was added in each well, after which the plates were incubated with continuous shaking for 30 min followed by centrifugation and washing. Next, 50µL streptavidin-phycoerythrin (PE) solution was added, and the plates were incubated with continuous shaking for 10 min at room temperature. 125µL of Bio-plex assay buffer was added, and Beadlyte™ readings were measured using a Luminex™ System and calculated using Bio-plex™ software (Bio-Rad).
Western blotting analysis
Prostrate tissue from C57BL/6 male mice fed with RD and HFD were processed for total, cytosolic and nuclear lysates as previously described [6]. The collected supernatant constituted the nuclear fraction. All the fractions were stored at −80°C and were used for Western blotting.
For Western blotting, 40µg proteins was resolved over 4–20% Tris-glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane. The blots were blocked using 5% nonfat dry milk and probed using appropriate primary antibodies overnight at 4°C. The membrane was then incubated with appropriate secondary antibody horseradish peroxidase conjugate (Santa Cruz Biotech) followed by detection using chemiluminescence ECL kit (GE Healthcare Biosciences). For equal loading of proteins, the membrane was probed with appropriate loading controls. Densitometric measurements of the bands in Western blot analysis were done using digitalized scientific software program using Kodak 2000R imaging system.
Immunoprecipitation
Total tissue lysate from the prostrate of mice fed with RD and HFD (200µg) was immunoprecipitated with 2µg appropriate primary antibody and was incubated at 4°C for 3 hrs. Protein A/G agarose beads (20µL) were added and incubated overnight at 4°C. Immunoprecipitated proteins were washed four times with lysis buffer, electrophoresed by SDS-PAGE, and analyzed by Western blotting as previously described [18].
Electrophoretic Mobility Shift Assay (EMSA)
EMSA for NF-κB, Stat-3 and Stat-3-κB domain were performed with the appropriate oligonucleotides using the Lightshift™ Chemiluminiscent EMSA kit (Pierce Biotechnology, Rockford, IL). Double stranded oligoneucleotides were used for NF-κB: Forward (5’-AGT TGA GGG GAC TTT CCC AGG C-3’) Reverse: (3’-TCA ACT CCC CTG AAA GGG TCC G-5’), Stat-3, Forward (5’-GAT CCT TCT GGG AAT TCC TAG ATC-3’) Reverse: (3’-CTA GGA AGA CCC TTA AGG ATC TAG-5’) and Stat-3-κB domain, Forward (AGT TGA GGG GAC TTT CCC AGG C), Reverse: (GAT CCT TCT GGG AAT TCC TAG ATC), respectively. The assay was performed following manufacturer’s instructions as previously described [19].
Prostate histology
Routine H&E-stained histological sections from the formalin-fixed paraffin-embedded whole mount mouse prostate specimens from animals fed with RD and HFD were evaluated and compared, with particular emphasis on assessing the degree of inflammation in each set of specimens.
Statistical Analysis
The values are expressed as mean ± SD. The significance of measured values between the groups with RD and HFD were performed by Student’s t-test for Western blotting densitometric analysis and by one-way analysis of variance (ANOVA) with 95% confidence limits for multiplex analysis. The p values less than 0.05 were taken as significant in experiments.
RESULTS
HFD increases plasma levels of pro-inflammatory cytokines in C57BL/6 mice
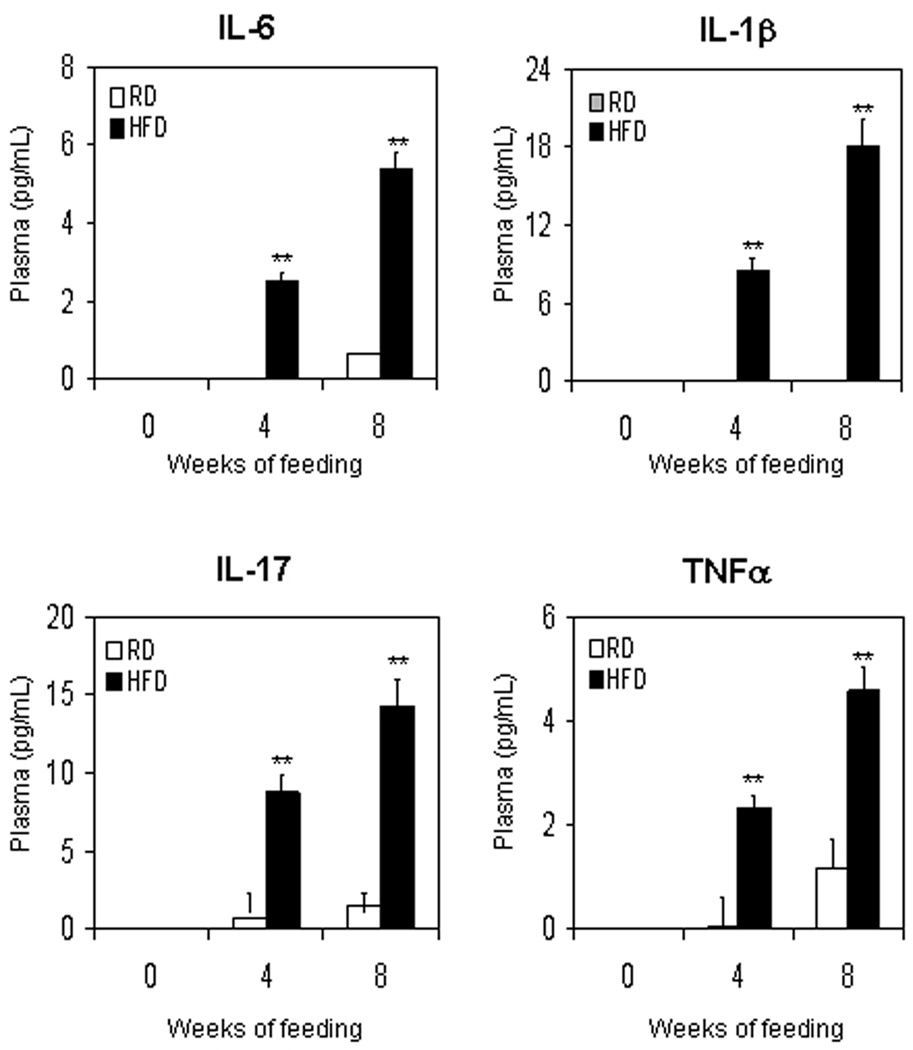
We have recently reported that HFD causes oxidative stress in the prostate leading to inflammation [6]. HFD has been reported to increase the expression of pro-inflammatory cytokines viz. IL-6, IL-1β, and TNFα in the hypothalamus and other peripheral tissues [20]. These pro-inflammatory cytokines are transcriptional target of NF-κB and Stat-3 [11–14]. We determined whether HFD feeding caused alterations in the plasma levels of IL-6, IL-1β, IL-17 and TNFα. We observed that HFD fed mice showed a significant increase in the levels of all these cytokines after 4 weeks of feeding that further increased after 8 weeks, compared to mice on RD diet. Compared to RD, HFD resulted in 2.5- and 8.1- fold increase (p<0.0001) in IL-6; 8.5- and 150- fold increase (p<0.0001) in IL-1β; 12.8- and 9.4- fold increase (p<0.0001) in IL-17; and 46.3- and 3.9- fold increase (p<0.0001) in TNFα after 4 and 8 weeks, respectively (Fig 1).
Figure 1.
Plasma cytokines levels in C57BL/6 mice after feeding with regular diet (RD) and high-fat diet (HFD). Plasma was collected from mice fed with RD or HFD for 4 and 8 weeks and were used for measuring the levels of IL-6, IL-1β, IL-17 and TNF-α by the Beadlyte™ mouse multi-cytokine detection assay. White bars indicate RD and black bars indicate HFD. Data are a mean of ± SD. **p<0.001, represents the significant changes in cytokine levels by HFD compared to its respective RD treated group.
HFD increases expression of IL-6, PKCε and p-Akt (Ser473) in the prostates of C57BL/6 mice
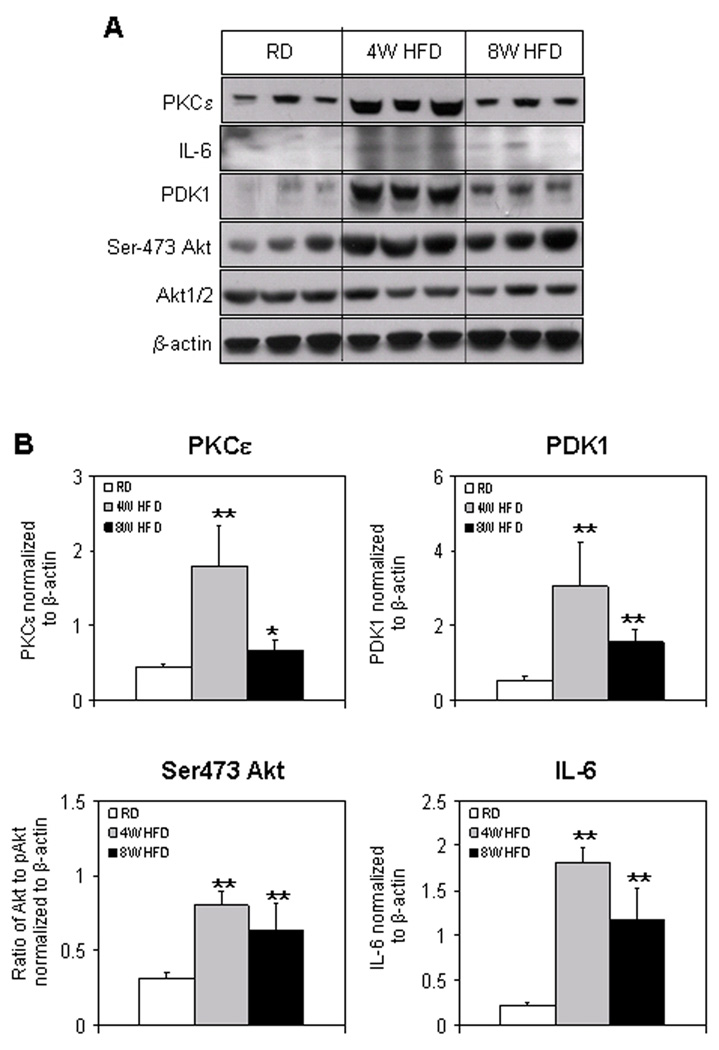
IL-6 and PKCε are reported to be upstream and activate Stat-3 [21], whereas Akt has been shown to induce NF-κB [22]. Therefore, next we determine the alterations in the levels of these proteins as a consequence of HFD in the prostate. We observed that HFD feeding caused an increase in the IL-6 protein expression. An 8.1-fold increase (p<0.001) at 4 weeks and 5.3-fold increase (p<0.04) at 8 weeks in the levels of IL-6 was observed in the HFD group compared with RD group. Similarly, a significant increase in the protein expression of PKCε was observed after HFD feeding. A 4.4-fold increase (p<0.005) was observed at 4 weeks which reduced to 1.47-fold (p<0.05) at 8 weeks of HFD feeding (Fig. 2A&B).
Figure 2.
Protein expression of PKCε, IL-6, PDK1, Akt and p-Akt (Ser473) in the prostrate of C57BL/6 mice fed with RD and HFD. (A) Total tissue extract were prepared as described in the Materials and Methods Section from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and β-actin was used as the loading control. (B) Densitometric quantification represents changes in the levels of PKCε, PDK1, p-Akt (Ser473), and IL-6. White bars indicate RD, gray bars indicate HFD for 4 weeks and black bars indicate HFD for 8 weeks. Data are a mean of ± SD and corrected for loading. The single asterisk (*) indicates changes (p<0.05) and double asterisk (**) indicates the significant changes (p<0.001) in HFD compared to RD fed controls.
To examine the changes in the protein expression of Akt and p-Akt (Ser473) and PDK1, we performed Western blotting using total tissue lysates of prostates from mice fed with HFD and RD, respectively. A 2.5-fold increase (p<0.001) in the phosphorylated form of Akt (Ser473) which was almost consistent to 2.2-fold (p<0.05) at 8 weeks, whereas no relevant changes were observed in the Akt protein expression after HFD feeding. Moreover, a significant increase in the levels of PDK1 was observed in HFD group. A 5.6-fold increase (p<0.001) at 4 weeks and 2.9-fold increase at 8 weeks in PDK1 was observed in HFD group, compared to RD group (Fig. 2A&B).
HFD causes intraprostatic activation of NF-κB and Stat-3 in C57BL/6 mice
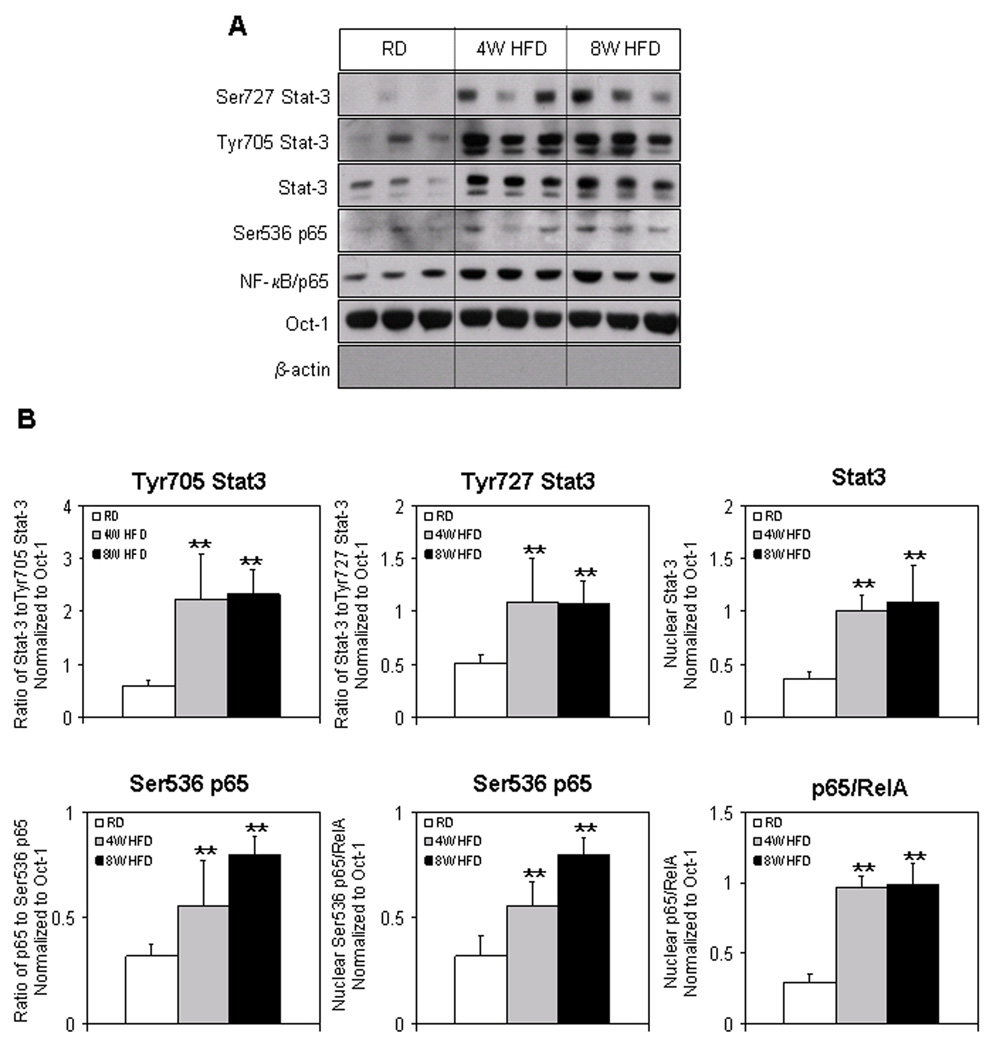
We recently reported that HFD increased the intraprostatic NF-κB in the nucleus, which was further confirmed by imaging studies [6]. Next our goal was to determine if HFD caused NF-κB activation was due to phosphorylation of NF-κB/p65 at Ser536. A significant increase in the phosphorylation of p65 at Ser536 was observed at 4 weeks (2.8-fold; p<0.05) and 2.5-fold (p<0.01) at 8 weeks, which corroborated with the increased nuclear levels of NF-κB/p65 in the prostate after HFD feeding (Fig. 3A&B).
Figure 3.
Protein expression of Ser727 Stat-3, Tyr705 Stat-3, Stat-3, Ser536 p65, NF-κB/p65 in the nuclear fraction extracted from the prostrate of RD and HFD fed C57BL/6 mice. (A) Nuclear extract were prepared as described in the Materials and Methods Section from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and Oct-1 was used as the loading control. (B) Densitometric quantification represents changes in the levels of Tyr705 Stat-3, Ser727 Stat-3, Stat-3, Ser536 p65, NF-κB/p65. White bars indicate RD, gray bars indicate HFD for 4 weeks and black bars indicate HFD for 8 weeks. Data are a mean of ± SD and corrected for loading. The double asterisk (**) indicates the significant changes (p<0.001) in HFD compared to RD fed controls.
It is well established that phosphorylation of Stat-3 at Tyr705 causes its translocation into the nucleus whereas phosphorylation at Ser727 results in its nuclear activation [21]. Next we determine the levels of Stat-3 and its phosphorylated form in the nucleus of HFD and RD fed groups. Feeding HFD to mice increased the nuclear Stat-3 levels and its activated forms in the prostates at 4 and 8 weeks. Stat-3 levels were elevated by 2.8-fold (p<0.01) after 4 weeks and were sustained after 8 weeks (3.0-fold; p<0.01) of HFD feeding. Similarly, phosphorylation of Stat-3 at Tyr705 was increased more than 3-fold (p<0.05) after 4 and 8 weeks of HFD feeding, compared to RD controls. A 2.0-fold increase (p<0.01) was observed in Stat-3 phosphorylation at Ser727 after 4 and 8 weeks of HFD feeding (Fig. 3A&B).
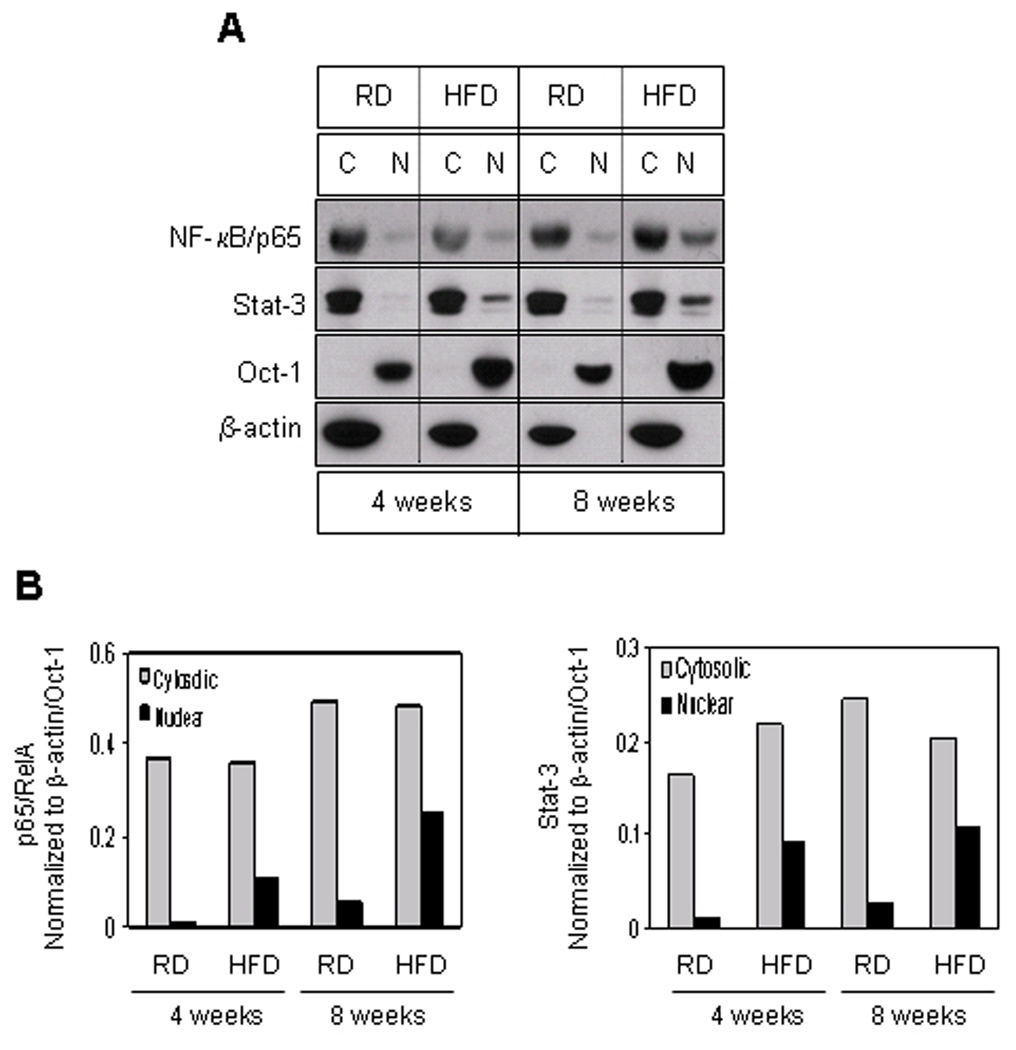
Next we determined the distribution of NF-κB/p65 and Stat-3 in the cytosolic and nuclear faction from the prostates of mice fed with HFD and RD. Compared to RD control, a significant increase in the translocation of Stat-3 and NF-κB/p65 was observed in the nucleus at 4 weeks, which further increased at 8 weeks as a consequence of HFD feeding (Fig. 4 A&B).
Figure 4.
Protein expressions of NF-κB/p65 and Stat-3 in the cytosolic and nuclear fraction extracted from the prostrate of RD and HFD fed C57BL/6 mice. (A) Cytosolic and nuclear extract were prepared as described in the Materials and Methods Section from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and Oct-1 and β-actin were used as the loading controls. (B) Densitometric quantification represents changes in the levels of NF-κB/p65 and Stat-3. Gray bars indicate cytosloic fraction and black bars indicate nuclear fraction.
HFD induced intraprostatic association of Stat-3 and NF-κB in C57BL/6 mice
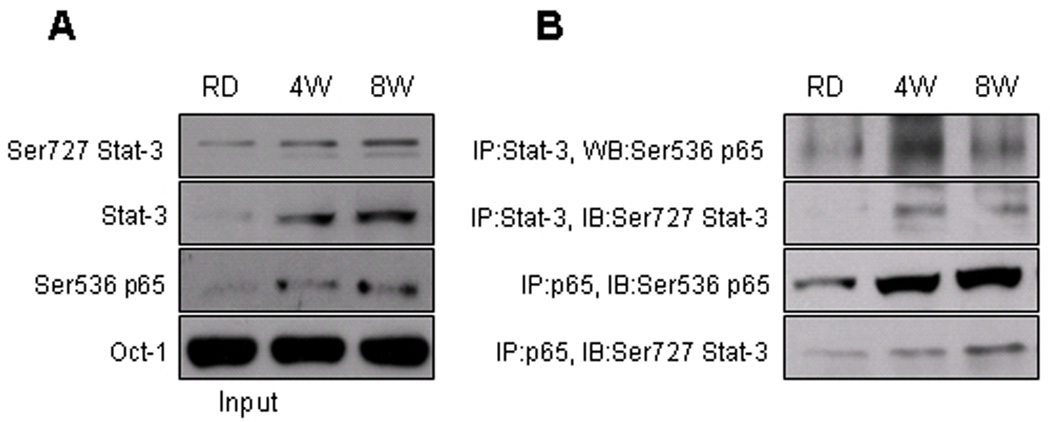
Previous studies have demonstrated the crosstalk between Stat-3 and NF-κB at multiple levels, this comprise activation of Stat-3 by NF-κB-regulated factors such as IL-6 and COX-2 [23, 24] and nuclear translocation of unphosphorylated NF-κB by unphosphorylated Stat-3 [25]. Recently it has been shown that NF-κB activity is determined, in part, by its interaction with activated Stat-3 [26]. In this study we examined if HFD caused an increase association between the activated Stat-3 and NF-κB/p65 in the prostate tissue. As shown in figure 5A, immunoprecipitation with Stat-3 in the nuclear fraction of HFD group considerably increased the levels of Ser536 p65, compared to RD control. HFD after 4 and 8 weeks of feeding increased the physical association between Ser727 Stat-3 and Ser536 p65. Similarly, immunoprecipitation with anti-p65 antibody also increased the association between Ser536 p65 and Ser727 Stat-3 which was more prominent at 8 weeks compared to 4 weeks of HFD and RD group, respectively (Fig. 5B).
Figure 5.
Protein expression of Ser727 Stat-3 and Ser536 p65 after immunoprecipitation with NF-κB/p65 and Stat-3 in the nuclear fraction extracted from the prostrate of RD and HFD fed C57BL/6 mice. Nuclear lysates were immunoprecipitated with anti-p65/RelA or anti-Stat-3 antibodies. Western blot was performed with immuno-complexes using the indicated antibodies as described in the Materials and Methods Section.
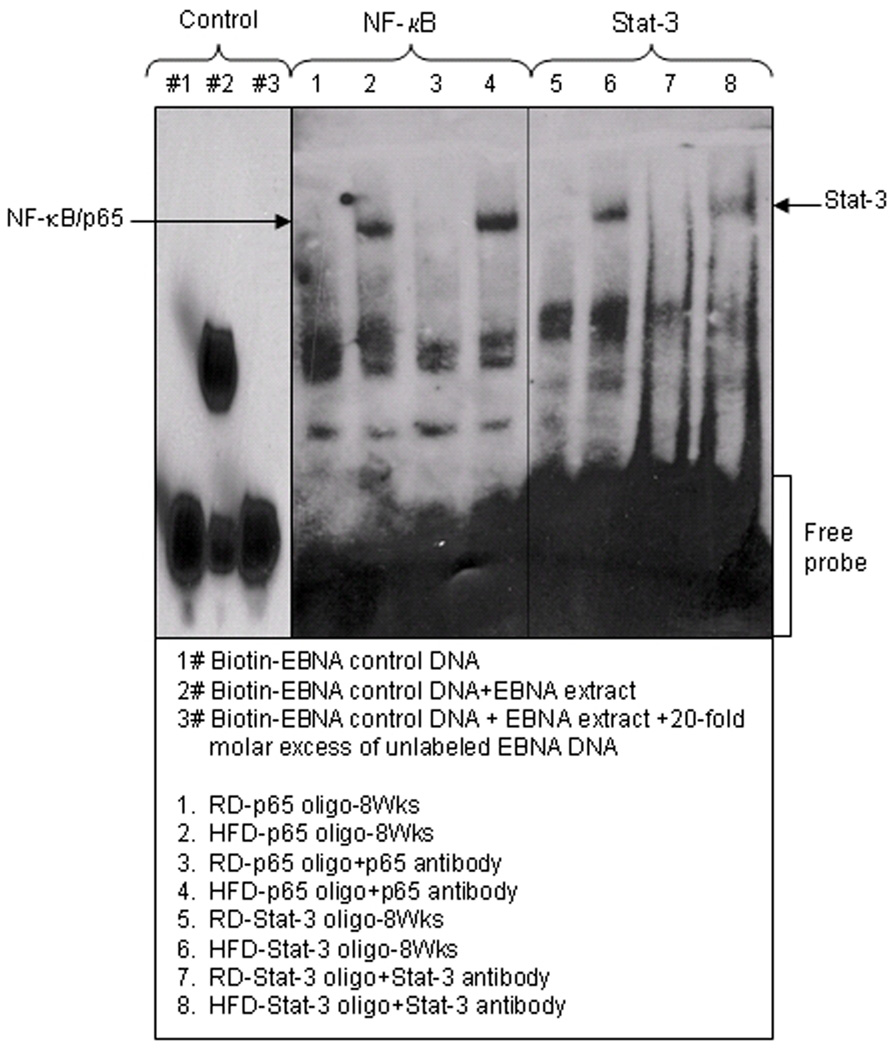
Next we performed EMSA in the nuclear fractions after 8 weeks of HFD feeding in order to confirm the increased nuclear translocation and DNA-binding activity for NF-κB and Stat-3. We observed that HFD caused an increased DNA binding activity of both NF-κB and Stat-3 in the nucleus whereas there was no DNA binding in the nuclear extract from prostrate of mice fed with RD. To confirm the specificity of the NF-κB and Stat-3 binding we used antibodies specific for p65 and Stat-3. We observed that addition of anti-p65 and anti-Stat-3 antibodies caused an increase in DNA binding as a result of HFD feeding (Fig 6).
Figure 6.
NF-κB and Stat-3 DNA-binding activity in the nuclear fraction extracted from the prostrate of RD and HFD fed C57BL/6 mice by EMSA. EMSA was performed to identify nuclear translocation of NF-κB and Stat-3 and their binding to DNA. The details are described in Materials and Methods Section. Controls: 1# biotin-EBNA control DNA; 2# biotin-EBNA control DNA + EBNA extract; and 3# biotin-EBNA control DNA + EBNA extract + 20-fold molar excess of unlabeled EBNA DNA.
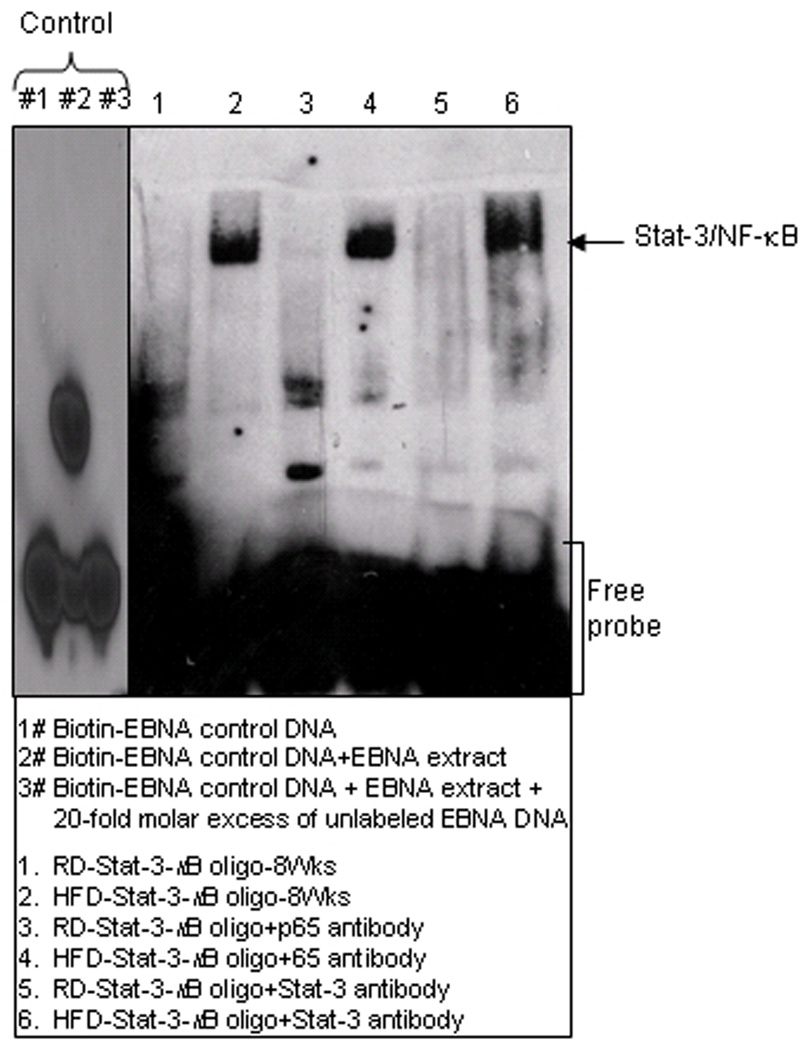
To determine the association between NF-κB and Stat-3, we used a Stat-3-κB consensus binding sequence in the EMSA assay to demonstrate that HFD enhances the binding of both the proteins. As shown in figure 7, HFD increased the DNA binding in the presence of the Stat-3-κB consensus sequence, compared to RD control, whereas addition of the respective antibodies caused further increase in the band intensity.
Figure 7.
Stat-3-κB DNA-binding activities in the nuclear fraction extracted from the prostrate of RD and HFD fed C57BL/6 mice by EMSA. EMSA was performed to identify interactions of Stat-3/NF-κB and their binding to DNA in the nuclear lysates. The details are described in Materials and Methods Section. Controls: 1# biotin-EBNA control DNA; 2# biotin-EBNA control DNA + EBNA extract; and 3# biotin-EBNA control DNA + EBNA extract + 20-fold molar excess of unlabeled EBNA DNA.
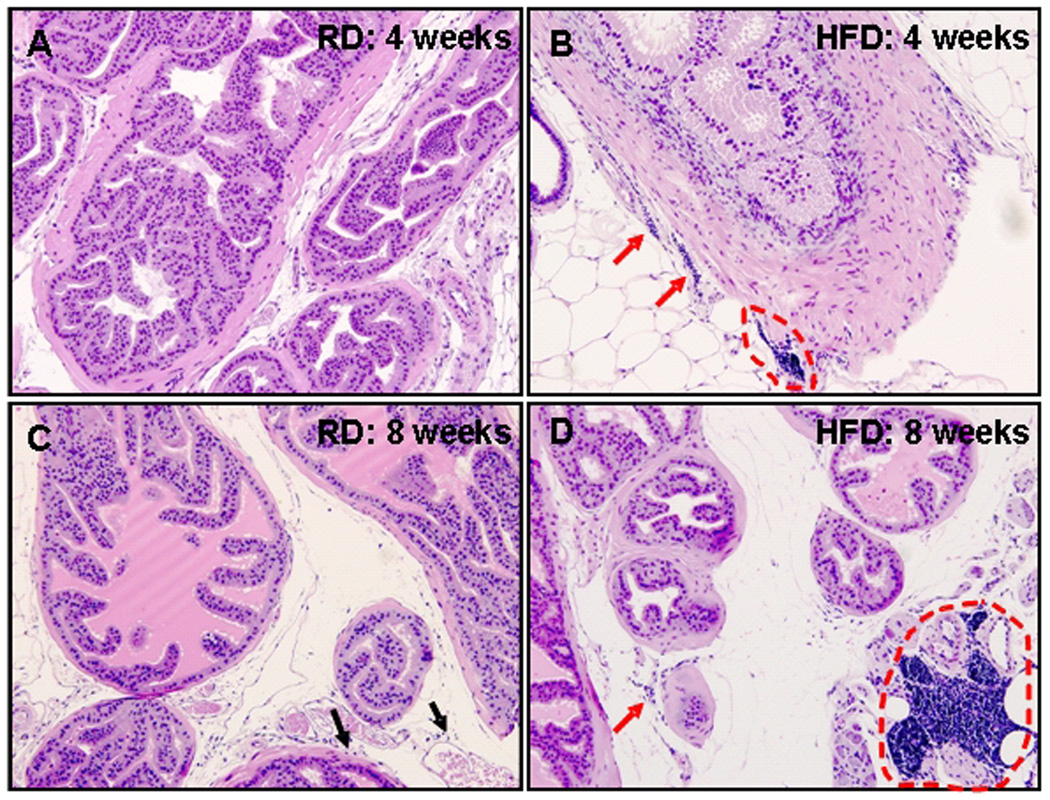
HFD induces intra-prostatic leukocyte accumulation in C57BL/6 mice
Leukocyte accumulation within tissues that usually lack this finding is an important sign of inflammation. As shown in figure 8A, prostate tissue of mice fed with RD for 4 wks was virtually leukocyte free. However, scattered small aggregates of lymphocytes were readily found in the dorsal-lateral prostate (Figure 8B) of animals after 4 weeks of HFD intake. Isolated lymphocytes were found in the stroma of mice fed with RD diet for 8 weeks, but in comparison, both isolated intra-stromal lymphocytes and very large stromal aggregates of lymphocytes were typically present in the dorsal-lateral prostate tissue in mice fed with HFD for 8 weeks (Fig. 8 C&D)
Figure 8.
H&E staining of formalin-fixed paraffin-embedded whole mounted prostates from mice fed with regular diet (RD) and high fat diet (HFD). (A) Lymphocytes are not readily seen in dorsal-lateral prostate tissue in animals fed 4 wks with RD; (B) Scattered small stromal aggregates of lymphocytes are readily found in the prostatic stroma of mice fed 4 wks with HFD (red arrows and red dotted circle). (C) Isolated stromal lymphocytes (black arrows) can be identified in the dorsal-lateral prostate of mice fed with RD for 8 wks; (D) Small and large stromal aggregates of lymphocytes are strikingly evident in the dorsal-lateral prostate in animals fed with HFD for 8 wks (arrow and red dotted circle). (all magnifications, ×200)
DISCUSSION
NF-κB signaling has been reported to play pivotal roles in a number of biologic processes, including oxidative stress, inflammation and response to external stimuli [8–10]. Our recent studies provide evidence that high dietary fat intake promoted oxidative stress, which may lead to prostatic inflammation by enhancing reactive oxygen species generation through elevated expression of NADPH oxidase subunits, leading to NF-κB activation and induction of NF-κB regulated genes viz. COX-2 and iNOS [6]. These results demonstrate that NF-κB is an important effector of both oxidative stress and inflammatory pathways and can be activated by HFD consumption. In the present study we investigated whether Stat-3 is involved in the activation of NF-κB in the prostate as a result of HFD feeding. Although previous studies have demonstrated crosstalk between NF-κB and Stat-3 at multiple levels, the interaction of activated forms of these transcription factors in the nucleus as a consequence of HFD has not been elucidated. Our studies demonstrate physical association between activated forms of Stat-3 and NF-κB in the nucleus after HFD feeding in the prostates of C57BL/6 mice.
Previous studies have shown that HFD feeding increases the levels of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 in the brain and peripheral tissues [20]. Our results demonstrate that HFD markedly increased the plasma levels of IL-1β, IL-6, IL-17 and TNF-α. Furthermore, in the prostate, 4 weeks of HFD feeding caused an increase in the protein expression of IL-6. It has been evident from previous studies that increase in the levels of pro-inflammatory cytokines like IL-1, IL-6, and TNF-α suggests the occurrence of a moderate degree of inflammation in high-fat diet fed rats [27]. We suggest that HFD induces elevated levels of IL-6, which in turn can predispose the prostate to inflammation and signaling involving Stat-3 and NF-κB, both of which play pivotal roles in promoting inflammation and facilitating tumor promotion and metastasis [12]. IL-17 has been reported to increase inflammation and support tumor growth involving IL-6 which in turn activates Stat-3 [28]. Therefore, changes in the levels of these pro-inflammatory cytokines as a consequence of HFD implicate the activation of both NF-κB and Stat-3 pathways in the prostrate.
Protein Kinase C epsilon (ε), a novel PKC, has been reported as a transforming factor and putative oncogene which is over-expressed in several human cancers [29]. In fact, in breast cancer it is shown to activate Akt to induce cell survival [30]. Both PKCε and PKCδ have been suggested to have distinctive roles in HFD-mediated glucose intolerance [31, 32]. In a HFD environment, PKCε is reported to play a critical role in the liver by mediating fat-induced hepatic insulin resistance [31]. PKCδ causes glucose intolerance upon HFD feeding and its deletion improves the glucose tolerance and lipid accumulation [32]. Association between PKCε and Stat-3 is suggested to be constitutive in prostate cancer cells, whereas PKCδ has been shown to phosphorylate Stat-3 at Ser727 in an IL-6-dependent manner [33]. In our studies, we sought to determine if HFD could alter the levels of PKCε as it has been reported to be an important mediator for Stat-3 activation [21]. Our studies demonstrate that PKCε levels were increased after HFD feeding in the prostate. This suggests that PKCε plays a role in the etiology of inflammation resulting from HFD and might be a causative factor in the development of prostatic neoplasia. Additional studies are required to determine if Stat-3 activation by PKCε in a HFD environment requires IL-6.
We further observed that HFD feeding caused an increase in the activation of Akt in the prostate. Akt has been reported to be upstream of NF-κB [22]. Akt is a negative regulator of the apoptotic machinery and NF-κB has been identified as a target of the Ras/PI3K/Akt pathway [34]. Our previous studies demonstrate that HFD caused increased phosphorylation of IKK and IκBα resulting in the activation of p65/RelA [6]. Reports so far have demonstrated the involvement of Akt in cancer development and progression. In this study, we demonstrated that HFD causes an increase in the levels of p-Akt (Ser473) in the prostate, potentiating NF-κB activation, and possible leading to the development of inflammation.
The IκB kinase IKK has been shown to play a dual role in the activation of NF-κB. IKK not only phosphorylates IκBα and its subsequent ubiquitination and degradation, but also activates p65/RelA by phosphorylating it at Ser536, resulting its nuclear import [35, 36]. Ser536 phosphorylation of p65/RelA is physiologically induced in response to a variety of pro-inflammatory stimuli and is essential for its transcriptional activity [36, 37]. We have previously demonstrated that HFD feeding caused an increased phosphorylation of IKKα, IKKβ and IκBα in the prostrate [23]. In this study, we report increased phosphorylation of Ser536 of NF-κB/p65 in the nuclear extract of the prostrate as a result of HFD. Furthermore, TNF-α is reported to activate NF-κB through direct phosphorylation of p65/RelA at Ser536, which in turn is reported to induce IL-6 synthesis [38, 39]. In our study we observed increased plasma levels of TNF-α and IL-6 suggesting that HFD feeding may cause sustained activation of NF-κB.
Several infectious and non-infectious agents are known to cause inflammation and involve Stat-3 activation by various distinct mechanisms [11, 12]. In particular, Stat-3 is activated by tyrosine phosphorylation at a single site close to the carboxy-terminus (Y705), as well as by serine phosphorylation at a site within the transactivation domain (S727). Tyrosine phosphorylation in response to cytokine stimulation is mediated by a Janus kinase, most often JAK1, whereas the single serine phosphorylation in the transcriptional activation domain is required for its full transcriptional activation [40, 41]. In our study we observed that HFD feeding caused significant increase in the levels of IL-6 with a concomitant increase in the levels of Stat-3 phosphorylation at Tyr705 and Ser727 in the prostate. This suggests that HFD is an important mediator of Stat-3 activation, involving PKCε and IL-6. We also observed increased plasma levels of IL-17, which may be due to IL-6-induced activation of Stat-3 resulting in increased levels of IL-17 as a result of HFD feeding. However, further studies are required to determine whether there is an HFD-mediated association between IL-6 and IL-17 during Stat-3 activation.
Association of NF-κB and Stat-3 has been well documented during inflammation, especially in a tumor microenvironment [14]. Stat-3 plays an important role in causing the nuclear retention of NF-κB through acetylation of NF-κB/p65 in the nucleus, thus maintaining its constitutive activation. This requires Ser727 and Tyr705 phosphorylation of Stat-3 along with the DNA-binding domain of Stat-3 protein [11, 14]. In the present study we hypothesized that HFD potentiates intraprostatic inflammation through an association between the activated forms of Stat-3 and NF-κB. Our studies demonstrate physical association between the active forms of NF-κB and Stat-3 during HFD feeding and provide direct evidence for such crosstalk and convergence. However, the exact compositions of the complexes and the ultimate functions of these interactions need further investigation.
Reports suggest that interaction between Stat-3 and NF-κB may have pathological implications [9, 14]. Constitutive activation of NF-κB causes oxidative stress in local tissues whereas Stat-3 deregulation is critical for inflammation [9]. A recent study has demonstrated that high-fat diet causes alterations in pathways related to glutathione metabolism, NRF-mediated oxidative stress response and NF-κB activation [42], similar to our previous findings [6]. Many Stat-3 regulated genes encode pro-inflammatory cytokines causing activation of the intrinsic inflammatory pathway [10]. Our studies demonstrate that high-fat diet feeding for 4 weeks resulted in increasing degrees of prostatic stromal accumulation of lymphocytes over an 8 week period of consumption of a high-fat diet, a finding that was far less prominent in the prostates of mice fed a regular diet during the same time period.
In summary, HFD is one of the risk factors for prostatic diseases in developed nations. Our findings demonstrate co-operativity between Stat-3 and NF-κB as a consequence of HFD and help us explain why both transcription factors appear to stimulate a highly overlapping repertoire of pro-survival, proliferative and inflammatory genes. Sustained inflammation as a result of Stat-3/NF-κB interaction may be the underlying cause of chronic prostatic inflammation. The relationships between chronic prostatic inflammation and other common prostatic diseases, such as benign prostatic hyperplasia and prostate cancer are currently not well understood, but are worthy of further investigation. For example, it would be interesting to know whether HFD-induced persistent chronic inflammation contributes to the oncogenic transformation of prostate epithelial cells.
Acknowledgments
Financial Support: This work was supported by grants from United States Public Health Services NCI RO1CA108512, NCCAM RO1AT002709, NIDDK P20DK090871 and the Sullivan Foundation for the study of prostatitis.
Abbreviations
- EMSA
electrophoretic mobility shift assay
- HFD
High Fat Diet
- RD
Regular Diet
- NF-κB
Nuclear Factor-kappa B
- iNOS
nitric oxide synthase
- COX-2
cyclooxygenase-2
- IL-6
interleukin-6
- PKC
protein kinase C
- PIA
proliferative inflammatory atrophy
- Stat-3
Signal transducer and activator of transcription-3
REFERENCES
- 1.Powell K. Obesity: the two faces of fat. Nature. 2007;447:525–527. doi: 10.1038/447525a. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep. 2009;9:26–32. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daneshgari F, Brown JS, Kusek JW, Nyberg LM. Urological complications of obesity and diabetes. J Urol. 2009;182:S1. doi: 10.1016/j.juro.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lophatananon A, Archer J, Easton D, Pocock R, Dearnaley D, Guy M, Kote-Jarai Z, O’Brien L, Wilkinson RA, Hall AL, Swayer E, Page E, Liu JF, Barratt S, Rahman AA. Dietary fat and early-onset prostrate cancer risk. British Journal of Nutrition. 2010;103:1375–1380. doi: 10.1017/S0007114509993291. [DOI] [PubMed] [Google Scholar]
- 6.Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S. High-fat diet increases NF-κB signaling in the prostate of reporter mice. Prostate. 2011;71:147–156. doi: 10.1002/pros.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: 'cause or effect'? Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi S, Ito H, Miyajima A. IkappaBeta, a nuclear IkappaB protein, positively regulates the NF-kappaB-mediated expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2010;107:11924–11929. doi: 10.1073/pnas.0913179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vykhovanets EV, Shukla S, MacLennan GT, Vykhovanets OV, Bodner DR, Gupta S. Il-1 beta-induced post-transition effect of NF-kappaB provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate. 2009;69:633–643. doi: 10.1002/pros.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Upregulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19:2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- 19.Han SS, Yun H, Son DJ, Tompkins VS, Peng L, Chung ST, Kim JS, Park ES, Janz S. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol Cancer. 2010;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 21.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 22.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 23.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Dalwadi H, Krysan K, Heuze-Vourc'h N, Dohadwala M, Elashoff D, Sharma S, Cacalano N, Lichtenstein A, Dubinett S. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11:7674–7682. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cano P, Cardinali DP, Ríos-Lugo MJ, Fernández-Mateos MP, Reyes Toso CF, Esquifino AI. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity. 2009;17:1866–1871. doi: 10.1038/oby.2009.200. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu D, Huang J, Basu A. Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem. 2006;281:22799–22807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- 31.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frangioudakis G, Burchfield JG, Narasimhan S, Cooney GJ, Leitges M, Biden TJ, Schmitz-Peiffer C. Diverse roles for protein kinase C delta and protein kinase C epsilon in the generation of high-fat-diet-induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C delta. Diabetologia. 2009;52:2616–2620. doi: 10.1007/s00125-009-1543-0. [DOI] [PubMed] [Google Scholar]
- 33.Jain N, Zhang T, Kee WH, Li W, Cao X. Protein kinase C delta associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem. 1999;274:24392–24400. doi: 10.1074/jbc.274.34.24392. [DOI] [PubMed] [Google Scholar]
- 34.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB Kinases Phosphorylate NF-κB p65 Subunit on Serine 536 in the Transactivation Domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 36.Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell co-stimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol. 2004;172:6336–6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 37.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J Neuroinflammation. 2010;7:16. doi: 10.1186/1742-2094-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 42.Sekine Y, Osei-Hwedieh D, Matsuda K, Raghavachari N, Liu D, Furuya Y, Koike H, Suzuki K, Remaley AT. High fat diet reduces the expression of glutathione peroxidase 3 in mouse prostate. Prostate. 2011 Mar 3; doi: 10.1002/pros.21365. [Epub ahead of print] PubMed PMID: 21374652. [DOI] [PMC free article] [PubMed] [Google Scholar]