Abstract
Some gender differences in the progression of human immunodeficiency virus (HIV) infection have been attributed to delayed treatment among women and the social context of poverty. Recent economic difficulties have led to multiple service cuts, highlighting the need to identify factors with the most influence on health in order to prioritize scarce resources. The aim of this study was to empirically rank factors that longitudinally impact the health status of HIV-infected homeless and unstably housed women. Study participants were recruited between 2002 and 2008 from community-based venues in San Francisco, California, and followed over time; marginal structural models and targeted variable importance were used to rank factors by their influence. In adjusted analysis, the factor with the strongest effect on overall mental health was unmet subsistence needs (i.e., food, hygiene, and shelter needs), followed by poor adherence to antiretroviral therapy, not having a close friend, and the use of crack cocaine. Factors with the strongest effects on physical health and gynecologic symptoms followed similar patterns. Within this population, an inability to meet basic subsistence needs has at least as much of an effect on overall health as adherence to antiretroviral therapy, suggesting that advances in HIV medicine will not fully benefit indigent women until their subsistence needs are met.
Keywords: health status, HIV, homeless persons, women
The National Health and Nutrition Examination Survey (NHANES) indicates that women and low-income persons are at increased risk of reporting poor health-related quality of life (1). Homeless persons, in particular, often suffer from serious mental and physical health problems that are attributed to numerous health risks, as well as low social support and a lack of social resources (2), particularly among women (3). It has been suggested that the face of homelessness is changing in some geographic areas from adult male alcoholics to an increasingly diverse population with complex medical illnesses, including psychiatric conditions, physical disability, consequences of drug and alcohol abuse, and violence (4). As part of this changing social landscape, female gender is now one of the strongest predictors of poor health among homeless persons (5, 6).
Human immunodeficiency virus (HIV) infection introduces another layer of complexity for vulnerable persons. HIV is increasingly characterized as a chronic condition that can be managed through adherence to a healthy lifestyle, complex drug regimens, and treatment monitoring; however, a person’s housing status can be a significant determinant of an individual’s ability to meet these requirements and achieve better health status (7). In addition, the effects of complex and interconnected risks facing homeless persons may be modified by gender. For instance, some gender differences in the progression of HIV have been attributed to delayed and fragmented treatment among women (8, 9) and the social context of poverty (10). This social context highlights issues such as drug use, which is an established risk factor for both HIV infection and homelessness among women (11, 12).
Improved antiretroviral medications have led to an era in which HIV has become a more manageable chronic illness, with increased attention to conditions beyond disease progression, such as the development of non-acquired immunodeficiency syndrome (AIDS)-defining cancers (13). The effects of exposures that change over time and influence one another, such as drug use and social support structures, now have the opportunity to influence a longer disease course. Few studies have considered the varying levels of these exposures over time among unstably housed persons. Additionally, few studies regarding the impact of competing needs on the health status of HIV-infected persons have considered data collected exclusively from unstably housed persons, and few have recruited participants from noninstitutional settings, which systematically excludes those outside the health-care system.
A better understanding of the numerous and changing health risks experienced by HIV-infected, unstably housed persons has the potential to improve the delivery of health and social services; however, funding for programs serving vulnerable populations, such as public health, mental health, and drug treatment programs, has been cut drastically (14–17). Understanding that each factor is important but recognizing program limitations, we aimed first in the current study to determine the extent to which changing risks influenced the health status of HIV-positive, unstably housed women over time and then empirically to rank risk exposures by their level of influence.
MATERIALS AND METHODS
Participants
From July 2002 to September 2008, a mobile outreach team recruited a probability sample of homeless and unstably housed biologic women from San Francisco homeless shelters, free-food programs, and a random sample of low-income hotels in 3 neighborhoods selected with probability proportional to the number of residents (18, 19). In addition, informational flyers were posted in these venues. All individuals who interfaced with outreach staff, obtained study information at these venues, or were referred by venue staff were eligible to participate in screening activities. The only inclusion criterion was HIV-positive serostatus. Study participants were part of “Shelter, Health, and Drug Outcomes among Women” (SHADOW), an ongoing cohort study that assesses the impact of competing risks on the health and well-being of poor and unstably housed women.
Procedures
Participants were asked to visit a community-based field site every 3 months. There, they provided blood for CD4 cell count and viral load assessments and completed a confidential interviewer-administered questionnaire to assess the presence and extent of factors that might influence health status. All study procedures were conducted with the approval of the Committee on Human Research at the University of California at San Francisco.
Outcome variables: health status
The Medical Outcome Study’s Short Form 36 (SF-36) (20) is a reliable and valid measure of health in this population (21) and was used to assess overall health; the Mental Composite Score and the Physical Composite Score ranged from 0 to 100, where higher scores indicated better health. In addition, given a more recent focus on gynecologic health that has been made relevant by improved HIV therapies (22), the presence of gynecologic symptoms was assessed. A binary variable was based on a panel of gynecologic symptoms and conditions developed by Wenzel et al. (23) and included abnormal vaginal discharge, severe pelvic pain, burning during urination, blood in the urine, skipped period, abnormally heavy periods, and new warts, sores, or lumps on the genitals. Thus, although better health was indicated by a unit increase in the Mental Composite Score and the Physical Composite Score, it was indicated by a decrease in (the absence of) gynecologic symptoms; that is, better health was indicated by higher linear estimates for the Mental Composite Score and the Physical Composite Score but lower odds ratios for gynecologic symptoms.
Main effects: competing health risks
Variables considered as having potential influence on health status have been previously established as important factors in predicting health. These factors included sociodemographic variables; unmet subsistence needs (difficulty gaining access to a bathroom, place to wash, clothing, food, or a place to sleep, regardless of where the respondent slept) (24); social support (having at least 1 close friend/confidant) and instrumental support (having someone who would lend the respondent money or give her a place to sleep) (25); heavy alcohol use (>1 drink/day) (26); any use of heroin, crack cocaine, or methamphetamine; symptoms of withdrawal from heroin, crack cocaine, methamphetamine, and alcohol detected in the Diagnostic Interview Schedule (27); exchange of sex for money or drugs (28); use of and adherence to antiretroviral therapy; and chronic health conditions experienced during the study (asthma, diabetes, heart disease, high blood pressure, and/or emphysema). The CD4 cell count and viral load are intermediate variables on the causal path between study variables and overall health status. Thus, in accordance with recommendations regarding overadjustment bias (29, 30), these variables were not considered potential candidates for the treatment model. Health services were not adjusted in the current study, as service use is a consequence of poor health and not a risk factor.
Statistical methods
Targeted variable importance (tVIM) was used to analyze data. tVIM assesses the effects of large numbers of variables with unknown or diverse correlation structures; it more accurately assesses effects when compared with techniques that rely on parametric regression models (31–34). tVIM estimates the effect of 1 variable at a time, which tailors the estimation approach toward the specific effect of interest, thereby providing more accurate effects and assessment of uncertainty. This approach is important for the analyses described herein, because different types and a broad spectrum of variables were analyzed for the current study and, thus, a single multivariate regression model approach would be impossible. tVIM involves 2 steps: the first step to estimate the population effect for each variable of interest by using a marginal structural model approach to estimate the parameters, and the second step to rank significant risk factors by the strength of the effect on the outcome.
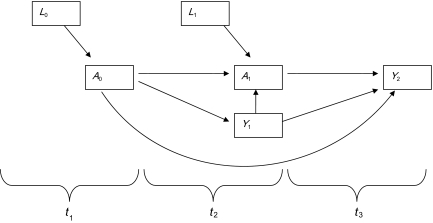
First, marginal structural models were used to estimate the population effect (35) of each risk factor, adjusting for confounding (36) via the general formula logit(P(Ya(t) = 1) = m(a(t − 1)|β) = β0 + β1a(t − 1) for binary variables and E(Ya(t)) = m(a(t − 1)|α) = α0 + α1a(t − 1) for continuous variables (Figure 1). Confounders were assessed for each risk factor separately to obtain estimates of the adjusted effects on health status. Also, variables were considered potential confounders in all models for which they were not being considered the primary effect. Standard errors were calculated through bootstrap methods, which do not treat weighting as implicitly fixed but as stochastic (37). In addition, the following variables were considered potential confounders for all analyses: 1) to account for secular trends, the year of study participation was considered; 2) to account for potential study effects, the number of study participation months was considered; 3) in recognition that age is an established predictor of health status, age was considered; and 4) in consideration of the potential cumulative effects of drug use, an individual’s drug use history was considered a potential confounder in order to focus on the impact of current drug use only. To ensure that the exposure preceded the outcome (an assumption of risk and the statistical models that estimate it), the effect of a change in the level of the exposure on the outcome in the current quarter was assessed (i.e., a 1-unit change for the Mental Composite Score and the Physical Composite Score or a change in odds ratios for gynecologic symptoms), adjusting for all other significant confounding variables from the prior quarters.
Figure 1.
Causal diagram for the effect of competing social needs on the health status of human immunodeficiency virus-positive, unstably housed women living in San Francisco, California, 2002–2008. A0 and A1 represent measurements of a single exposure variable at 2 consecutive timepoints (t1 and t2). L0 and L1 denote measured confounders that are associated with both A0 and A1 and, therefore, are included in the treatment model. Y1 and Y2 represent the health status at 2 consecutive timepoints (t2 and t3). It is important to note that marginal structural models account for changes in confounding over time and account for the fact that the outcome can influence the exposure.
Next, tVIM techniques were applied such that the risk factor-specific effects obtained by the marginal structural models were ranked by P value, which was appropriate for the current study because the exposure variables (i.e., risk factors) had different units of measure. Because the population and sample size were consistent between models, ranking variables based on the P value was a standardized approach to ranking effect estimates (i.e., signal-to-noise ratio). Thus, ranking is not from the most negative to the most positive effect; rather, it is from the variable with the largest population-level effect on the outcome to that with the smallest. With this approach, variables with the strongest individual influence will not necessarily have the largest population-level effect if their prevalence is low. If all covariates represent all confounding, then the tVIM estimate can be interpreted as the average causal effect of that exposure on the outcome. If this assumption is untenable, then the tVIM estimate can be interpreted as a summary measure of the importance of the variable after controlling for covariates.
Because participants were recruited into the study at different time points, a staggered entry approach was used where follow-up time began at the time of study entry for each individual. Because individuals belonged to different comparison groups as the risk factors changed, estimates yielded by each model represented the overall average effect of each risk factor, adjusting for measured confounders. To account for missing values, we used a forward imputation method in which the most recent previously observed value for that participant was analyzed, which is a common approach in analyses that include marginal structural modeling (38–40). If no previous value was observed, the median population value was used for continuous variables, and categorical variables were imputed at the category with the highest proportion of observed values. This approach was chosen because it does not rely on modeling assumptions to predict missing information and ensures that original data are preserved.
RESULTS
Among the 249 female adults who were screened for study participation, 133 were confirmed as HIV infected and, thus, eligible to participate. A 3% refusal rate resulted in a cohort of 129 HIV-infected women. Loss to follow-up was approximately 2% per year, and the mortality rate was approximately 0.5% per year; the median follow-up time was 10 months per person. Outcome variables did not contain any missing values, and missing data for exposure variables did not exceed 6.5%.
The median age of study participants was 44 years, 42% were African American, 29% Caucasian, 5% Latina, and 14% “other” (Table 1). At baseline, 33% of respondents reported the use of crack cocaine, and 20% reported sleeping on the street or in a homeless shelter during the past 3 months. Unmet subsistence needs were reported by 25% of the population. Having no sources of instrumental support was reported by 20% of study participants; any insurance coverage was reported by 97% of participants (89% Medicaid; 15% Veterans; 10% private). At baseline, the median viral load was 1,105 copies/mL, the median CD4 cell count was 385 cells/μL, and 57% of participants reported at least 1 chronic health condition. Highlighting the importance of change over time, 52% of participants took antiretroviral therapy at baseline, while 71% took antiretroviral therapy at some point during the study; 31% of those on antiretroviral therapy at baseline reported ≥90% adherence, while 72% were >90% adherent at some point in the study; 3% experienced cocaine withdrawal, and 1% experienced alcohol withdrawal at baseline, while 11% and 20% experienced cocaine and alcohol withdrawal, respectively, at some point in the study.
Table 1.
Baseline Characteristics and Behaviors (Past 3 Months) of HIV-infected Homeless and Unstably Housed Women (n = 129) Living in San Francisco, California, 2002–2008
| Median | % | |
| Socioeconomic | ||
| Age, years | 44 | |
| Graduated from high school | 40 | |
| Race | ||
| African American | 52 | |
| Caucasian | 29 | |
| Latina | 5 | |
| Other | 14 | |
| Minor children living at home | 7 | |
| Overnight jail/prison stay | 7 | |
| Slept on the street or in a homeless shelter | 20 | |
| Employed | 5 | |
| Any income from SSI or SSDI | 80 | |
| Monthly income, $ | 812 | |
| Drug and alcohol use (past 3 months) | ||
| Crack cocaine | 33 | |
| Heroin | 15 | |
| Methamphetamine | 1 | |
| Cocaine withdrawal | 3 | |
| Heavy alcohol use (>1 drink/day) | 14 | |
| Alcohol withdrawal | 1 | |
| Subsistence needs and social support (past 3 months) | ||
| Difficulty meeting basic subsistence needsa | 25 | |
| At least one close friend/confidant | 69 | |
| No sources of instrumental support | 20 | |
| Health | ||
| CD4 cell count, cells/μL | 385 | |
| Viral load, copies/mL | 1,105 | |
| Taking antiretroviral therapy | 52 | |
| >90% adherence (among those taking ART) | 31 | |
| Any chronic health conditionb | 57 | |
| Physical health composite scorec | 41/100 | |
| Mental health composite scorec | 42/100 | |
| Any gynecologic symptomsd | 43 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; SSDI, Social Security Disability Insurance; SSI, Supplemental Security Income.
Access to a bathroom, sufficient food, clothing, or a place to sleep.
Asthma, diabetes, heart disease, high blood pressure, or emphysema.
General population median = 50/100.
Abnormal vaginal discharge, severe pelvic pain, burning during urination/blood in the urine, skipped period, abnormally heavy periods, or new warts, sores, or lumps on the genitals.
Exploratory factor analysis of the 5-item subsistence needs variable measured at baseline was performed with Mplus, version 6, software (Muthen & Muthen, Los Angeles, California). A single dominant factor emerged (eigenvalue = 4.68) with all other eigenvalues being negligible in size (≤0.25). The fit of a 1-factor model to the data was excellent, as indicated by the χ2 test of exact fit (χ25df = 3.64; P = 0.60). Factor loadings for all items exceeded 0.85, demonstrating strong factor-item relations for all 5 items. Reliability for this factor’s items was excellent (Cronbach’s α = 0.90). These items were, therefore, considered to comprise a subsistence needs scale whose composite score was used in the analyses described below.
Overall mental health
The population median for the Mental Composite Score was 42 (of a possible 100; median for the general population = 50). With adjustment for all significant study confounders, unmet subsistence needs was the most important explanatory variable (i.e., had the largest effect) among the study’s estimated effects on the overall mental health of women in this population (Table 2). On average, women reporting unmet subsistence needs had Mental Composite Scores that were 5.37% lower than those who did not (P = 0.000027), after adjustment for all other significant study confounders. In separate models and, in order of the population effect on the Mental Composite Score, ≥90% antiretroviral therapy adherence and having a close friend/confidant were the second and third most important explanatory variables for overall mental health, corresponding to a 5.07% (P = 0.0006) and a 3.20% (P = 0.0014) higher Mental Composite Score, respectively; subsequently ranked variables were crack use with a score of −4.55% (P = 0.0018), any drug use with a score of −4.15% (P = 0.0018), sleeping on the street with a score of −2.92% (P = 0.0036), experiencing cocaine withdrawal with a score of −3.02% (P = 0.011), heavy alcohol use with a score of −2.78% (P = 0.032), and having no sources of instrumental support with a score of −2.26% (P = 0.040).
Table 2.
Ranked Influence of a Unit Increase in Competing Needs During the Past 3 Months on the Overall Mental Health (Score, 0–100)a of HIV-positive Homeless and Unstably Housed Women Living in San Francisco, California, 2002–2008 (n = 129)b
| Main Effect | Crude Population Effect | Crude 95% CI | Adjusted Population Effect | Adjusted 95% CI | tVIM Rank | P Value |
| Unmet subsistence needs | −8.23 | −11.31, −5.05 | −5.37 | −7.34, −2.35 | 1 | 0.000027 |
| ≥90% ART adherence | 7.16 | 4.12, 10.07 | 5.07 | 2.12, 7.76 | 2 | 0.0006 |
| Has a close friend/confidant | 3.97 | 1.70, 6.08 | 3.20 | 1.05, 4.98 | 3 | 0.0014 |
| Crack use | −7.58 | −10.47, −4.58 | −4.55 | −7.2, −1.69 | 4 | 0.0018 |
| Any drug use | −7.43 | −10.3, −4.43 | −4.15 | −6.81, −1.6 | 5 | 0.0018 |
| Slept on the street | −10.06 | −14.35, −5.76 | −2.92 | −4.47, −0.49 | 6 | 0.0036 |
| Cocaine withdrawal | −9.32 | −12.98, −5.69 | −3.02 | −4.95, −0.34 | 7 | 0.011 |
| Heavy alcohol use | −4.57 | −7.43, −1.66 | −2.78 | −5.44, −0.38 | 8 | 0.032 |
| No reported sources of instrumental support | −4.49 | −6.65, −2.19 | −2.26 | −4.49, −0.15 | 9 | 0.040 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; SF-36, Short Form 36; tVIM, targeted variable importance.
SF-36 mental health composite score in which a higher score indicates better health.
Each row represents a separate model in which a single main linear effect is estimated, adjusting for the potential confounding of all other significant study variables.
Overall physical health
The population median for the Physical Composite Score was 41 (of a possible 100; median for the general population = 50). With adjustment for all significant study confounders, crack use was the most important explanatory variable among the study’s estimated effects on the overall physical health of women in this population (Table 3). On average, women reporting crack use had Physical Composite Scores that were 3.62% lower (P = 0.0052) than those who did not, after adjustment for all other significant study confounders. In separate models, and in order of their adjusted population effects on physical health status scores, any drug use and unmet subsistence needs also had significant negative impact on overall physical health, with −3.1% (P = 0.013) and −2.9% (P = 0.016) scores, respectively.
Table 3.
Ranked Influence of a Unit Increase in Competing Needs During the Past 3 Months on the Overall Physical Health (Score, 0–100)a of HIV-positive Homeless and Unstably Housed Women Living in San Francisco, California, 2002–2008 (n = 129)b
| Main Effect | Crude Population Effect | Crude 95% CI | Adjusted Population Effect | Adjusted 95% CI | tVIM Rank | P Value |
| Crack use | −3.89 | −6.63, −1.08 | −3.62 | −5.89, −0.94 | 1 | 0.052 |
| Any drug use | −3.59 | −6.15, −0.88 | −3.1 | −5.5, −0.6 | 2 | 0.013 |
| Unmet subsistence needs | −3.02 | −5.31, −0.8 | −2.91 | −5.33, −0.61 | 3 | 0.016 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; SF-36, Short Form 36; tVIM, targeted variable importance.
SF-36 physical health composite score in which a higher score indicates better health.
Each row represents a separate model in which a single main linear effect is estimated, adjusting for the potential confounding of all other significant study variables.
Gynecologic symptoms and conditions
At least 1 gynecologic symptom or condition was reported by 43% of this population. With adjustment for all significant study confounders, unmet subsistence needs was the most important explanatory variable for the presence of gynecologic symptoms and conditions in this population (Table 4). On average, the odds of reporting gynecologic symptoms were more than 2-fold higher among women reporting unmet subsistence needs (P = 0.0000028). In addition, in order of their adjusted population effects, the odds of reporting gynecologic symptoms were lower among women with ≥90% antiretroviral therapy adherence (odds ratio (OR) = 0.48; P = 0.0032), higher for those reporting heavy alcohol use (OR = 1.95; P = 0.0078) or a close friend/confidant (OR = 2.70; P = 0.017), lower for those reporting no sources of instrumental support (OR = 0.59; P = 0.022) and, continuing in order of rank, higher for individuals reporting heroin (OR = 3.09; P = 0.031) and alcohol withdrawal (OR = 4.36; P = 0.043).
Table 4.
Ranked Exponential Influence of Competing Needs During the Past 3 Months on the Presence of Any Gynecologic Symptoms or Conditions Among HIV-positive Homeless and Unstably Housed Women Living in San Francisco, California, 2002–2008 (n = 129)a
| Main Effect | Crude Odds Ratio | Crude 95% CI | Adjusted Odds Ratio | Adjusted 95% CI | tVIM Rank | P Value |
| Unmet subsistence needs | 3.37 | 2.31, 4.94 | 2.38 | 1.65, 3.40 | 1 | 0.0000028 |
| ≥90% ART adherence | 0.48 | 0.32, 0.73 | 0.54 | 0.35, 0.81 | 2 | 0.0032 |
| Heavy alcohol use | 1.95 | 1.30, 2.86 | 1.66 | 1.16, 2.44 | 3 | 0.0078 |
| Has a close friend/confidant | 2.70 | 1.84, 4.19 | 1.60 | 1.15, 2.43 | 4 | 0.017 |
| No reported sources of instrumental support | 0.48 | 0.31, 0.73 | 0.59 | 0.37, 0.90 | 5 | 0.022 |
| Heroin use | 3.09 | 1.77, 5.28 | 1.34 | 1.00, 1.71 | 6 | 0.031 |
| Alcohol withdrawal | 4.36 | 1.40, 23.34 | 1.65 | 1.00, 2.19 | 7 | 0.043 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; tVIM, targeted variable importance.
Each row represents a separate model in which a single main exponential effect is estimated, adjusting for the potential confounding of all other significant study variables.
DISCUSSION
To our knowledge, this is the first study to rank the population effects of competing risks on the health status of community-recruited, HIV-positive homeless and unstably housed women. In this sample, women’s unmet subsistence needs have the most empirical influence on mental and gynecologic health, while crack cocaine use has the most empirical influence on physical health. These analyses do not necessarily indicate the highest health priorities for specific individuals; instead, they indicate that unmet subsistence needs and the use of crack cocaine have the largest population-level effects on the health of unstably housed HIV-positive women and that the biggest population-wide impact on health would be made by focusing on these issues. The results presented here support evidence from previous research indicating that the social context of poverty often changes the priorities of impoverished persons, frequently relegating important issues such as adherence to medication as less concerning than meeting basic subsistence needs (10).
Our findings are consistent with those of Schlossstein et al. (41), who found that competing priorities (finding housing or employment, keeping welfare appointments, and finding child care) were barriers to keeping medical appointments in a sample of homeless women. Taken together, these results indicate that competing priorities are important nonfinancial barriers to the use of both services and optimal health status and, thus, the results provide compelling support for the consideration of subsistence issues as a core aspect of health care (42).
All primary variables had similar patterns for their effects on the different outcomes (i.e., correlations with both the Mental Composite Score and the Physical Composite Score indicating worse overall health and relative odds of gynecologic symptoms indicating worse gynecologic health), with the exception of reporting a close friend/confidant. Women who reported a confidant had better mental health but worse gynecologic health. Potential confounding of this association by sex work/exchange was accounted for by the chosen statistical methods, but it was not among the most influential variables associated with gynecologic symptoms. Taken together, these results may indicate that the social support homeless women are able to receive largely comes from intimate partners (i.e., those engaged in nontransactional sex) and, although such partnerships may be associated with better emotional health, they may come at the cost of compromising gynecologic health. Continued counseling and testing for additional sexually transmitted infections are prudent among HIV-positive, unstably housed women for both the health of the woman and potential secondary infections among her contacts.
Income, race, and education are widely attributed to poor health in the general population (43–45), yet they did not have significant longitudinal influence in this extremely vulnerable sample of HIV-positive, unstably housed women; however, drug use did. Even after adjustment for the potential cumulative effects of long-term drug use via drug use history, current use of crack, heroin, and alcohol had strong effects on health status. In addition, cocaine withdrawal ranked seventh among the influential factors of mental health, and alcohol withdrawal ranked seventh among the influential factors of gynecologic symptoms. These findings support previous research showing that withdrawal is associated with a more severe clinical history of substance dependence (46). Given that all the factors considered in this study have been previously found to significantly compromise health, this ranking is noteworthy. Drug use and complications of drug use must receive attention to improve health in this population.
The results of this study should be considered in light of potential limitations. First, study participants may have underreported behaviors such as drug use, because of recall bias or social desirability; however, this would have biased results toward the null, indicating that effect sizes are at least as extreme as those reported. Second, models used in this study assumed that there were no unmeasured confounders related to health status, and it is possible that residual confounding existed from unmeasured effects. This limitation is inherent to all modeling techniques, and our inclusion of factors that have been previously established as important correlates of health status was intended to minimize this potential limitation. Third, the data used in the current study came from a single city and may not be generalizeable to other metropolitan areas. There is, however, evidence to suggest similar findings between US studies regarding health research among impoverished populations (7, 10, 47–51). Thus, generalizeability may not be drastically compromised from studying indigent individuals in a single city. The first major strength of this study was a community-recruited sample of indigent women transitioning in and out of homelessness, a population that is known to contend with high rates of competing and unmet needs, yet is underrepresented in the literature. The second strength was frequent data collection over the observation period. Fourth, statistical control for time-dependent confounding was addressed, and population-level effects of competing risks on health status were estimated.
With the transformation of HIV/AIDS from an acute, fatal disease to a chronic disease managed through new advances/medications, it will be increasingly important from a service delivery perspective to better understand the influence that long-term exposures and chronic conditions have on the health of people with HIV/AIDS. Additionally, in an increasingly constrained fiscal environment, studies such as this will be important to assist policymakers in effectively allocating limited resources. The results presented herein suggest that, on a population level, advances in HIV medicine will not fully benefit indigent women until their subsistence needs are met.
Acknowledgments
Author affiliations: Department of Medicine, University of California, San Francisco, California (Elise D. Riley, Jacqueline P. Tulsky, Torsten B. Neilands); Department of Psychiatry, University of California, San Francisco, California (James L. Sorensen); Department of Biostatistics, University of California, Berkeley, California (Kelly Moore); and the Initiative for Global Health, Harvard University, Boston, Massachusetts (David R. Bangsberg).
This work was supported by National Institutes of Health grants DA15605, MH54907, and UL1 RR024131.
The authors thank the study team, Jennifer Cohen, Sujana Bhattacharyya, Kara Marson, Shemena Campbell, and Deb Schneider, as well as collaborating researchers, Richard Clark, John Day, Nelia Dela Cruz, Carina Flores, Minoo Gorji, David Guzman, Scot Hammond, Jackie Haslam, Zizi Hawthorne, Jay Jankowski, Rhonda Johnson, Mac McMaster, Sandra Monk, Rebecca Packard, Joyce Powell, Kathleen Ragland, Mathew Reynolds, Paul Rueckhaus, Jacqueline So, John Weeks, and Kelly Winslow.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- HIV
human immunodeficiency virus
- OR
odds ratio
- tVIM
targeted variable importance
References
- 1.Zahran HS, Kobau R, Moriarty DG, et al. Health-related quality of life surveillance—United States, 1993–2002. Centers for Disease Control and Prevention (CDC) MMWR Surveill Summ. 2005;54(4):1–35. [PubMed] [Google Scholar]
- 2.Hwang SW, Kirst MJ, Chiu S, et al. Multidimensional social support and the health of homeless individuals. J Urban Health. 2009;86(5):791–803. doi: 10.1007/s11524-009-9388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell JM, Smith K. HIV infected women and women’s services. Health Care Women Int. 1998;19(2):131–139. doi: 10.1080/073993398246467. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull J, Muckle W, Masters C. Homelessness and health. CMAJ. 2007;177(9):1065–1066. doi: 10.1503/cmaj.071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley ED, Wu AW, Perry S, et al. Depression and drug use impact health status among marginally housed HIV-infected individuals. AIDS Patient Care STDS. 2003;17(8):401–406. doi: 10.1089/108729103322277411. [DOI] [PubMed] [Google Scholar]
- 6.Gelberg L, Linn LS. Demographic differences in health status of homeless adults. J Gen Intern Med. 1992;7(6):601–608. doi: 10.1007/BF02599198. [DOI] [PubMed] [Google Scholar]
- 7.Leaver CA, Bargh G, Dunn JR, et al. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007;11(suppl 6):85–100. doi: 10.1007/s10461-007-9246-3. [DOI] [PubMed] [Google Scholar]
- 8.Chaisson RE, Keruly JC, Moore RD. Race, sex, drug use, and progression of human immunodeficiency virus disease. N Engl J Med. 1995;333(12):751–756. doi: 10.1056/NEJM199509213331202. [DOI] [PubMed] [Google Scholar]
- 9.Sohler NL, Li X, Cunningham CO. Gender disparities in HIV health care utilization among the severely disadvantaged: can we determine the reasons? AIDS Patient Care STDS. 2009;23(9):775–783. doi: 10.1089/apc.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley ED, Gandhi M, Hare C, et al. Poverty, unstable housing, and HIV infection among women living in the United States. Curr HIV/AIDS Rep. 2007;4(4):181–186. doi: 10.1007/s11904-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 11.Montoya ID. Infectious diseases and anemia in a sample of out-of-treatment drug users. Am J Manag Care. 1998;4(9):1257–1264. [PubMed] [Google Scholar]
- 12.Lum PJ, Sears C, Guydish J. Injection risk behavior among women syringe exchangers in San Francisco. Subst Use Misuse. 2005;40(11):1681–1696. doi: 10.1080/10826080500222834. [DOI] [PubMed] [Google Scholar]
- 13.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Ann Intern Med. 2008;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 14.SMHA Budget Shortfalls: FY 2009, 2010 & 2011. Results Based on Forty-Two States Responding. Alexandria, VA: National Association of State Mental Health Program Directors Research Institute, Inc; 2008. [Google Scholar]
- 15.Johnson N, Oliff P, Williams E. An Update on State Budget Cuts. At least 42 States Have Imposed Cuts That Hurt Vulnerable Residents; Federal Economic Recovery Funds and State Tax Increases Are Reducing the Harm. Washington, DC: Center on Budget and Policy Priorities; 2009. [Google Scholar]
- 16.Casey J. Oregon drug treatment cuts: a lot to lose. The Oregonian. March 30, 2009 [Google Scholar]
- 17.Klas M. Millions in state budget cuts criticized as shortsighted. St. Petersburg Times. January 15, 2009 [Google Scholar]
- 18.Burnam MA, Koegel P. Methodology for obtaining a representative sample of homeless persons: the Los Angeles Skid Row Study. Eval Rev. 1988;12:117–152. [Google Scholar]
- 19.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 21.Riley ED, Bangsberg DR, Perry S, et al. Reliability and validity of the SF-36 in HIV-infected homeless and marginally housed individuals. Qual Life Res. 2003;12(8):1051–1058. doi: 10.1023/a:1026166021386. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox R. Gynecological considerations are more important now that HIV therapies have improved. HIV Clin. 2001;13(2):1–3. [PubMed] [Google Scholar]
- 23.Wenzel SL, Andersen RM, Gifford DS, et al. Homeless women’s gynecological symptoms and use of medical care. J Health Care Poor Underserved. 2001;12(3):323–341. doi: 10.1353/hpu.2010.0797. [DOI] [PubMed] [Google Scholar]
- 24.Gelberg L, Gallagher TC, Andersen RM, et al. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217–220. doi: 10.2105/ajph.87.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gielen AC, McDonnell KA, Wu AW, et al. Quality of life among women living with HIV: the importance violence, social support, and self care behaviors. Soc Sci Med. 2001;52(2):315–322. doi: 10.1016/s0277-9536(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 26.The Physician’s Guide to Helping Patients With Alcohol Problems. Washington, DC: National Institute on Alcohol Abuse and Alcoholism; 1995. (NIH publication no. 95-3769) [Google Scholar]
- 27.Zimmerman M, Coryell W. The validity of a self-report questionnaire for diagnosing major depressive disorder. Arch Gen Psychiatry. 1988;45(8):738–740. doi: 10.1001/archpsyc.1988.01800320050006. [DOI] [PubMed] [Google Scholar]
- 28.Weiser SD, Dilworth SE, Neilands TB, et al. Gender-specific correlates of sex trade among homeless and marginally housed individuals in San Francisco. J Urban Health. 2006;83(4):736–740. doi: 10.1007/s11524-005-9019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day NE, Byar DP, Green SB. Overadjustment in case-control studies. Am J Epidemiol. 1980;112(5):696–706. doi: 10.1093/oxfordjournals.aje.a113042. [DOI] [PubMed] [Google Scholar]
- 31.van der Laan MJ, Rubin D. Targeted maximum likelihood learning [electronic article] Int J Biostat. 2006;2(1) ( http://www.bepress.com/cgi/viewcontent.cgi?article=1043&context=ijb) [Google Scholar]
- 32.van der Laan MJ. Statistical inference for variable importance [electronic article] Int J Biostat. 2006;2(1) ( http://www.bepress.com/ijb/vol2/iss1/2) [Google Scholar]
- 33.Bembom O, Petersen ML, Rhee SY, et al. Biomarker discovery using targeted maximum-likelihood estimation: application to the treatment of antiretroviral-resistant HIV infection. Stat Med. 2009;28(1):152–172. doi: 10.1002/sim.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KL, van der Laan MJ. Covariate adjustment in randomized trials with binary outcomes: targeted maximum likelihood estimation. Stat Med. 2009;28(1):39–64. doi: 10.1002/sim.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics [electronic article] Stat Appl Genet Mol Biol. 2004;3(1) doi: 10.2202/1544-6115.1069. ( http://www.bepress.com/cgi/viewcontent.cgi?article=1069&context=sagmb) [DOI] [PubMed] [Google Scholar]
- 37.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 38.Cole SR, Hernán MA, Margolick JB, et al. Marginal structural models for estimating the effect of highly active antiretroviral therapy initiation on CD4 cell count. Am J Epidemiol. 2005;162(5):471–478. doi: 10.1093/aje/kwi216. [DOI] [PubMed] [Google Scholar]
- 39.Petersen ML, Wang Y, van der Laan MJ, et al. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45(7):908–915. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edmonds A, Lusiama J, Napravnik S, et al. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38(6):1612–1621. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlossstein E, St Clair P, Connell F. Referral keeping in homeless women. J Community Health. 1991;16(6):279–285. doi: 10.1007/BF01324513. [DOI] [PubMed] [Google Scholar]
- 42.Shubert V, Bernstine N. Moving from fact to policy: housing is HIV prevention and health care. AIDS Behav. 2007;11(suppl 6):172–181. doi: 10.1007/s10461-007-9305-9. [DOI] [PubMed] [Google Scholar]
- 43.Shi L, Tsai J, Higgins PC, et al. Racial/ethnic and socioeconomic disparities in access to care and quality of care for US health center patients compared with non-health center patients. J Ambul Care Manage. 2009;32(4):342–350. doi: 10.1097/JAC.0b013e3181ba6fd8. [DOI] [PubMed] [Google Scholar]
- 44.Peckham E, Wyn R. Health disparities among California’s nearly four million low-income nonelderly adult women [policy brief] Los Angeles, CA: UCLA Center for Health Policy Research; 2009. ( http://www.healthpolicy.ucla.edu/pubs/files/Disparities_4Mil_Women_PB_11-09.pdf) [PubMed] [Google Scholar]
- 45.Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75–90. [PMC free article] [PubMed] [Google Scholar]
- 46.Schuckit MA, Daeppen JB, Danko GP, et al. Clinical implications for four drugs of the DSM-IV distinction between substance dependence with and without a physiological component. Am J Psychiatry. 1999;156(1):41–49. doi: 10.1176/ajp.156.1.41. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel SL, Tucker JS, Elliott MN, et al. Sexual risk among impoverished women: understanding the role of housing status. AIDS Behav. 2007;11(suppl 6):9–20. doi: 10.1007/s10461-006-9193-4. [DOI] [PubMed] [Google Scholar]
- 48.Kim TW, Kertesz SG, Horton NJ, et al. Episodic homelessness and health care utilization in a prospective cohort of HIV-infected persons with alcohol problems [electronic article] BMC Health Serv Res. 2006;6:19. doi: 10.1186/1472-6963-6-19. . ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1421395/pdf/1472-6963-6-19.pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidder DP, Wolitski RJ, Campsmith ML, et al. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health. 2007;97(12):2238–2245. doi: 10.2105/AJPH.2006.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis JH, Andersen RM, Gelberg L. Health care for homeless women. J Gen Intern Med. 2003;18(11):921–928. doi: 10.1046/j.1525-1497.2003.20909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterk CE, Elifson KW, Theall KP. Individual action and community context: the Health Intervention Project. Am J Prev Med. 2007;32(suppl 6):S177–S181. doi: 10.1016/j.amepre.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]