Abstract
Evaluating early changes in cerebral metabolism in hydrocephalus can help in the decision making and the timing of surgical intervention. This study was aimed at examining the tricarboxylic acid (TCA) cycle rate and 13C label incorporation into neurotransmitter amino acids and other compounds 2 weeks after rats were subjected to kaolin-induced progressive hydrocephalus. In vivo and ex vivo magnetic resonance spectroscopy (MRS), combined with the infusion of [1,6-13C]glucose, was used to monitor the time courses of 13C label incorporation into the different carbon positions of glutamate in the forebrains of rats with hydrocephalus as well as in those of controls. Metabolic rates were determined by fitting the measured data into a one-compartment metabolic model. The TCA cycle rate was 1.3 ± 0.2 μmoles/gram/minute in the controls and 0.8 ± 0.4 μmoles/gram/minute in the acute hydrocephalus group, the exchange rate between α-ketoglutarate and glutamate was 4.1 ± 2.5 μmoles/gram/minute in the controls and 2.7 ± 2.6 μmoles/gram/minute in the hydrocephalus group calculated from in vivo MRS. There were no statistically significant differences between these rates. Hydrocephalus caused a decrease in the amounts of glutamate, alanine and taurine. In addition, the concentration of the neuronal marker N-acetyl aspartate was decreased. 13C Labelling of most amino acids derived from [1,6-13C]glucose was unchanged 2 weeks after hydrocephalus induction. The only indication of astrocyte impairment was the decreased 13C enrichment in glutamine C-2. This study shows that hydrocephalus causes subtle but significant alterations in neuronal metabolism already early in the course of the disease. These sub-lethal changes, however, if maintained and if ongoing might explain the delayed and programmed neuronal damage as seen in chronic hydrocephalus.
Keywords: In vivo 13C MR spectroscopy, Astrocytes, Neurons, Glucose, Metabolism
Introduction
In hydrocephalus the intracranial cerebrospinal fluid (CSF) volume is increased followed by compression of brain tissue. Kaolin-induced hydrocephalus is an intensively studied model of progressive hydrocephalus first described in 1932 [1]. In this model kaolin is injected under microscopic guidance into the cisterna magna resulting in a sterile meningeal inflammation. This leads to an obstruction of the foramina of Luschka and Magendi and thus to hydrocephalus and syringomyelia [2]. Clinical symptoms of acute hydrocpehalus are evoked by an increase in intracranial pressure and outflow resistance. Treatment of chronic hydrocephalus is operative, but decision making concerning the appropriate timing of surgical treatment is difficult. Despite enormous efforts in hydrocephalus research, this issue is still not satisfactorily solved. Thus, non-invasive techniques to assess development of hydrocephalus have to be established. A possible solution might be to monitor changes in cerebral metabolism which has been shown to take place in hydrocephalus [3]. In vivo MRS can provide valuable information about cerebral metabolites and tricarboxylic acid (TCA) cycle rates and thus, such studies of the brain with hydrocephalus could be of significant clinical importance.
13C MRS has been employed extensively to study cerebral metabolism and TCA cycle activity in animals after the injection of 13C labelled substrates. Since 13C labelled metabolites such as glutamate and aspartate are in equilibrium with TCA cycle intermediates, the level of labelling of these measured metabolites reflect TCA cycle activity. The present study is the first to describe the in vivo TCA cycle rate in rats subjected to hydrocephalus. Hydrocephalus was induced by kaolin-instillation into the cisterna magna of Sprague–Dawley rats and 2 weeks later [1,6-13C]glucose was infused and in vivo MRI and 13C MRS were preformed. The incorporation of 13C label from [1,6-13C]glucose into [4-13C]- and [3-13C]glutamate was thus observed. The time courses of 13C label incorporation into glutamate in the brains of rats with hydrocephalus as well as in controls were fitted using a one-compartment metabolic model allowing non-invasive determination of the neuronal TCA cycle rate and the exchange rate between α-ketoglutarate and glutamate. Since most glutamate is located in neurons [13], metabolic rates obtained using this model primarily reflects neuronal metabolism. Thereafter, labelling of different metabolites in the cortex was measured by ex vivo 13C MRS and amino acid concentrations were determined using high performance liquid chromatography (HPLC) of tissue extracts.
Experimental Procedures
Animals
All experimental procedures were approved by the Norwegian Experimental Animal Board. Animals had free access to water and food and were kept under standard laboratory conditions (room temperature 22°C, 66% humidity, 12/12 h light/dark cycle). Five Sprague–Dawley rats (2 months old, 200 g, Mollergaard Breeding Centre, Copenhagen, Denmark) were anaesthetized with ketamine (100 mg/kg, ketalar, Warner Lambert, Nordic AB, Solna, Sweden) and medotomidinehydrochlorid (0.5 mg/kg, Janssen-Cilag Ltd., Sollentuna, Sweden) and made hydrocephalic by microsurgical instillation of 0.1 ml kaolin (4 g/ml 0.9% saline, NMD, Oslo, Norway) into the cisterna magna. Five rats were sham-operated and served as controls. Postoperatively, rats were kept one per cage at a light–dark shift of 12 h and received water and food ad libitum. In vivo MRS was performed in all animals, but due to technical problems only 3 spectra of sufficient signal to noise ratio of the peaks in the 13C MR spectra were obtained for each group. In addition, 4 rats were used to determine the input function of glucose.
Determination of the Input Function of Glucose
The time courses of incorporation of labelling in [4-13C]- and [3-13C]glutamate in the brain were fitted into a one-compartment metabolic model (see section below) which yielded the rate of the TCA cycle and the exchange rate between α-ketoglutarate and glutamate. The time courses of incorporation of 13C labelling into glutamate C-4 and C-3 depend on the kinetics of 13C labelling of glucose in the blood. The latter time course is called the input function and was determined by measuring the 13C enrichment of glucose in blood during the infusion. Without knowledge of the input function, the rate of 13C labelling of glutamate cannot be interpreted, since it is not known how quickly the 13C labelled substrate enters the system. Since blood cannot be drawn easily inside the magnet in rats with a small blood volume, additional measurements were performed on the bench using the same infusion protocol as for the MRS experiment to determine the input function and to assess arterial pH, pCO2, pO2, glucose and lactate. Four animals were fasted for 18–20 h prior to the experiment; their weight was 258 ± 4 g after fasting. Spontaneously breathing animals were anesthetized with 3.5% isoflurane in 30/70% O2/N2 using a small animal ventilator (Harvard Apparatus, MA, USA) in a closed chamber. During surgery and also later during glucose infusion the animals were spontaneously breathing 2% isoflurane in 30/70% O2/N2 delivered though a face mask. Body temperature was maintained using a feed-back controlled heating table connected to a rectal probe. One catheter was introduced into the right femoral vein, and one in the right femoral artery. The insertion was sprayed with lidocain (10 mg/doses) and closed, with the catheters emerging from the middle and posterior end of the incision. Arterial blood samples from the control animals were drawn 5 min before the start of the intravenous glucose infusion, at the start of the infusion and thereafter then 5, 10, 15, 30, 45, 60 and 80 min after the start of the infusion. Approximately 300 μl arterial blood was drawn at each time point. One hundred micro liter was used for the measurement of pH, plasma lactate and glucose as well as blood gasses using a blood gas analyzer (Siemens RapidLab 1200, Siemens Health Care, USA). Two hundred micro liter of blood was transferred to an Eppendorf tube and centrifuged at 1,000 rpm for 5 min and at 4°C. Plasma samples were extracted with 1 ml ice-cold 7% perchloric acid and centrifuged for 10 min at 4°C and 4,000g. Each sample was neutralized with 2 M KOH, centrifuged and lyophilized. The lyophilized plasma samples were dissolved in 400 μl D2O (99%, Cambridge Isotope Laboratories, Woburn, Massachusetts, USA) and analyzed with high resolution 1H MRS to determine the fractional 13C enrichment of glucose. 1H NMR spectra were acquired on a Bruker DRX500 spectrometer 11.7T, (Bruker Biospin GmbH, Rheinstetten, Germany) with the following acquisition parameters: 90° pulse angle, an acquisition time of 1.36 s and a relaxation delay of 10 s, 512 scans were accumulated for each sample. Water suppression was achieved by applying a low-power pre-saturation pulse at the water frequency. The glucose peaks in the 1H spectra were quantified by integration in XWINNMR in relation to a known amount of ethylene glycol added to the sample.
In Vivo Magnetic Resonance Protocol
In vivo 13C MRI and MRS were performed on hydrocephalus and sham-operated rats 2 weeks after surgery. The rats were fasted 18–20 h before the experiment. On the day of the experiment the rats with induced hydrocephalus weighed 197 ± 25 g (n = 3) and the control group weighed 300 ± 5 g (n = 3). The spontaneously breathing animals were anesthetized with 3.5% isoflurane (Baxter, Norway) in 30/70% O2/N2 using a small animal ventilator (Harvard Apparatus, MA, USA) in a closed chamber. During surgery the animals were spontaneously breathing 2% isoflurane in 30/70% O2/N2 delivered though a face mask. Body temperature was maintained using a feed-back controlled heating blanket connected to a rectal probe. First a catheter was introduced into the right femoral vein. The insertion was sprayed with lidocain (10 mg/doses) (AstraZeneca, Norway) and closed, with the catheter emerging from the posterior end of the incision. Subsequently the scalp was surgically removed to minimize lipid signal contamination. The skull was sprayed with lidocain (10 mg/doses) and a layer of plastic wrap was placed over the skull to prevent desiccation. During in vivo MRI and MRS core body temperature was maintained between 36.5 and 38°C by placing the animals in prone position on a pad heated with circulating water (Bruker Biospin, Ettlingen, Germany).
In vivo MRI and MRS were performed on a 7 Tesla Bruker Biospec 70/20 magnet (Bruker Biospin, Ettlingen, Germany) operating at 75.50 MHz (13C) and 300.30 MHz (1H) with a 72-mm volume coil for transmission and an actively decoupled quadrature rat head surface coil (20-mm) for receive-only. Water-cooled BGA-12 (400 mT/m) gradients were used. Coronal gradient-echo images (RARE-sequence with a RARE factor of 16) were acquired with TR = 6,000 ms, TE = 71.85 ms, number of averages = 8. A total of 30 slices were acquired with FOV = 5 × 5 cm and a matrix of 256 × 256. Total MRI scan time was 12 min and 48 s.
To monitor cerebral 13C label incorporation into cortical metabolites (glutamate) an infusion of 1 M [1,6-13C] glucose (99% 13C enriched, Cambridge Isotope Laboratories, Woburn, Massachusetts, USA) was administered. An initial bolus infusion of 105 mg/kg/min for 2 min was followed by a steady-state infusion of 35 mg/kg/min. The total length of the infusion was approximately 80 min. The in vivo 13C MR spectra were acquired using a homemade 10 mm circular singular transmit/receive 13C surface coil, whereas the 72-mm transmit volume coil was used for 1H decoupling. Nuclear Overhauser effect (NOE) enhanced 1H decoupled 13C MR spectra were obtained from a non-localized volume. Waltz 16 was used for decoupling protons. Magnetic field homogeneity was optimized by first and second order shims using FASTMAP [4] on a selected voxel that included most of the cortical area detected by the surface coil. 13C MR spectra were summed in batches of 256 averages; giving a temporal resolution of 6 min and 24 s. 13C MR spectra were acquired continuously throughout the glucose infusion. Before the infusion of labelled glucose a baseline spectrum was acquired for each animal. This baseline spectrum was subtracted from each post infusion spectrum in order to eliminate the broad lipid signal in the spectral region of interest. The dimensions of the coil were such as that the majority of signal was acquired from the cortical tissue. The relative amount of [4-13C]glutamate and [3-13C]glutamate at each time point was quantified by using the signal to noise function in XWINNMR. At the last time point the signal intensities were set to the concentration of [4-13C]glutamate and [3-13C]glutamate which was determined by ex vivo MRS on the extracted tissue samples, see the below section.
Ex Vivo 13C MRS and HLPC Analysis of Brain Tissue Samples
After the in vivo 13C MRS experiment the animals were sacrificed by micro-wave fixation, 4 kW, 2.2 s (Model GA5013, Gerling Applied Engineering, California, USA). The cortex was dissected, weighed and frozen at −80°C. The dissected cortical tissue was homogenized with an ultrasound sonicator Vibra Cell sonicator (Model VCX 750, Sonics & Materials, Newtown, CT, USA) in ice-cold 0.7% perchloric acid and thereafter centrifuged for 20 min at 4°C and at 10,000g. Ten micro liter of supernatant was stored at −20°C for HPLC analysis. The rest of the supernatant was transferred into a new tube, neutralized with 1 M KOH and centrifuged for 20 min at 4°C and at 10,000g. The supernatants were thereafter lyophilized and dissolved in 400 μl D2O (99%, Cambridge Isotope Laboratories, Woburn, Massachusetts, USA) containing 0.1% ethylene glycol as an internal reference signal and pH was adjusted to values between 6.8 and 7.0. Proton decoupled 13C MR spectra were acquired on a 11.7T Bruker DRX500 spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany), and relevant peaks in the 13C spectra were quantified by integration in XWINNMR in relation to the known amount of ethylene glycol added to the sample. The following acquisition parameters were applied; 30° pulse angle, acquisition time of 1.3 s and a relaxation delay of 2.5 s. The number of scans was typically 15,000. Some spectra were also broad band decoupled only during acquisition and accompanied by a relaxation delay of 20 s, to achieve fully relaxed spectra without NOE. From several sets of spectra, correction factors were obtained and applied to the integrals of the individual peaks.
The total amounts of glutamate, glutamine, GABA, alanine and aspartate in the extracts were quantified by HPLC analysis after derivatization with o-phthaldialdehyde essentially as described by Geddes and Wood [5], using a Hewlett Packard 1100 apparatus (Agilent, Palo Alto, CA, USA).
Percent enrichment in the 13C labelled metabolites was calculated from the following formula:
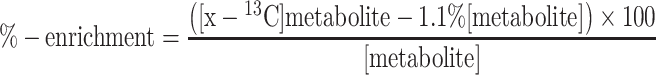 |
[Metabolite] represents the total amount of a given metabolite measured by HPLC. [X-13C]metabolite represent the concentration of a 13C isotopomer of a given metabolite measured by 13C MRS in ex vivo tissue extracts. This calculation was done for each peak in the ex vivo 13C spectrum obtained for each rat individually.
A t test was thereafter used to compare the means of the HPLC and ex vivo 13C MRS data (n = 5 in each group), and the statistical significance was set to P < 0.05.
Metabolic Model
The time course of plasma glucose 13C enrichment and the time courses for incorporation of 13C label into [4-13C]glutamate and [3-13C]glutamate from the in vivo MRS experiment were fitted with a comprehensive mathematical metabolic model described in Henry et al. [6]. The metabolic model consists of a set of differential equations which can be solved numerically using SAAM II (The SAAM institute, Seattle, WA). Since most glutamate is located in neurons [13], metabolic fluxes obtained using this model primarily reflect neuronal metabolism. Furthermore, the concentrations of glutamate, glutamine and aspartate measured by HPLC were also fitted into the model. Based on literature values the concentration of oxaloacetate was assumed to be 1 μmol/g and that of α-ketoglutarate was assumed to be 0.5 μmol/g in brain tissue [7]. In the one-compartment model used there were four unknown parameters; (1) a rate constant describing the neuronal TCA cycle (VTCA) (2) the exchange rate between α-ketoglutarate and glutamate (VX) (3) the rate constant describing the dilution of labelled 13C lactate through exchange with unlabelled lactate (VDIL) and (4) a dilution flux to account for loss of 13C-labelled glutamine through exchange with unlabelled glutamine (VEFF) [6]. Furthermore, the model described in Henry et al. [6] assumes that VGLN = 0.41 × VTCA based on previous studies [7] and since [1,6-13C]glucose was infused the rate of glycolysis VGLY was assumed to be the same as VTCA. Each animal was modelled individually and mean values of the rate of the neuronal TCA cycle and the exchange rate between α-ketoglutarate and glutamate were then calculated for each group. The dilution flux to account for loss of 13C-labelled glutamine through exchange with unlabelled glutamine could not be determined with accuracy for the animals in the hydrocephalus group, and the rate constant describing the dilution of labelled 13C lactate through exchange with unlabelled lactate could not be determined at all since this parameter reached the lowest limit constraint in the model which was 0.01. A non-parametric Mann–Whitney test was thereafter applied to compare the means, and the statistical significance was set to P < 0.05.
Results
All the rats subjected to kaolin injection were indeed hydrocephalic as evidenced by ventriculomegaly involving all ventricles and compression of cortical tissue (Fig. 1). The bench experiment (n = 4, values are mean ± SD) showed that the pH (7.35 ± 0.007), pO2 (31.3 ± 2.1 kPa) and pCO2 (5.17 ± 0.43 kPa) were all at normal values throughout the glucose infusion. Glucose concentration rose from 5.8 ± 1.0 mmol/l at the start of the infusion to 36.5 ± 2.6 mmol/l at the end of the infusion, i.e. after 80 min. The concentration of plasma lactate was measured to be 1.00 ± 0.04 mmol/l prior to the infusion and rose to 3.31 ± 0.51 mmol/l during the infusion.
Fig. 1.

Coronal T2-weighted MR images of controls and rats with acute hydrocephalus 2 weeks after kaolin treatment are shown
The incorporation of 13C label into [4-13C]glutamate and [3-13C]glutamate in the brain during glucose infusion is shown in Fig. 2. As seen from the figure this incorporation was lower in the hydrocephalus group as compared with the control group. These time courses were fitted with a one-compartment model to determine the rate constant describing the neuronal TCA cycle and the exchange rate between α-ketoglutarate and glutamate, which are shown in Table 1. The dilution flux to account for loss of 13C-labelled glutamine through exchange with unlabelled glutamine was 0.49 ± 0.02 μmoles/gram/minute in the control group, but could not be determined in the hydrocephalus animals since the standard deviation of the mean was larger than the mean value itself. The rate constant describing the dilution of labelled 13C lactate through exchange with unlabelled lactate hit lower limit.
Fig. 2.
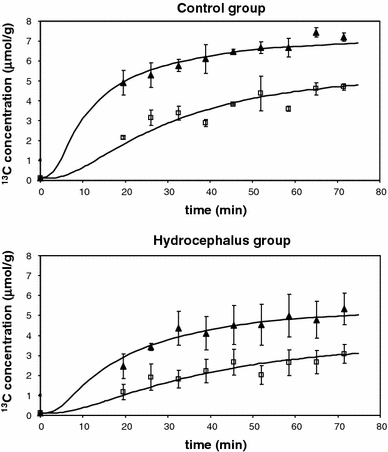
Incorporation 13C into [4-13C]glutamate (black triangles) and [3-13C]glutamate (open squares) for the control group and the hydrocephalus group (μmol/gram/min). The error bars at each experimental point denotes the SEM of three individual animals in each group. The best fits of the metabolic model to the data are shown as continuous lines and the values of the rate of the neuronal TCA cycle and the exchange rate between a-ketoglutarate and glutamatecorresponds to the best fit. These metabolic rates are given in Tabel 1
Table 1.
Values of of the rate of the neuronal TCA cycle and the exchange rate between α-ketoglutarate and glutamate derived from the fit of the experimental data with the metabolic model
| Control (n = 3) | Hydrocephalus (n = 3) | P-value | |
|---|---|---|---|
| Rate of neuronal TCA cycle | 1.3 ± 0.2 | 0.8 ± 0.4 | 0.275 |
| Exchange rate between α-ketoglutarate and glutamate | 4.1 ± 2.5 | 2.7 ± 2.6 | 0.513 |
All results are expressed as mean ± standard deviation (μmoles/gram/minute).of 3 control animals and 3 rats with induced hydrocephalus. Statistical differences between the two groups were analyzed using non-parametric Mann–Whitney test
* The level of significance was set at P < 0.05
Oedema was assessed by comparing wet weight tissue sample before extraction and the freeze dried pellet after extraction and the ratio between them was 5.19 ± 0.15 for controls and 5.61 ± 0.43 for the hydrocephalus group (P = 0.075). Thus, there might be a slight accumulation of water in the tissue of the animals with hydrocephalus.
Table 2 gives the concentration of metabolites that were measured in the cortical tissue samples at the end of the experiment corrected for wet weight. The contents of glutamate, taurine, alanine and N-acetyl aspartate (NAA) were decreased in the brains of the animals with hydrocephalus compared with the control group when corrected for wet weight.
Tabel 2.
The amount of metabolites in the brain tissue of hydrocephalus animals and controls
| Control (n = 5) | Hydrocephalus (n = 5) | P-value | |
|---|---|---|---|
| Glutathione | 1.12 ± 0.16 | 1.02 ± 0.20 | 0.89 |
| Aspartate | 1.93 ± 0.20 | 1.69 ± 0.17 | 0.11 |
| Glutamate | 9.20 ± 0.50 | 7.86 ± 0.97* | 0.03 |
| Glutamine | 5.90 ± 0.61 | 5.5 ± 0.51 | 0.15 |
| GABA | 0.96 ± 0.18 | 0.88 ± 0.04 | 0.33 |
| Taurine | 5.29 ± 0.63 | 4.54 ± 0.33* | 0.04 |
| Alanine | 0.93 ± 0.07 | 0.69 ± 0.15* | 0.02 |
| NAA | 11.73 ± 1.03 | 9.41 ± 1.76* | 0.03 |
| Myo-inositol | 4.13 ± 0.55 | 4.12 ± 1.29 | 0.99 |
| Lactate | 4.58 ± 1.46 | 3.73 ± 1.80 | 0.43 |
All results are expressed as mean ± standard deviation of 5 control animals and 5 rats with hydrocephalus. All concentrations were corrected for wet weight (μmol/g tissue). Statistical differences between the two groups were analyzed using unpaired two-tailed Student’s t test
* The level of significance was set at P < 0.05
The content of 13C labelled metabolites and the %-13C enrichment (for calculation see materials and methods) were very similar in most metabolites except for glutamine, where both percent enrichment (28 ± 5% for controls and 22 ± 4% for hydrocephalus rats, P = 0.05) and amount of label (1.65 ± 0.28 μmol/gram for controls and 1.17 ± 0.32 for hydrocephalus rats P = 0.03) were decreased in the C-2 position.
Discussion
Several studies have used 31P, 1H and ex vivo 13C MRS to study hydrocephalus and they all provide evidence for both a disturbance of cerebral energy metabolism and neuronal and/or myelin dysfunction [8–10]. The current study is the first one in which cerebral metabolism has been investigated using in vivo 13C MRS in a rodent model of hydrocephalus. This allows the assessment of the TCA cycle rate based on measurements of 13C labelling time courses in individual animals. Thus, the number of animals used is reduced in contrast to metabolic rate determination using ex vivo 13C MRS. Despite the poorer resolution in spectra obtained using in vivo MRS, uncertainties in the measurements related to post mortem changes and possible errors in the extraction procedures are avoided.
The rate of the neuronal TCA cycle was not significantly different in the rats with hydrocephalus compared to the control group in the present study. This is in line with results obtained in an ex vivo 13C MRS study, where no differences were found in 13C incorporation in glutamate, glutamine, GABA and aspartate [11]. The fact that there were no significant differences in the rate of the neuronal TCA cycle and the exchange rate between α-ketoglutarate and glutamate between the groups indicates that the rats with hydrocephalus were indeed able to maintain normal TCA cycle metabolism. However, due to the small number of animals in each group, only large differences in metabolism will be detected. Also glycolysis appears to be unchanged, since lactate levels were similar in the two groups, in agreement with a previous study [3].
Subtle alterations in neuronal metabolism were detected in rats with hydrocephalus compared with controls in the present study. NAA is a neuronal marker [12], and most glutamate is located in neurons [13]. Concentrations of both these metabolite were decreased in cortex of rats with hydrocephalus compared to those in control rats, pointing to decreased neuronal function in the early stage (acute phase) of hydrocephalus. A similar decrease in glutamate level in rats 2 weeks after hydrocephalus induction was observed by Kondziella et al. 2009 [3] using in vivo 1H MRS. However, Kondziella et al. 2002 [14] analyzed metabolites in the entire cerebrum at 2 weeks after hydrocephalus induction and found no such difference. Thus, it can be hypothesized that the largest changes in glutamate concentration take place in the cortex, as both the most recent studies analyzed cortical tissue and not the entire brain. While the NAA level was decreased in the present study, Kondziella et al. 2003 and 2009 [3, 11] reported unchanged levels 2 weeks after kaolin induced hydrocephalus. A similar discrepancy was obtained with alanine, which was decreased in the present study, but not in the aforementioned publications. This might point to slight variations in the induction and possibly severity of the kaolin model of hydrocephalus. Indeed results from 1H MRS of brain extracts from Texas rats with hydrocephalus, a genetic model of neonatal hydrocephalus due to aqueduct stenosis with onset in late gestation, revealed a decrease in many metabolites (about 50% reduction in glutamine, glutamate, aspartate, creatinine, NAA, taurine and alanine) compared to controls [15–17], pointing to marked differences on metabolites in different hydrocephalus models.
The present study provides important translational evidence for an understanding of long-term sequels in chronic hydrocephalus. It offers evidence to the fact that hydrocephalus causes subtle but significant alterations in neuronal metabolism already early in the course of the disease. These sub-lethal changes, however, if maintained and if ongoing might explain the delayed and programmed neuronal damage as seen in chronic hydrocephalus.
Furthermore, in the present study there is indication of reduction also in astrocyte metabolism, since there was a decrease in labelling and % 13C enrichment in the C-2 position of glutamine. This position is labelled by repeated turns in the TCA cycle with the same intensity as C-3 and by pyruvate carboxylase, an astrocyte specific enzyme [18, 19]. Since only C-2 was affected and not C-3, the pyruvate carboxylase activity appeared to be slightly decreased in the hydrocephalus group. This is in line with a decrease in astrocyte metabolism observed at 4 weeks after hydrocephalus induction using ex vivo MRS [11]. However, at 6 weeks (chronic hydrocephalus) astrocyte metabolism had returned to controls levels [11].
Ventriculomegaly and brain tissue compression was confirmed with MRI of rats with hydrocephalus which was in line with previous results [3, 9]. Considerable oedema has been detected 4 weeks after kaoline injection [14]. However, at 2 weeks after kaoline injection only a tendency towards elevated tissue water content was seen in the current study, but this did not reach statistical significance. An additional indication of disturbed tissue water content was the finding of altered levels of the osmolyte taurine. However, the amount of taurine was significantly decreased in the hydrocephalus group compared with controls, similar to the findings in Kondziella et al. 2002 [14]. Altered taurine levels can be used to control osmolyte balance in the event of changes in water content.
In conclusion, the present results show that in acute experimental hydrocephalus there is slightly reduced neuronal and astrocytic metabolism, but still the hydrocephalic brain is able to maintain its overall metabolism as measured by the rate of the neuronal TCA cycle. This supports the hypothesis that in hydrocephalus disturbance of CSF dynamics is not the only pathophysiologic factor but that metabolic impairment is also of significance [20]. Sublethal deterioration of brain function in the acute course of hydrocephalus has been shown by experimental and clinical studies of blood flow [21] and the present study adds metabolic evidence to it. Possibly, modern metabolic spectroscopy might be used in the future as a diagnostic tool in human hydrocephalus and assist decision making on treatment.
Acknowledgments
The authors would like to thank Tore W. Meisingseth, Sivert H. Sandvik and Tina B. Pedersen for practical help with the bench experiment. Pierre-Gilles Henry wishes to acknowledge support from NIH grant R01 NS38672.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- CSF
Cerebrospinal fluid
- HPLC
High performance liquid chromatography
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- NAA
N-acetyl aspartate
- TCA
Tricarboxylic acid
References
- 1.Dixon WE, Heller H. Experimentelle Hypertonie durch Erhöhung des intrakraniellen Druckes. Arch Exp Pathol Pharmacol. 1932;166:265–275. doi: 10.1007/BF01860670. [DOI] [Google Scholar]
- 2.Hochwald GM, Nakamura S, Camins MB. The rat in experimental obstructive hydrocephalus. Z Kinderchir. 1981;34:403–410. doi: 10.1055/s-2008-1063383. [DOI] [PubMed] [Google Scholar]
- 3.Kondziella D, Eyjolfsson EM, Saether O, et al. Gray matter metabolism in acute and chronic hydrocephalus. Neuroscience. 2009;159:570–577. doi: 10.1016/j.neuroscience.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 5.Geddes JW, Wood JD. Changes in the amino acid content of nerve endings (synaptosomes) induced by drugs that alter the metabolism of glutamate and gamma-aminobutyric acid. J Neurochem. 1984;42:16–24. doi: 10.1111/j.1471-4159.1984.tb09691.x. [DOI] [PubMed] [Google Scholar]
- 6.Henry PG, Criego AB, Kumar A, et al. Measurement of cerebral oxidative glucose consumption in patients with type 1 diabetes mellitus and hypoglycemia unawareness using (13)C nuclear magnetic resonance spectroscopy. Metabolism. 2010;59:100–106. doi: 10.1016/j.metabol.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–E112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- 8.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 9.McAllister JP, 2nd, Chovan P. Neonatal hydrocephalus. Mechanisms and consequences. Neurosurg Clin N Am. 1998;9:73–93. [PubMed] [Google Scholar]
- 10.Braun KP, van Eijsden P, Vandertop WP, et al. Cerebral metabolism in experimental hydrocephalus: an in vivo 1H and 31P magnetic resonance spectroscopy study. J Neurosurg. 1999;91:660–668. doi: 10.3171/jns.1999.91.4.0660. [DOI] [PubMed] [Google Scholar]
- 11.Kondziella D, Qu H, Ludemann W, et al. Astrocyte metabolism is disturbed in the early development of experimental hydrocephalus. J Neurochem. 2003;85:274–281. doi: 10.1046/j.1471-4159.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- 12.Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/A:1023250721185. [DOI] [PubMed] [Google Scholar]
- 13.Ottersen OP, Storm-Mathisen J. Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- 14.Kondziella D, Ludemann W, Brinker T, et al. Alterations in brain metabolism, CNS morphology and CSF dynamics in adult rats with kaolin-induced hydrocephalus. Brain Res. 2002;927:35–41. doi: 10.1016/S0006-8993(01)03320-0. [DOI] [PubMed] [Google Scholar]
- 15.Harris NG, Jones HC, Williams SC. MR imaging for measurements of ventricles and cerebral cortex in postnatal rats (H-Tx strain) with progressive inherited hydrocephalus. Exp Neurol. 1992;118:1–6. doi: 10.1016/0014-4886(92)90016-J. [DOI] [PubMed] [Google Scholar]
- 16.Harris NG, Plant HD, Briggs RW, et al. Metabolite changes in the cerebral cortex of treated and untreated infant hydrocephalic rats studied using in vitro 31P-NMR spectroscopy. J Neurochem. 1996;67:2030–2038. doi: 10.1046/j.1471-4159.1996.67052030.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones HC, Harris NG, Rocca JR, et al. Progressive changes in cortical metabolites at three stages of infantile hydrocephalus studied by in vitro NMR spectroscopy. J Neurotrauma. 1997;14:587–602. doi: 10.1089/neu.1997.14.587. [DOI] [PubMed] [Google Scholar]
- 18.Shank RP, Bennett GS, Freytag SO, et al. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 19.Yu AC, Drejer J, Hertz L, et al. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 20.Kondziella D, Sonnewald U, Tullberg M, et al. Brain metabolism in adult chronic hydrocephalus. J Neurochem. 2008;106:1515–1524. doi: 10.1111/j.1471-4159.2008.05422.x. [DOI] [PubMed] [Google Scholar]
- 21.Klinge PM, Samii A, Muhlendyck A, et al. Cerebral hypoperfusion and delayed hippocampal response after induction of adult kaolin hydrocephalus. Stroke. 2003;34:193–199. doi: 10.1161/01.STR.0000048820.17198.15. [DOI] [PubMed] [Google Scholar]


