Abstract
This study tested the hypothesis that chronic widespread pain (CWP) would predict low levels of physical activity (PA). Pain status and PA levels were ascertained at baseline and 32 months in community subjects. Three PA questions were used: “in comparison with others your own age, is your PA “the same” (referent), “more-much more” or “less-much less””, and “during the past month on average how many days/week have you taken exercise that has (i) lasted at least 20 min? and (ii) made you sweat?: “4–7” (referent), “1–3” or “none””. Multinomial logistic regression models quantified the relationship between baseline CWP and PA at follow-up (relative risk ratios (RRR) (95% confidence intervals)). Two thousands one hundred and eighty-two subjects participated and provided complete pain and PA information at both timepoints. CWP was reported by 18% (n = 429) of participants at baseline. Compared to subjects who were free of CWP at baseline, those with CWP had an increased odds of reporting “less-much less” PA at follow-up (RRR = 4.5 (3.2–6.2)). This relationship remained after adjustment for confounders (RRR = 1.9 (1.3–2.9)). A similar association was observed with exercise that lasted at least 20 min (RRR = 1.9 (1.3–2.8)). The current study suggests that low self-reported levels of physical activity are a consequence of having CWP.
Keywords: Chronic widespread pain, Physical activity, Psychosocial, Population-based, Prospective
1. Introduction
Individuals with fibromyalgia, a common disorder characterised by chronic widespread pain (CWP), widespread tenderness and additional physical and psychological symptoms (Wolfe et al., 1990) have been shown to be sedentary (Busch et al., 2008) to have low levels of physical functioning (Jones et al., 2008) and compromised cardiorespiratory fitness (Busch et al., 2008). These studies have examined the cross-sectional relationship between CWP and/or fibromyalgia. Whether CWP is associated with a consequent decrease in physical activity is not known. Nevertheless, the complex presentation of the disorder requires a multidisciplinary approach to treatment (Arnold et al., 2008) with an increase in levels of physical activity generally recommended. The role of low levels of physical activity in the aetiology of CWP and fibromyalgia is unclear and it has been recommended that the aim of increasing physical activity and exercise for individuals with fibromyalgia should be to target secondary symptoms (general fitness, physical function, emotional well being) rather than the pain per se. A recent review of randomised clinical trials of physical activity for the treatment of fibromyalgia (Karmisholt and Gotzsche, 2005) reported that of four “high quality” trials none reported an improvement in pain although improvements in cardiovascular fitness and health related quality of life were noted. A meta analysis of six studies confirmed the improvement in cardiovascular fitness and suggested a positive effect on pain and tender points (Busch et al., 2008).
We have previously reported, from a population-based study, that subjects who reported CWP had an increased mortality risk (Macfarlane et al., 2001, McBeth et al., 2003). The increased risk was primarily associated with deaths from cancer. We have replicated the finding with cancer and reported an increased risk of death associated with cardiovascular disease (McBeth et al., 2009). Other studies have reported no association (Macfarlane et al., 2007, Smith et al., 2003). If the relationship is true, one possible mechanism of association is through reduced levels of physical activity. It is plausible to hypothesise that individuals with CWP are more likely to report reduced levels of physical activity. Reduced levels of physical activity are associated with an increased risk of breast (Rockhill et al., 1999) and prostate (Thune and Lund, 1996) cancer, the two cancers increased in our previous work, and with cardiovascular disease (Prasad and Das, 2009). The aim of the current study was to test the hypothesis that CWP would predict lower physical activity levels when compared to pain free subjects and that these relationships would be independent of putative confounding psychological factors.
2. Materials and methods
2.1. Study design
The EPIFUND (epidemiology of functional disorders) study was a prospective population based cohort study. All subjects were contacted by post and asked to complete a questionnaire that included a detailed assessment of current pain status and physical activity levels. The questionnaire also included an assessment of psychological state and illness behaviour. Subjects who provided full baseline information on pain status, physical activity and putative confounders were followed up 32 months later and asked again about their pain status and physical activity levels.
2.2. Study subjects
Subjects, aged 25–65 years, were randomly recruited from the general practice registers of three areas in the northwest of England. The areas represented three diverse socio-economic areas (median (95% CI) Townsend scores (Townsend et al., 1988) were −3.6 (−3.8, −3.6), 0.7 (0.2, 1.2), and 3.8 (3.6, 3.8)). The study had received full ethical approval from the Local Research Ethics Committees and the Research Ethics Committee of The University of Manchester.
2.3. Assessment of baseline pain status
Subjects were asked whether they had experienced pain lasting for one day or longer in the past month. If their response was positive subjects were asked to indicate the location(s) of their pain, by shading on blank body manikins (front, back, left and right sides). In addition subjects were asked whether they had been aware of their pain for 3 months or longer. Subjects were classified into one of three groups based on their pain reports: those reporting no pain were the “no pain” group, those reporting pain that did not satisfy criteria for CWP were classified as “some pain” and those who satisfied the American College of Rheumatology criteria for “CWP” used in their definition of fibromyalgia (pain in contra-lateral body quadrants, above and below the waist, and on the left and right-hand sides of the body that has been present for at least 3 months) (Wolfe et al., 1990).
2.4. Assessment of physical activity levels
Identical methods to assess physical activity levels were used in both the baseline and follow-up questionnaires. Participants were asked to rate three questions addressing different aspects of physical activity:
-
1.In comparison to others of your own age, do you think your physical activity is?
-
–Much more/more/the same/less/much less.
-
–
-
2.During the past month, on average, on how many days per week have you taken exercise that lasts at least 20 min?
-
–Every day/4–6 days/2–3 days/1 day/none.
-
–
-
3.During the past month, on average, on how many days per week have you taken exercise that makes you sweat?
-
–Every day/4–6 days/2–3 days/1 day/none.
-
–
2.5. Baseline questionnaire
Alongside pain and physical activity information, the baseline questionnaire also gathered data on psychosocial status, using a number of validated scales, and other measures that may act as potential confounders to any association found between pain and subsequent physical activity levels.
2.6. Psychosocial status
2.6.1. General Health Questionnaire (GHQ) (Goldberg and Williams, 1988)
The 12-item version of the GHQ has been used in previous population-based studies of pain to assess levels of psychological distress. Items are answered on a Likert scale of 1–4, which are subsequently dichotomised to 0, for a negative answer, and 1 for a positive, hence giving a score of 0–12, with a higher score indicating greater levels of psychological distress. Items include “in the past few weeks have you felt constantly under strain”.
2.6.2. Hospital Anxiety and Depression Scale (HAD) (Zigmond and Snaith, 1983)
The HAD is a 14-item scale originally designed to assess anxiety and depression in patients with physical illness. It has since been common to find it used in population surveys. All items are scored on a Likert scale of 0–3, the two subscales are scored separately, thus providing a maximum score of 21 for both anxiety and depression. Scores on each subscale of 10–11 represent a high probability of an anxiety or depression disorder being present.
2.6.3. Threatening life events (Brugha et al., 1985)
This is a 12-item scale adapted from Tennant and Andrews (1976) 67-item life events inventory (Tennant and Andrews, 1976). It gathers information on 12 events, including death of a spouse, financial crisis, court appearance and marital difficulties, which are thought to reflect a significant contextual threat. The inventory is scored as the number of events experienced during the past 6 months.
2.7. Other potential confounding factors
To adjust for area level deprivation we used study area as a proxy variable with subjects in the relatively affluent area (Townsend score −3.6) classified as the referent group. Subjects’ marital status was classified as single, married/co-habiting, separated/divorced, or widowed. Employment status was grouped into three categories: working, not working due to ill health, and other. “Other” represented subjects who classified themselves as unemployed but seeking work, a student, semi-retired or retired.
Information on alcohol consumption and smoking history were collected. For the purposes of this analysis, having ever smoked cigarettes regularly for a period of 1 month or longer and having ever drank alcohol regularly for one month or longer was used.
2.8. Statistical analysis
Subjects who provided complete physical activity and pain data at follow-up formed the cohort for this analysis. The three physical activity questions at follow-up were the three outcomes of interest in the analysis. For analysis responses to the question “In comparison to others your own age do you think your physical activity is?” were collapsed into “more/much more” and “less/much less” with “the same classified as the referent category. Responses to the other questions were classified as “none”, “1–3 days” with “4–7 days classified as the referent category. Multinomial logistic regression models were used to quantify the relationship between baseline pain status and subsequent physical activity levels 32 months later. All models were adjusted for age and gender. In order to assess the importance of potential baseline confounders measured, each was added into the models individually and a likelihood ratio test conducted and variables making a significant contribution to the model retained. This method of modelling has been suggested as an alternative to forward stepwise regression in which the software is in control of the data (Henderson and Velleman, 1981). Each of the three models was then adjusted for the same baseline physical activity question. Results are presented as Relative risk ratios (RRR) and 95% confidence intervals (95% CI). Finally, to take account of those subjects who did not respond to the follow-up questionnaires, i.e. non-response, age–sex stratum specific sampling probability weights were applied to each subject and “weighted” multinomial logistic models were fitted. All statistical analyses were carried out using STATA version 9 (STATA, 2003).
3. Results
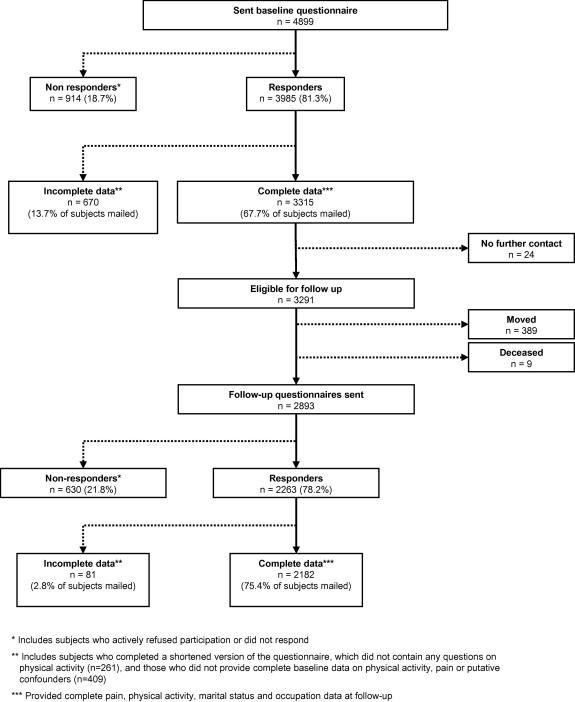
A total of 4899 subjects were mailed a baseline questionnaire of who 3985 (81.3%) returned a questionnaire (Fig. 1). However of those who responded 670 subjects did not provide complete data and were removed from the dataset, leaving 3315 (67.7% of the original mailed sample) for analysis. When compared to those who participated and provided full data (1) nonresponders were younger and from a more affluent area and (2) those that returned a questionnaire but had missing data did not significantly differ with respect to age, gender or area of residence. A total of 2893 were mailed a follow-up questionnaire (after removing from the study the 24 who had requested no further contact, 389 subjects who had moved and the nine who had died in the interim period) (Fig. 1). Of those 2387 returned a questionnaire and 2182 provided full data that could be used in the current analysis. This provided a participation rate of 75.4% of those subjects who were mailed a follow-up questionnaire.
Fig. 1.
Flowchart of baseline and follow-up participation.
3.1. Baseline analysis
Of the 2182 participants 911 (41.8%) reported no pain, 889 (40.7%) some pain and 382 (17.5%) satisfied the criteria for CWP at baseline. Subjects with some pain and those with CWP were more likely to be female, older, not be married or co-habiting, not working due to ill health, less likely to consume alcohol but more likely to smoke when compared to those who were pain free (data not shown). Those reporting some pain and CWP also had higher GHQ and HAD scores, reported experiencing more stressful life events in the 6 months prior to completing the study questionnaire and had lower mental and physical quality of life.
Having some pain and CWP at baseline was associated with being in the poorest category for all three measures of physical activity at follow-up (less/much less physical activity compared to others your own age, no exercise lasting at least 20 min, and no exercise that has made you sweat) (Table 1). Women were more likely than men to report low levels of activity. Subjects who were separated/divorced and those reporting not working due to ill health were also more likely to report low levels. While regular alcohol use for >1 month was associated with an increased likelihood of physical activity, ever smoking for >1 month was associated with a decreased likelihood.
Table 1.
Comparison of subject characteristics by follow-up physical activity levels.
| Category | Physical activity levels at follow-up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In comparison to others your own age do you think your physical activity is |
During the past month, on average, how many days per week have you taken exercise that lasts at least 20 min? |
During the past month on average on how many days per week have you taken exercise that has made you sweat? |
||||||||||
| The same |
More – much more |
Less – much less |
4–7 days |
1–3 days |
None |
4–7 days |
1–3 days |
None |
||||
| n (%) | n (%) | n (%) | P-valuea | n (%) | n (%) | n (%) | P-valuea | n (%) | n (%) | n (%) | P-valuea | |
| Baseline pain status | ||||||||||||
| No pain | 357 (39.2) | 455 (50.0) | 99 (10.8) | 433 (47.5) | 352 (38.7) | 126 (13.8) | 219 (24.1) | 434 (47.6) | 258 (28.3) | |||
| Some pain | 360 (40.5) | 359 (40.4) | 170 (19.1) | <0.01 | 381 (42.9) | 330 (37.1) | 178 (20.0) | <0.01 | 181 (20.4) | 388 (43.6) | 320 (36.0) | <0.01 |
| CWP | 118 (30.9) | 119 (31.1) | 145 (38.0) | 141 (36.9) | 132 (34.6) | 109 (28.5) | 77 (20.2) | 144 (37.7) | 161 (42.1) | |||
| Gender | ||||||||||||
| Male | 302 (33.5) | 434 (48.2) | 165 (18.3) | <0.01 | 394 (43.7) | 345 (38.3) | 162 (18.0) | 0.57 | 210 (22.3) | 435 (48.3) | 256 (28.4) | <0.01 |
| Female | 533 (41.6) | 499 (39.0) | 249 (19.4) | 561 (43.8) | 469 (36.6) | 251 (19.6) | 267 (20.8) | 531 (41.5) | 483 (37.7) | |||
| Age (median (95% CI)) | 48.5 (47.2–49.3) | 51.1 (50.0–52.2) | 48.8 (47.0–50.2) | <0.01 | 50.9 (49.8–51.9) | 47.8 (47.0–48.9) | 50.0 (49.0–51.1) | <0.01 | 48.6 (47.2–50.2) | 48.6 (47.4–49.5) | 51.5 (50.4–52.8) | <0.01 |
| Marital Statusc | ||||||||||||
| Single | 89 (41.0) | 74 (34.1) | 54 (24.9) | <0.01 | 94 (43.3) | 78 (36.0) | 45 (20.7) | 0.03 | 48 (22.1) | 95 (43.8) | 74 (34.1) | 0.01 |
| Married/co-habiting | 646 (39.0) | 730 (44.1) | 281 (16.9) | 741 (44.7) | 626 (37.8) | 290 (17.5) | 369 (22.3) | 758 (45.7) | 530 (32.0) | |||
| Separated/divorced | 64 (29.0) | 95 (43.0) | 62 (28.0) | 81 (36.7) | 79 (35.7) | 61 (27.6) | 44 (19.9) | 81 (36.7) | 96 (43.4) | |||
| Widowed | 36 (41.4) | 34 (39.1) | 17 (19.5) | 39 (44.8) | 31 (35.6) | 17 (19.6) | 16 (18.4) | 32 (36.8) | 39 (44.8) | |||
| Employment statusc | ||||||||||||
| Working | 621 (41.4) | 663 (44.1) | 218 (14.5) | <0.01 | 629 (41.9) | 595 (39.6) | 278 (18.5) | <0.01 | 343 (22.8) | 688 (45.8) | 471 (31.4) | <0.01 |
| Not working due to ill health | 22 (16.8) | 18 (13.7) | 91 (69.5) | 40 (30.5) | 36 (27.5) | 55 (42.0) | 17 (13.0) | 38 (29.0) | 76 (58.0) | |||
| Other | 192 (35.0) | 252 (45.9) | 105 (19.1) | 286 (52.1) | 183 (33.3) | 80 (14.6) | 117 (21.3) | 240 (43.7) | 192 (35.0) | |||
| Ever drank regularly for >1 month? | ||||||||||||
| No | 193 (41.8) | 151 (32.7) | 118 (25.5) | <0.01 | 203 (43.9) | 145 (31.4) | 114 (24.7) | <0.01 | 89 (19.3) | 165 (35.7) | 208 (45.0) | <0.01 |
| Yes | 642 (37.3) | 782 (45.5) | 296 (17.2) | 752 (43.7) | 669 (38.9) | 299 (17.4) | 388 (22.5) | 801 (46.6) | 531 (30.9) | |||
| Ever smoked for >1 month? | ||||||||||||
| No | 461 (39.5) | 525 (45.0) | 181 (15.5) | <0.01 | 532 (45.6) | 470 (40.3) | 165 (14.1) | <0.01 | 261 (22.4) | 559 (47.9) | 347 (29.7) | <0.01 |
| Yes | 374 (36.8) | 408 (40.2) | 233 (23.0) | 423 (41.7) | 344 (33.9) | 248 (24.4) | 216 (21.3) | 407 (40.1) | 392 (38.6) | |||
| Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
P-Valueb |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
P-Valueb |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
P-Valueb |
|
| Psychosocial scales | ||||||||||||
| GHQ | 0 (0–0) | 0 (0–0) | 1 (1–2) | <0.01 | 0 (0–0) | 0 (0–0) | 1 (0–1) | <0.01 | 0 (0–0) | 0 (0–0) | 0 (0–1) | <0.01 |
| HAD anxiety | 5 (4–5) | 4 (4–5) | 6 (6–6) | <0.01 | 5 (4–5) | 5 (4–5) | 5 (5–6) | <0.01 | 5 (4–5) | 5 (4–5) | 5 (5–6) | 0.01 |
| HAD depression | 2 (2–3) | 2 (2–2) | 5 (4–6) | <0.01 | 2 (2–2) | 2 (2–3) | 4 (3–5) | <0.01 | 2 (2–3) | 2 (2–2) | 3 (3–4) | <0.01 |
| Life events | 0 (0–1) | 0 (0–1) | 1 (1–1) | <0.01 | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.29 | 1 (0–1) | 0 (0–1) | 1 (0–1) | 0.02 |
By Chi-squared test.
By Kruskall Wallis test.
Measured at follow-up.
3.2. Prospective analysis
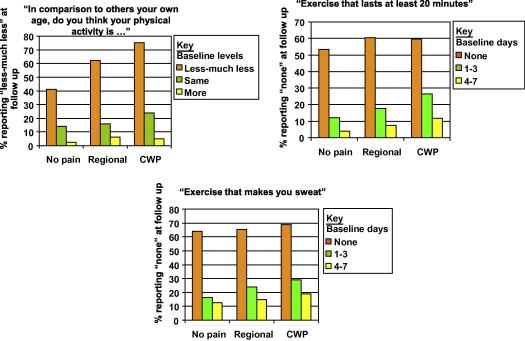
Compared to those with no pain at baseline, having regional or CWP was associated with an increased likelihood of reporting poorer levels of physical activity at follow-up. As an example we have included a figure that shows stability or movement into the lowest level of activity for all three physical activity variables (see Fig. 2). This shows, for example, that of those with no pain at baseline and who reported less-much less “physical activity in comparison to others your own age”, 41% continued to report less-much less at follow-up. Of those with no pain who reported the same or more-much more “physical activity in comparison to others your own age” at baseline 14% and 3% respectively reported less-much less at follow-up. These figures were significantly higher for those with CWP at baseline: 75% continued to report less-much less, while 27% and 5% of those reporting the same or more-much more respectively were in the lowest category at follow-up.
Fig. 2.
Relationship between baseline pain and reporting physical inactivity at follow-up.
When we examined the relationship between baseline pain status and physical activity levels at follow-up (Table 2) we found that after adjusting for age and gender, compared to subjects with no pain at baseline those with some pain were 70%, and those with CWP over four times more likely to report “less/much less” physical activity in comparison to others their own age. Similarly reporting pain was associated with an increased odds of reporting no days in the past month for exercise lasting at least 20 min or exercise that has made you sweat. The relationships with “less/much less” physical activity in comparison to others their own age and no days in the past month for exercise lasting at least 20 min persisted, albeit attenuated, after adjustment for putative confounders (deprivation, depression, traumatic life events, smoking, alcohol consumption, and occupation). Physical activity levels at follow-up may simple reflect those at baseline. However, after adjustment for baseline levels of physical activity, reporting some pain remained moderately associated while having CWP remained associated with an almost 2-fold increased odds of reporting low levels of activity at follow-up.
Table 2.
Impact of baseline pain on self-reported physical activity levels at follow-up.
| Adjustments | Baseline pain statusa | In comparison to others your own age do you think your physical activity isd |
During the past month, on average, how many days per week have you taken exercise that lasts at least 20 min?e |
During the past month on average on how many days per week have you taken exercise that has made you sweat?f |
|||
|---|---|---|---|---|---|---|---|
| More-much more | Less-much less | 1–3 days | None | 1–3 days | None | ||
| RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | ||
| Age and gender | No pain | Referent | Referent | Referent | Referent | Referent | Referent |
| Some pain | 0.8 (0.6–0.9) | 1.7 (1.3–2.3) | 1.1 (0.9–1.3) | 1.6 (1.2–2.1) | 1.1 (0.9–1.4) | 1.4 (1.1–1.9) | |
| CWP | 0.8 (0.6–1.02) | 4.5 (3.2–6.2) | 1.2 (0.9–1.6) | 2.7 (1.9–3.7) | 0.95 (0.7–1.3) | 1.6 (1.2–2.3) | |
| + Putative confoundersb | No pain | Referent | Referent | Referent | Referent | Referent | Referent |
| Some pain | 0.8 (0.6–0.98) | 1.4 (1.04–1.9) | 1.1 (0.9–1.4) | 1.4 (1.1–1.9) | 1.1 (0.9–1.4) | 1.3 (0.98–1.7) | |
| CWP | 0.9 (0.6–1.2) | 2.5 (1.7–3.5) | 1.2 (0.9–1.6) | 1.7 (1.2–2.4) | 0.96 (0.7–1.3) | 1.1 (0.8–1.6) | |
| + Baseline physical activity levelsc | No pain | Referent | Referent | Referent | Referent | Referent | Referent |
| Some pain | 0.8 (0.6–1.01) | 1.3 (0.97–1.8) | 1.1 (0.9–1.4) | 1.5 (1.1–2.0) | 1.04 (0.8–1.3) | 1.2 (0.9–1.6) | |
| CWP | 0.9 (0.6–1.3) | 1.9 (1.3–2.8) | 1.2 (0.9–1.7) | 1.7 (1.1–2.6) | 0.95 (0.7–1.3) | 1.0 (0.7–1.5) | |
Adjusted for age and gender.
Adjusted for age, gender, area of residence and confounding factors specific to each of the three physical activity models.
Adjusted for age, gender area of residence and confounding factors specific to each of the three physical activity models, and baseline physical activity levels.
Depression, smoking history, alcohol consumption, occupation at follow-up.
Depression, smoking history, alcohol consumption, occupation at follow-up.
Depression, traumatic life events, alcohol consumption, occupation at follow-up.
Finally we were concerned about the impact non-response may have had on our results and so compared the baseline measures between those who did and did not participate at follow-up (Table 3). Non-participants were younger, had lower levels of physical activity at baseline, less likely to drink alcohol, but more likely to smoke. Non-participants were also more likely to score higher on all psychosocial scales except that assessing the number of recent traumatic life events. To examine the effect of non-participation on our results we conducted a weighted analysis that indicated no effect of non-participation (data not shown).
Table 3.
Comparison of baseline measures between non-participants and full-participants at follow-up.
| Category | Non-participantsa | Full participants | |
|---|---|---|---|
| n = 711 (24.6%) | n = 2182 (75.4%) | ||
| n (%) | n (%) | P-valueb | |
| Gender | |||
| Male | 319 (44.9) | 901 (41.3) | 0.09 |
| Female | 392 (55.1) | 1281 (58.7) | |
| Age (median, 95% CI) | 45.6 (44.5–47.3) | 49.5 (48.9–50.2) | <0.01 |
| Baseline CWP status | |||
| No pain | 263 (37.0) | 911 (41.8) | 0.11 |
| Some pain | 300 (42.2) | 889 (40.7) | |
| CWP | 148 (20.8) | 382 (17.5) | |
| In comparison to others your own age do you think your physical activity is | |||
| The same | 282 (39.7) | 859 (39.4) | <0.01 |
| More-much more | 256 (36.0) | 955 (43.8) | |
| Less-much less | 173 (24.3) | 368 (16.9) | |
| During the past month, on average, how many days per week have you taken exercise that lasts at least 20 min? | |||
| 4–7 days | 258 (36.3) | 927 (42.4) | <0.01 |
| 1–3 days | 300 (42.2) | 909 (41.7) | |
| None | 153 (21.5) | 346 (15.9) | |
| During the past month on average on how many days per week have you taken exercise that has made you sweat? | |||
| 4–7 days | 133 (18.7) | 456 (20.9) | <0.01 |
| 1–3 days | 303 (42.6) | 1042 (47.8) | |
| None | 275 (36.7) | 684 (31.3) | |
| Ever drank regularly for >1 month? | |||
| No | 181 (25.5) | 462 (21.2) | 0.02 |
| Yes | 530 (74.5) | 1720 (78.8) | |
| Ever smoked for >1 month? | |||
| No | 321 (45.2) | 1167 (53.5) | <0.01 |
| Yes | 390 (54.8) | 1015 (46.5) | |
| Median (95% CI) |
Median (95% CI) |
||
| Psychosocial scales | |||
| GHQ | 0 (0–1) | 0 (0–0) | 0.01 |
| HAD anxiety | 5 (5–6) | 5 (5–5) | 0.01 |
| HAD depression | 3 (3–4) | 3 (2–3) | <0.01 |
| Life events | 1 (0–1) | 1 (0–1) | 0.33 |
Non-participants include subjects who did not respond or who did not provide full data (including participants of the short and telephone questionnaires) at follow-up.
Chi-squared test, except for age and psychosocial scales, which was by Mann–Whitney U test.
4. Discussion
In the current study we sought to examine the hypothesis that having CWP would predict low levels of self-reported physical activity. We found that subjects who reported having some pain or CWP had an increased risk of low levels of physical activity 32 months later. Specifically, after adjusting for age, gender, and other putative confounding factors subjects with some pain reported less physical activity than their peer group and were more likely to report not exercising for at least 20 min in the past month. These relationships were also evident among subjects with CWP, although to a greater degree. Interestingly these relationships were independent of baseline levels of physical activity, depression, smoking history, alcohol consumption, occupation status and level of deprivation. There are likely to be other factors that may explain the observed relationship including body mass index and co-morbidities but that we have not measured. Future studies should aim to include such factors in a comprehensive assessment to determine the true relationship between CWP and physical inactivity.
The current study has a number of strengths: it was a large study of an unselected population with a comprehensive assessment of putative confounders. The tools used to assess pain are routinely used in population-based studies of pain and have been shown to have construct validity (Hunt et al., 1999). However, levels of physical activity were based upon self-report and these are likely to be influenced by an individual’s pain state at the time of questionnaire completion. Our data indicate that 40% of the study population report exercising for at least 20 min per day on 4–7 days per week. While these levels of activity appear to be high, they are in fact entirely consistent with reported levels of physical activity in other general population samples. For example data from the Health Survey for England (http://www.ic.nhs.uk/pubs/hse08trends) show that 34% of adults report an even more rigorous exercise regime of 30 min or more of moderate or vigorous activity on at least 5 days a week. The study results indicate that the likelihood of reporting physical inactivity at follow-up was independent of baseline levels of activity suggesting a direct relationship with pain status. Future studies could usefully assess the relationship between self-report physical activity and actual activity levels in subjects with musculoskeletal pain using objective tools of assessment such as actigraphy. The direction of the relationship between CWP and physical activity levels at baseline is also unclear. While we have shown that CWP leads to physical inactivity, it is equally plausible that low levels of physical activity increases the risk of developing CWP. While we have not tested the latter hypothesis in this study, it must remain a possibility. Irrespective of the direction of the relationship between CWP and physical activity at baseline, we have shown that having CWP at baseline increased self-reported physical inactivity at follow-up and this relationship was independent of baseline levels of activity.
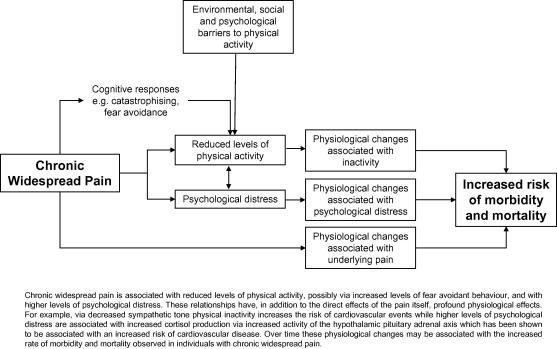
What is the relationship between musculoskeletal pain and subsequent low levels of physical activity? One potential mechanism is via fear avoidant behaviour (Vangronsveld et al., 2007). Individuals with pain who tend to catastrophise their symptoms (i.e. perceive their pain to have negative consequences) are likely to avoid behaviours that they believe will increase either their pain or associated negative consequences (see Fig. 3). While in the short term such behaviour may be beneficial in limiting the pain experience, long term avoidant behaviour may lead to lowering levels of physical activity, consequent physical deconditioning and a paradoxical increase in pain. We were unable to examine that potential causal pathway in the current study. However data from other sources would support that hypothesis: individuals with fibromyalgia and CWP have higher levels of catastrophising when compared to patients with rheumatoid arthritis (Hassett et al., 2000). Higher levels of catastrophising are associated with increased levels of pain and persistence of pain behaviourally via hypervigilance to symptoms (Rhydderch et al., 2006), and physiologically via amplification of cortical activation in relation to pain (Campbell and Edwards, 2009) and/or dysregulation of endogenous opioids (Campbell and Edwards, 2009). These increased levels of pain may be associated with an increase in avoidant behaviours such as lowering physical activity levels. Similar mechanisms have been observed in patients with non-specific low back pain (Elfving et al., 2007).
Fig. 3.
Hypothesised pathway illustrating route from chronic widespread pain to increased risk of morbidity and mortality.
A recent study (Andersson, 2009) has explored the relationship between CWP and mortality and replicated our previous reports of an increased risk of cardiovascular deaths (Hazard ratio (HR) = 2.2, 95% CI (1.1, 4.2)) although not with cancer deaths (HR = 1.2, 95% CI (0.5, 2.6)). In addition to reporting current pain subjects in that study also completed questions on smoking habits (never/past/current) and physical activity levels (range: “no activity” to “hard activity at least twice a week”). After adjusting for these lifestyle factors and perceived stress, body mass index and insomnia, the relationship between CWP and cardiovascular death was attenuated (HR = 1.1, 95% CI (0.6, 1.9)). In that analysis low physical activity significantly predicted the mortality risk (HR = 1.7, 95% CI (1.1, 2.4)) suggesting that the relationship between pain and cardiovascular death is moderated by low levels of physical activity. However, the direction of the relationship between CWP and physical inactivity remains unclear. The data we present is novel, having determined for the first time that having CWP increases the risk of physical inactivity and may be the driver of the increased mortality risk rather than CWP simply being a path variable between physical inactivity and mortality. The physiological implications of long term physical inactivity for health are clear. Physical activity decreases cardiovascular disease risk independently of confounding factors possibly via an improvement in cardiovascular risk factors, enhanced fibrinolysis, improved endothelial function, and decreased sympathetic tone (Prasad and Das, 2009). Physical activity is also protective against cancer, particularly breast and colo-rectal cancers (Hardman, 2001). Compared to subjects classified as “least active”, those classified as “most active” had a reduced likelihood of developing colo-rectal cancer of up to 40–50% (Levi et al., 1999, Tavani et al., 1999) and this reduced risk is not explained by physical activity being a marker for a healthier lifestyle (Hardman, 2001). Rather physical activity appears to exert an independent protective effect in men and women, although this effect appears to be strongly related to the colon and less so to the rectum. Higher levels of physical activity are also associated with a reduction of up to 30% in the risk of breast cancer (Hardman, 2001) although this finding is not consistent. It is colo-rectal and breast cancer that we have previously found to be in excess among subjects who report pain (Macfarlane et al., 2001, McBeth et al., 2003). Although unclear, potential mechanisms of association include systemic influences (e.g. reduced body fat), improved immunosurveillence, improved gut transit time (colon cancer) and decreased exposure to oestrogen (breast cancer). Our previous studies of the long term outcome of CWP have shown strong associations with cancer incidence and subsequent mortality (Macfarlane et al., 2001, McBeth et al., 2003) and with cardiovascular deaths (McBeth et al., 2009). These relationships were independent of factors known to increase the rate of both cancer and cardiovascular disease: age, gender, smoking and socio-economic status. It is plausible to hypothesise that the increased rate of physical inactivity in subjects with CWP may explain the relationship with mortality. Of course there are other pathways through which CWP may influence survival including (see Fig. 3) increasing levels of psychological distress and the consequent physiological changes associated with those increased levels, and the physiological changes associated with pain itself.
To summarise, the current study has shown that individuals in a population-based study of musculoskeletal pain were more likely when compared to those with no pain to report physical inactivity 32 months later. This increased risk of physical inactivity may in turn increase the risk of colo-rectal and breast cancer among subjects with pain and offers a plausible mechanism via which pain may be directly associated with an increased mortality risk. Future studies should seek to confirm these findings through objective measurement of physical activity levels. Current evidence (Busch et al., 2008) suggests that physical activity benefits both symptoms and cardiovascular fitness and so the individual health benefits of recommending increased levels of physical activity among these individuals are clear.
Funding
This study was supported by the Arthritis Research Campaign, Chesterfield, United Kingdom. Grant No. 17552.
Disclosure statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank the doctors, staff and participants at the three general practices for their participation in the study. Thanks also to Joanne Bradley, Karen Schaftheutle and Ruth Fullam for data collection and administration.
References
- Andersson H.I. Increased mortality among individuals with chronic widespread pain relates to lifestyle factors: a prospective population-based study. Disabil Rehabil. 2009;31:1980–1987. doi: 10.3109/09638280902874154. [DOI] [PubMed] [Google Scholar]
- Arnold L.M., Bradley L.A., Clauw D.J., Glass J.M., Goldenberg D.L. Multidisciplinary care and stepwise treatment for fibromyalgia. J Clin Psychiat. 2008;69:e35. doi: 10.4088/jcp.1208e35. [DOI] [PubMed] [Google Scholar]
- Brugha T., Bebbington P., Tennant C., Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. 1985;15:189–194. doi: 10.1017/s003329170002105x. [DOI] [PubMed] [Google Scholar]
- Busch A.J., Schachter C.L., Overend T.J., Peloso P.M., Barber K.A. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35:1130–1144. [PubMed] [Google Scholar]
- Campbell C.M., Edwards R.R. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfving B., Andersson T., Grooten W.J. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. 2007;12:14–24. doi: 10.1002/pri.355. [DOI] [PubMed] [Google Scholar]
- Goldberg DP, Williams P. A user’s guide to the General Health Questionnaire. Windsor: Nfer-Nelson; 1988.
- Hardman A.E. Physical activity and cancer risk. Proc Nutr Soc. 2001;60:107–113. doi: 10.1079/pns200076. [DOI] [PubMed] [Google Scholar]
- Hassett A.L., Cone J.D., Patella S.J., Sigal L.H. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum. 2000;43:2493–2500. doi: 10.1002/1529-0131(200011)43:11<2493::AID-ANR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Henderson H.V., Velleman P.F. Building multiple regression models interactively. Biometrics. 1981;37:391–411. [Google Scholar]
- Hunt I.M., Silman A.J., Benjamin S., McBeth J., Macfarlane G.J. The prevalence and associated features of chronic widespread pain in the community using the ‘Manchester’ definition of chronic widespread pain. Rheumatology (Oxford) 1999;38:275–279. doi: 10.1093/rheumatology/38.3.275. [DOI] [PubMed] [Google Scholar]
- Jones J., Rutledge D.N., Jones K.D., Matallana L., Rooks D.S. Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues. 2008;18:406–412. doi: 10.1016/j.whi.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Karmisholt K., Gotzsche P.C. Physical activity for secondary prevention of disease. Systematic reviews of randomised clinical trials. Dan Med Bull. 2005;52:90–94. [PubMed] [Google Scholar]
- Levi F., Pasche C., Lucchini F., Tavani A., La V.C. Occupational and leisure-time physical activity and the risk of colorectal cancer. Eur J Cancer Prev. 1999;8:487–493. doi: 10.1097/00008469-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Macfarlane G.J., Jones G.T., Knekt P., Aromaa A., McBeth J., Mikkelsson M. Is the report of widespread body pain associated with long-term increased mortality? Data from the Mini-Finland Health Survey. Rheumatology (Oxford) 2007;46:805–807. doi: 10.1093/rheumatology/kel403. [DOI] [PubMed] [Google Scholar]
- Macfarlane G.J., McBeth J., Silman A.J. Widespread body pain and mortality: prospective population based study. Br Med J. 2001;323:662–665. doi: 10.1136/bmj.323.7314.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeth J., Silman A.J., Macfarlane G.J. Association of widespread body pain with an increased risk of cancer and reduced cancer survival: a prospective, population-based study. Arthritis Rheum. 2003;48:1686–1692. doi: 10.1002/art.10973. [DOI] [PubMed] [Google Scholar]
- McBeth J., Symmons D.P., Silman A.J., Allison T., Webb R., Brammah T. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology (Oxford) 2009;48:74–77. doi: 10.1093/rheumatology/ken424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D.S., Das B.C. Physical inactivity: a cardiovascular risk factor. Indian J Med Sci. 2009;63:33–42. [PubMed] [Google Scholar]
- Rhydderch M., Edwards A., Marshall M., Elwyn G., Grol R. Developing a facilitation model to promote organisational development in primary care practices. BMC Fam Pract. 2006;7:38. doi: 10.1186/1471-2296-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill B., Willett W.C., Hunter D.J., Manson J.E., Hankinson S.E., Colditz G.A. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;159:2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- Smith B.H., Elliott A.M., Hannaford P.C. Pain and subsequent mortality and cancer among women in the Royal College of General Practitioners Oral Contraception Study. Br J Gen Pract. 2003;53:45–46. [PMC free article] [PubMed] [Google Scholar]
- Stata Statistical Software. Stata. (Release 8.0). TX: Stata Corporation; 2003.
- Tavani A., Braga C., La V.C., Conti E., Filiberti R., Montella M. Physical activity and risk of cancers of the colon and rectum: an Italian case-control study. Br J Cancer. 1999;79:1912–1916. doi: 10.1038/sj.bjc.6690304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant C., Andrews G. A scale to measure the stress of life events. Aust New Zeal J Psychiat. 1976;10:27–32. doi: 10.3109/00048677609159482. [DOI] [PubMed] [Google Scholar]
- Thune I., Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73:1134–1140. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend P, Phillimore P, Beattie A. Health and deprivation: inequalities and the North. London: Croom Helm; 1988.
- Vangronsveld K., Peters M., Goossens M., Linton S., Vlaeyen J. Applying the fear-avoidance model to the chronic whiplash syndrome. Pain. 2007;131:258–261. doi: 10.1016/j.pain.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Smythe H.A., Yunus M.B., Bennett R., Bombardier C., Goldenberg D.L. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]