Abstract
Asthma can be exacerbated by environmental factors including airborne particulate matter (PM) and environmental tobacco smoke (ETS). We report on a study designed to characterize PM levels and the effectiveness of filters on pollutant exposures of children with asthma. 126 households with an asthmatic child in Detroit, Michigan, were recruited and randomized into control or treatment groups. Both groups received asthma education; the latter also received a free-standing high efficiency air filter placed in the child’s bedroom. Information regarding the home, emission sources, and occupant activities was obtained using surveys administered to the child's caregiver and a household inspection. Over a one-week period, we measured PM, carbon dioxide (CO2), environmental tobacco smoke (ETS) tracers, and air exchange rates (AERs). Filters were installed at midweek. Before filter installation, PM concentrations averaged 28 µg m−3, number concentrations averaged 70,777 and 1,471 L−1 in 0.3–1.0 and 1–5 µm size ranges, respectively, and the median CO2 concentration was 1,018 ppm. ETS tracers were detected in 23 of 38 homes where smoking was unrestricted and occupants included smokers and, when detected, PM concentrations were elevated by an average of 15 µg m−3. Filter use reduced PM concentrations by an average of 69 to 80%. Simulation models representing location conditions show that filter air flow, room volume and AERs are the key parameters affecting PM removal, however, filters can achieve substantial removal in even "worst" case applications. While PM levels in homes with asthmatic children can be high, levels can be dramatically reduced using filters.
Keywords: indoor environment, free-standing HEPA air filters, asthmatic children, particulate matter, exposures
1 Introduction
Airborne particulate matter (PM) is a environmental trigger of asthma [1–3] and has been linked to adverse health impacts including aggravation of respiratory conditions and premature death [4, 5]. Often, attention focuses on the PM fraction that is small enough to enter deep into the respiratory tract, e.g., PM2.5 consisting of particles smaller than 2.5 µm dia [6, 7]. Exposure to environmental tobacco smoke (ETS), to which a large fraction (60%) of asthmatic children in the U.S. is exposed [8], is associated with increased frequency and 2 severity of asthma attacks, prolonged duration of symptoms, and decreased lung function [9, 10]. Children in urban areas are especially exposed to elevated levels allergens and indoor air pollutants, including PM2.5 [7].
Indoor environments dominate exposures of many pollutants, including PM, because most people spend the bulk of their time indoors, e.g., U.S. adults and children respectively are indoors 87 and 85% of the time [11]. Indoor PM concentrations are determined by both indoor emission sources, e.g., tobacco smoke, gas stoves, cooking, vacuuming, and outdoor (ambient) sources, e.g., suspended soils, pollen and traffic exhaust [12–14]. Ambient PM2.5 can easily penetrate building envelopes [7, 18–20] and it represents an important component of indoor exposure [15]. ETS is an important source of PM as well as gaseous pollutants [9, 16]. In addition to the types and strengths of indoor and outdoor sources [14, 17], indoor concentrations are affected by building characteristics [17, 18], air exchange rates (AER) [19], air mixing characteristics [20, 21], heating/cooling system type [22], and the presence, if any, of PM filters [19, 23–25].
1.1 Application and evaluation of air filters
Air filters can substantially reduce indoor concentrations and exposures to PM, as demonstrated in both modeling [23, 25] and field studies [19, 22, 26–31]. Free-standing (semi-portable or “room”) filters are low in cost and easily installed in spaces where people spend large amounts of time. Both high-efficiency particle arrestor (HEPA) filters, which are rated for a minimum removal of 99.97% for particles 0.3 µm dia [25], as well as conventional low efficiency filters can provide effective PM removal since air makes multiple passes over the filter. Several studies have suggested that such filters can reduce asthma symptoms of children and adults [3, 18, 22–24, 27–29, 32–35].
In practice, filter performance is affected by many factors. First, filter performance decreases as AERs increases, since a smaller fraction of air in the space will be treated by the filter. AERs can increase due to opened windows, large indoor-outdoor temperature gradients, high wind speeds [36, 37], and coupling to other building zones. Unfortunately, few filter studies have measured AERs. Second, indoor emission rates can be highly variable and are rarely measured or controlled, which can confound performance evaluations [38, 39]. Third, filter performance can decrease if filters become loaded and air flow rates decrease. Filter performance can also be affected by the quality of ventilation air, the placement of the filter, and mixing of air in the room and building.
Most studies investigating PM removals using free-standing filters have focused on symptom outcomes [25, 27–29, 35] or have employed small sample sizes and thus may not be representative of either local or global conditions. Few studies have assessed PM removal rates, filter usage, and factors affecting filter performance. Previous tests of HEPA filters in 4 cigarette smoker’s homes showed that PM concentrations decreased by 30–70% depending on size fraction and occupant activities [19]. Several larger filter studies have been conducted that have focused on specific allergens, but have not evaluated PM removal. In the Netherlands, HEPA filters were provided to 15 of 45 homes studied, and filters were dismantled after 6 months to quantify dust and house dust mite allergen loadings [33], however, airborne PM measurements were not collected. In seven U.S. cities, allergens in bed and surface dust sampled in 425 homes equipped with filters in the child's bedroom were compared to levels in 444 control homes [35]. Unfortunately, information regarding filter size and use is unavailable, and again, PM was not measured. In a study of 35 cat-allergic adults, half received HEPA filters placed in their bedrooms (including a timer to document use), and 1-hr monitoring conducted monthly over a season showed gradual reductions (up to 40%) of airborne levels of Fel d1 compared to the placebo group, but again, PM was not measured [29]. In Buffalo, NY and Hagerstown, MD, HEPA room filters were placed in bedrooms of 32 adults with symptoms of perennial rhinitis, and particle number counts (PNCs) showed reductions in most (85%) homes, with an overall reduction of 73% for >0.3 µm PNCs [28]. This study was conducted in winter; short-term measurements (duration unspecified) were utilized; and homes where active smoking took place were omitted. In a study of 93 asthmatic children living in mostly row homes in Baltimore, MD, HEPA filters were placed in half of the children’s bedrooms, and PM2.5 and PM10 concentrations in the bedrooms decreased by about 39%; comparable reductions were seen for some allergens [27]. In upstate New York, a system integrating a HEPA filter, air conditioner and energy recovery ventilator reduced 0.5–10 µm PM by 72% in bedrooms of 30 children with asthma [22]. This system was sophisticated, and the tests were designed to boost AERs, isolate and positively pressurize the bedroom. Overall, evaluations of stand-alone filters have been modest in scope and limited by incompletely known or controlled variables including, most importantly, indoor emissions, outdoor concentrations, and AERs.
1.2 Objectives
The objectives of this study are to characterize PM and ETS exposures of children with asthma in Detroit city homes, and to evaluate the effectiveness of stand-alone filters in reducing PM concentrations. In this intervention study, HEPA filters were placed in the bedrooms of children with asthma, one of the "microenvironments" where children spend considerable time. Our approach and sample size were designed to obtain representative and robust results. This study was motivated by the need to understand pollutant exposures of children, and to recognize the significance of the time spent at home. It was conducted as community-based participatory research by the Community Action Against Asthma (CAAA) partnership, which includes community-based organizations, health and human service organizations, and university researchers.
2 Materials and methods
2.1 Participant recruitment and schedule
The study sample consisted of households recruited to participate in a study investigating the effect of providing HEPA filters and asthma education provided by community health workers on symptoms and lung function of asthmatic children. Households in Detroit, Michigan, with a child between the age of 6 – 12 and identified as having persistent asthma were eligible. Detroit is a city of about 900,000 that contains numerous industrial facilities, rail yards and highways. The study area is predominantly African American and Latino, a large fraction of households have low incomes, and asthma hospitalization rates are moderately high to very high [40].
A short screening questionnaire was distributed to caregivers of Detroit children ages 6–12 through recruitment activities at community-based organizations, schools, community fairs and other venues. Households were deemed initially eligible if they had at least one child who reported symptoms or medication use consistent with persistent asthma. Exclusion criteria were: participation in a previous CAAA intervention study; living outside of Detroit; intention to move in the next 6 months; family monolingual in a language other than English or Spanish; and child with physical or mental handicap that would preclude successful spirometry measurements.
Our final sample included 126 households that were randomized to one of three groups: a control group receiving only community health worker (CHW) home education visits (n=37); the standard intervention group receiving a filter and CHW visits (n=47); and an enhanced intervention group receiving the filter, the CHW visits, plus an air conditioner (n=42). Households entered the study on a rolling basis, and baseline monitoring and filter deployment occurred between March, 2009 to February, 2010. Table 1 shows the number of households completing initial visits by season. Following the baseline visit, two or three follow-up visits approximately 3 months apart to each home were planned. Two families moved prior to their next (seasonal) visit. In these cases, the baseline visit in their new home was repeated. The number of homes sampled each week varied from about 6 to 10. This paper presents results from the baseline measurements, and includes the enhanced intervention (air conditioner) group prior to the provision of air conditioners. (Seasonal visits will be discussed in a subsequent paper.)
Table 1.
Number of baseline visits by season in the three groups. “CHW” =control; “AF” = CHW+air filter; “AF/AC” = CHW+air filter+air conditioner; “Total” includes sum of three groups.
| Season | CHW | AF | AF/AC | Total |
|---|---|---|---|---|
| Spring (Mar–May) | 6 | 5 | 6 | 17 |
| Summer (Jun–Aug) | 14 | 3 | 3 | 20 |
| Fall (Sep–Nov) | 6 | 21 | 15 | 42 |
| Winter (Dec–Feb) | 11 | 18 | 18 | 47 |
| Total | 37 | 47 | 42 | 126 |
The child's primary caregiver provided informed and written consent, following protocols approved by the University of Michigan Institutional Review Board. Incentives included $5 gift certificates to complete the initial asthma screening instrument, asthma education materials and a resource guide of services, $15 to complete a screening visit to assess the physical features of the house, and $15 for each data collection home visit. Households receiving filters and/or air conditioners could keep these units along with cash compensation for electricity consumed during the study; households not receiving these units received $100.
2.2 Walkthrough and caregiver survey
A technician completed a walkthrough inspection in each home to collect information on its characteristics and condition. The inspection evaluated general building characteristics, e.g., presence of an attached garage, design and integrity of roofing, and the type of heating and cooling system. Rooms frequented by the child, e.g., bedroom or sleeping area, play area, kitchen and bathroom, were inspected for: evidence of water damage, mold, chipping paint, etc., on ceilings, walls and floors; number of windows; type of covering on floors, furniture and windows; and the presence of emission sources such as candles, incense and room deodorizers, etc., using a comprehensive checklist. If present and accessible, basements were inspected, including an assessment of the furnace. The dimensions and volume of the home and the child's bedroom were measured. These data were collected using a handheld computer.
Participants completed a survey at each visit that included questions about health status, features of their home, indoor PM-emitting activities, e.g., frequency of cigarette smoking, cooking activities, and cleaning. We also conducted a focus group and surveyed each participant to identify factors that influenced the household’s use of the filters, which will be reported in a subsequent paper.
2.3 Filter type and operation
A stand-alone HEPA air filter (Whispure 510, Whirlpool Corporation, Benton Harbor, MI) was installed in each home that was randomized to the air filter/air conditioner group. This 61 × 52 × 25 cm unit features a carbon-impregnated pre-filter, a pleated HEPA-type filter, a squirrel cage fan, 4 fan speeds, indicator lights for fan speed and replacement times for filters, a maximum clean air delivery rating (CADR) of 330 CFM (0.156 m3 s−1), quiet operation, and an upward discharge. We previously tested the same model in four homes containing smokers [19]. A circuit and data logger was added to the filter to monitor and record filter usage and fan speed, along with air temperature and humidity. A data logger (OM-44, Omega Engineering, Stamford, CT, USA) recorded the fan speed of the air filter, along with air temperature and humidity, by continuously recording a DC voltage produced by a simple circuit installed in the filter, which consisted of a 0.2 Ω 5W 5% resistor in series with one leg of the fan's 120 V line, with the voltage across this resistor rectified by a signal diode and smoothed using an RC circuit (two 2.2 kΩ resistors, one 47 µF capacitor). Individual calibrations were performed for each data logger-air purifier pair. These calibrations were very stable.
Participants were instructed on the recommended placement and operation of the filter. A technician then placed the filter in the room in which the child normally slept, typically a bedroom but occasionally a living area. Filters were positioned within reach of an electrical outlet and away from doors, play and high traffic areas. To provide the best performance, participants were instructed to operate the filters continuously at the highest speed, or the highest speed tolerable for noise and comfort reasons, and to close the door to the bedroom as much as practical. Participants were shown how to remove and clean the pre-filter. Our technician replaced the pre-filter and the HEPA filter element after 6 months of operation.
During the initial baseline visit, environmental monitoring was conducted for one week (see below). The HEPA filter was installed near the middle of this week, thus, depending on the timing of the installation, we obtained 2 to 4 days of monitoring data without the filter, followed by 2 to 4 days with the filter. During these baseline visits, filter usage was logged every 5 min. Due to memory constraints of the logger, 2 hr sampling intervals were used during the period between baseline and seasonal visits.
2.4 Environmental monitoring
Baseline and seasonal assessments included week-long measurements of air quality and ventilation parameters in the child’s bedroom and the living area of each home. In the bedroom, air samplers were placed on the floor with inlets at ~1.3 m height above the floor and 0.3 m from a wall or other surface. PM concentrations were measured as sequential 24-hr samples, collected on 1 µm rated 47 mm dia PTFE filters (225–2749, SKC, Eighty-Four, PA, USA) installed in static-free polypropylene cassettes (Omega Specialty Instruments Co., Houston, TX, USA). The inlets on these cassettes are not designed to be size selective, and are equivalent to open-face filter sampling, which essentially captures the total suspended particulate (TSP) fraction. The cassettes protected the filters during handling in the field, and allowed automated sequential sampling at modest cost, an important concern since up to 16 sampling systems, each sampling 7 filters, were simultaneously deployed. PM concentrations were significantly correlated with 0.3 to 5 µm dia particle number counts (see below), which were also measured to provide continuous and size-selective information. Filter PM samples were collected at 15 L min−1; flow rates were continuously measured (AWM5000 Microbridge mass airflow sensor, Honeywell, Freeport IL) and logged. Filters were conditioned before and after use at 25 °C and 34% relative humidity, and weighed to 1 µg using a microbalance (ME-5, Sartorius AG, Goettingen, Germany). In the laboratory, filters were installed in labeled cassettes for transport and use, and stored in heavy sealable plastic bags. The integrated sampling volume and sampling start and stop times were recorded and used to determine the sampled volume. Particle number counts (PNCs) in 0.3–1.0 µm and 1–5 µm dia size ranges were measured and recorded every 1-min using an optical particle counter (GT-521, MetOne, Grants Pass, OR). The smaller size range is largely due to combustion-related particles, e.g., ETS; the larger includes mechanically-generated emissions, e.g., entrained dusts and pollens [41].
Quality assurance activities for PM measurements included the use of detailed written standard operating protocols (SOPs), standardized data sheets, and weekly performance reviews of field monitoring results. For the PM filter samples, a field filter was collected and analyzed along with the 7 sequential sample filters at each house, as well as a laboratory blank. The flow control and measurement systems were regularly calibrated using a piston-type flow meter (DryCal, Bios International Corp., Butler, NJ). Unloaded and loaded filters were each weighed three times and averaged; any variation above 3 µg was noted and filters reweighed. Temperature and relative humidity during filter conditioning and weighing was continuously recorded, and stayed within narrow limits. PM concentrations were calculated only for samples that obtained 75% or more of the intended volume. (For example, sometimes occupants would unplug pumps, which was easily noted in plots trending flow rates and the calculated sample volume.) For the PNCs, all 16 instruments used were new or recently calibrated by the manufacturer, who recommends a yearly calibration. We conducted side-by-side and simultaneous tests of all PNC units prior and after the study and found that agreement averaged 7% among the instruments for the 0.3–1.0 µm dia channel and 6 to 14% for the 1–5 µm dia channel. The lower agreement cited for the 1–5 µm channel was due to low PNC counts in a second test, and appears inflated. Excluding this test, the maximum disagreement was 23% in both size channels. Each season, units were tested with a comparison unit that reflected typical responses; these tests showed similar agreement. The occasional pump failure or other fatal flaw in the PNCs was recorded by the instrument's log file.
As a measure of occupancy and ventilation, carbon dioxide (CO2) concentrations were measured every 5 min using an IR sensor (C7632A, Honeywell Corp., Morristown, NJ). All 16 units used in the study were new and recently calibrated by the manufacturer, however, in this case we saw considerable variation. Initially, each CO2 unit was calibrated by blending zero air and a certified CO2 gas (at 1011 ppm, Scott Specialty Gases, Plumstead, PA) using calibrated flow controllers and multiple concentrations between 0 and 1011 ppm. Because the CO2 sensors showed very linear responses, final calibrations used two-point calibrations at 0 and 1011 ppm. Calibrations for these instruments are specified by the manufacturer to be valid for 5 years, but each unit was rechecked after approximately 6 months. The average and maximum variation from initial calibration was 6.5 and 21%, respectively.
Temperature and relative humidity were recorded every 5 min using a miniature logger (Hobo H08, Onset Computer Corporation, Bourne, MA). These loggers (including 89 units used to monitor air purifier fan speed and approximately 40 others used to monitor environmental conditions) were all checked in the laboratory under controlled conditions. Temperature and relative humidity measurements for these units were found to be within manufacturer's specification and very reliable.
Perfluorocarbon tracers (PFTs) for air exchange rate (AER) determinations, and ETS tracers were measured using passive samplers containing Tenax-GR and Carbosieve SIII adsorbents [42]. These samples were collected in duplicate in the child's bedroom. A third sampler was placed in the living area in an inconspicuous location, e.g., bookshelf away from children, pets, etc., along with a logger for temperature and humidity, which was used to calculate the sampling rate. A blank was also taken in each home. Two tracers of environmental tobacco smoke (ETS) were measured, namely, 2,5-dimethyl furan and 3-ethenyl pyridine (3-EP), both of which are unique to ETS and never detected in non-smoking environments [43]. Due to detection limit issues, these tracers were primarily qualitative in nature, thus ETS levels were not quantified, and false negatives are possible. However, false positives are very unlikely, thus ETS is nearly certainly present if the tracers were detected. Samples were analyzed by thermal desorption, cryofocusing and GC-MS following well-developed protocols [44]. Duplicate measurements, which were usually within 20%, were averaged.
AERs in the home and child's sleeping area, and air flows between these zones, were derived using the multizone constant injection method, two different PFTs, and measurements in the bedroom and living room. The selected PFTs are inert, do not exist naturally, and were sampled and analyzed with high sensitivity [45]. Technicians placed two passive emitters of hexafluorobenzene (HFB) in the living area, and two octafluorotoluene (OFT) emitters in the sleeping area, typically in opposite corners, each releasing these compounds at a constant rate (between 0.7 and 2 mg hr−1, depending on PFT) over the week-long sampling period. Emitters were individually calibrated, periodically checked, and re-filled as needed. AERs for the house and child's sleeping area, and flows between these zones, were determined using PFT concentrations measured at the two locations, the measured volumes of the house and bedroom, and methods presented elsewhere [45].
3 Calculation
Variables from the walkthough and participant surveys that might affect PM levels and/or filter performance were selected for analysis. We also derived several composite variables that summarized building conditions, e.g., total number of locations where deteriorating paint or water damage in the home was noted.
Short term measurements (PNC, CO2, etc.) were reduced to 1 hr averages. 24-hr averages were calculated using a sampling period starting at 6 am, which better corresponded to the time the child was expected to be in the sleeping area. Each average required a minimum of 75% of values (18 hours) to be valid. Non-parametric statistical tests were emphasized to account for the right-skewed distributions of pollutant measurements.
For homes receiving a filter, PM, PNC and CO2 concentrations were determined for the portion of the sampling week prior to filter deployment. For homes not receiving a filter, average concentrations were determined for sampling days 2 to 3, days 5 to 7, and for the entire week. Paired t-tests were used to test for differences within the week and for seasonal effects. PM, PNC, and CO2 levels with filters deployed and operating were calculated for those days when the filter was used at least 75% of the time (at any fan speed), based on filter use monitoring. The removal percentage R (%) of PM attributable to the filter was calculated for PM and PNC measurements in each house as:
| (1) |
where Cf and Cn = concentrations with and without the filter operating, respectively.
A steady-state fully-mixed one-zone box model was used to determine the sensitivity of PM removals to changes in AERs, filter air flow rates, and other variables. The removal rate can be reduced to [19]:
| (2) |
where AER = air exchange rate (h−1); Qr = filter air flow rate (m3 h−1); ηr = filter PM capture efficiency (dimensionless); V = effective volume of the space (m3), and Kp = deposition loss rate coefficient (h−1). All of these parameters vary by residence. Nominal, best- and worst-case scenarios were selected to reflect the study homes. The relative sensitivity RSi (dimensionless) was calculated for each parameter i and scenario as:
| (3) |
where Ri,T = removal achieved for a 10% increase in model parameter Xi (Kp, AER, Qr, V, ηr) from its nominal value for the scenario, Xi,nom, and Rnom = removal rate attained for the nominal parameter value Xnom. RSi reflects the proportional change in removal for a change in input Xi, while holding other parameters at their nominal values.
4 Results and discussion
4.1 Home characteristics
Table 2 summarizes characteristics of the study homes. Most (78%) were relatively small (average area = 147 ± 58 m2) single family homes containing 2 to 4 bedrooms. While only a fraction of caregivers (n = 29) knew the age of their home, the responses show a wide range of age of homes, e.g., 21% were >110 years old, 14% were 80–109 years old, 38% were 10–79 years old, and 28% were <9 years old. Home heating systems were mostly forced air (88%); the remainder used steam or hot water radiators. Few (30%) homes had central air conditioning. Most (87%) homes had some water damage (mostly in basements), 92% had at least one wall, floor or ceiling defect (most commonly in basements), and 26% had visible mold or mildew (most commonly in bathrooms or kitchens). The child’s bedroom tended to be small (average area = 12 ± 5 m2), and about half (46%) had hard floor coverings (e.g., wood, tile, linoleum), while the rest had carpeting or rugs; all rooms had windows. Few of the rooms homes had cloth-covered chairs (13%) or couches (3%). Of all the variables in Table 2, only the number of children in the home showed significant (p<0.05) differences between control and treatment (filter) groups: the control group had an average of 2.8 children in the home compared to 2.2 in the treatment group; both groups had the same range (1–8). Given that 37 characteristics were tested, this single difference can be attributed to chance, a result of the multiple comparisons performed.
Table 2.
Characteristics of the 126 homes in the study.
| Home characteristics | Unit | Average | Range | Percent | |
|---|---|---|---|---|---|
| House | Floor area | m2 | 147 | 63 – 483 | - |
| Interior house volume | m3 | 368 | 156 – 1192 | - | |
| Single story | - | - | 24 | ||
| Single family house | - | - | 78 | ||
| Duplex or flat | - | - | 15 | ||
| Apartment building | - | - | 3 | ||
| No. of bedrooms | n | 3.0 | 1 – 6 | - | |
| Heating system (Forced air) | - | - | 88 | ||
| Central AC | - | - | 30 | ||
| Ventilation fan | - | - | 19 | ||
| Furnace filter change frequency times/yr | 2.5 | 1 – 12 | 46 | ||
| Roof : slope (not flat) | - | - | 99 | ||
| Interior damage | Water | n | 1.7 | 1 – 7 | 87 |
| Mold | n | 2.0 | 1 – 6 | 26 | |
| Condition | n | 3.5 | 1 – 11 | 92 | |
| Child's sleeping area | Floor area | m2 | 12 | 4 – 46 | - |
| Room volume | m3 | 28 | 13 – 110 | - | |
| No. of windows | n | 1.7 | 1 – 5 | 97 | |
| Wood, tile or linoleum floor | - | - | 46 | ||
| Wall to wall carpeting | - | - | 48 | ||
| Rugs on floor | - | - | 3 | ||
| Occupancy and activities | No. of adults | n | 1.7 | 1 – 5 | 97 |
| No. of children | n | 2.4 | 1 – 8 | 95 | |
| Dogs present | 1.2 | 1 – 3 | 31 | ||
| Cats present | 1.2 | 1 – 2 | 9 | ||
| Either dogs or cats | - | - | 36 | ||
| Smoking | Never any smokers indoors | - | - | 54 | |
| Any smokers in household | - | - | 60 | ||
| No. of smokers | n | 1.7 | 1 – 5 | 60 | |
| Rule 1: No smoking indoors | n | - | - | 70 | |
| Rule 2: Reduce smoking indoors | n | - | - | 48 | |
| Rule 3: Limit to one room | n | - | - | 52 | |
| Cleaning | Use a vacuum cleaner | - | - | 44 | |
| Swept or dusted CSA in the last 2 weeks | 3.1 | 1 – 5 | - | ||
| Swept or dusted CSA in the last 2 weeks | - | - | 100 | ||
| AER | Living room | h−1 | 0.76 | 0.10–3.06 | - |
| Bedroom | h−1 | 1.67 | 0.22–9.52 | - | |
AERs in the homes averaged 0.76 ± 0.52 h−1 (median = 0.65 h−1 and interquartile range = 0.37 to 0.99 h−1). These AERs are high relative to those in suburban homes in Michigan where the AER averaged 0.34 ± 0.18 h−1, which were measured recently using the same technique, [45] and they are slightly below AERs measured in California and New Jersey homes in 1999–2001 where the median AERs were 0.87 and 0.88 h−1, respectively. [46] Generally low AERs are expected in Detroit given the local climate, but AERs reflect many factors, e.g., building age, condition and weatherization measures. AERs in the bedrooms averaged 1.67 ± 1.47 h−1 (median = 1.24 h−1 and interquartile range = 0.71 to 2.08 h−1). (The reported AERs exclude flows between two zones, as discussed later.)
The average occupancy of the homes was 1.7 ± 0.8 adults and 2.4 ± 1.4 children; the highest was 5 adults and 8 children. Over half (60%) of the households included adult smokers. Many households limited indoor smoking by not allowing or by reducing the amount of indoor smoking, or restricting smoking to one room. 36% of the homes had dogs or cats. Approximately half of the caregivers (44%) used vacuum cleaners, and all (100%) of the child's sleeping areas had been cleaned in the past two weeks by either vacuuming, sweeping or dusting.
4.2 Pollutant levels prior to filter installation
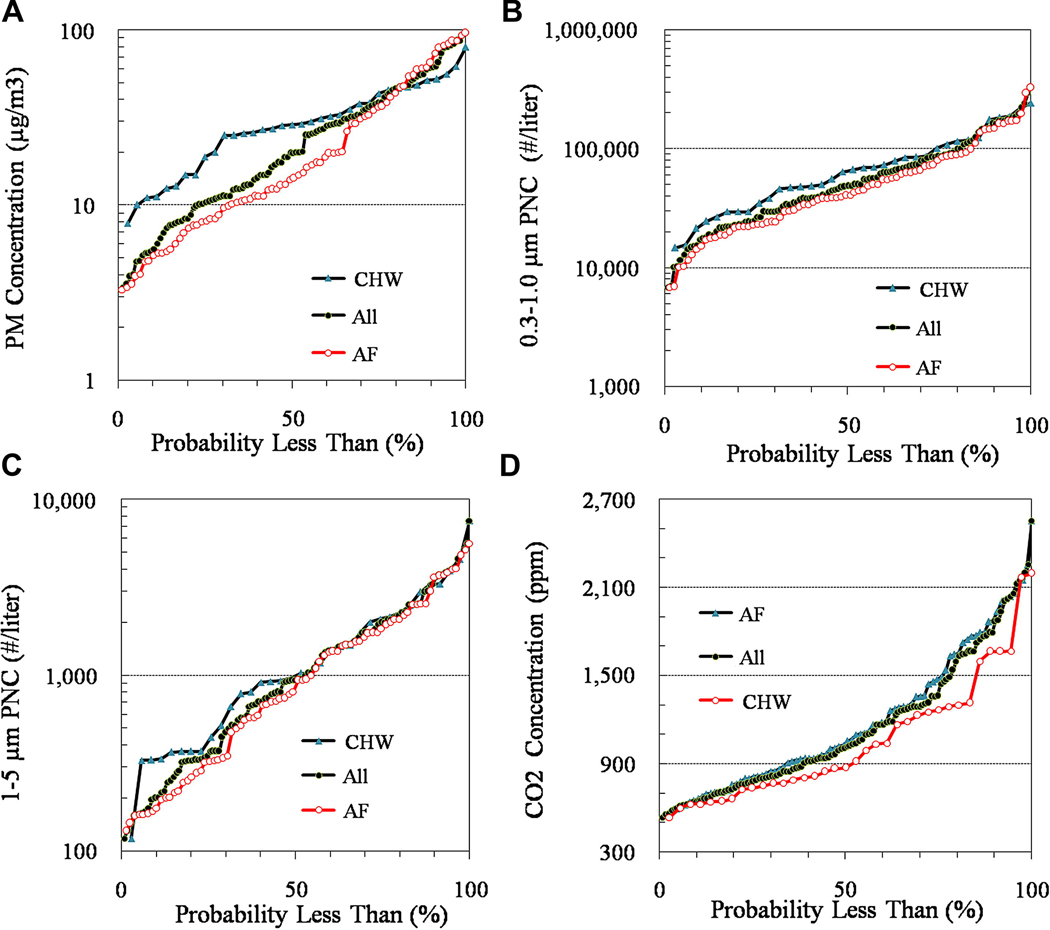
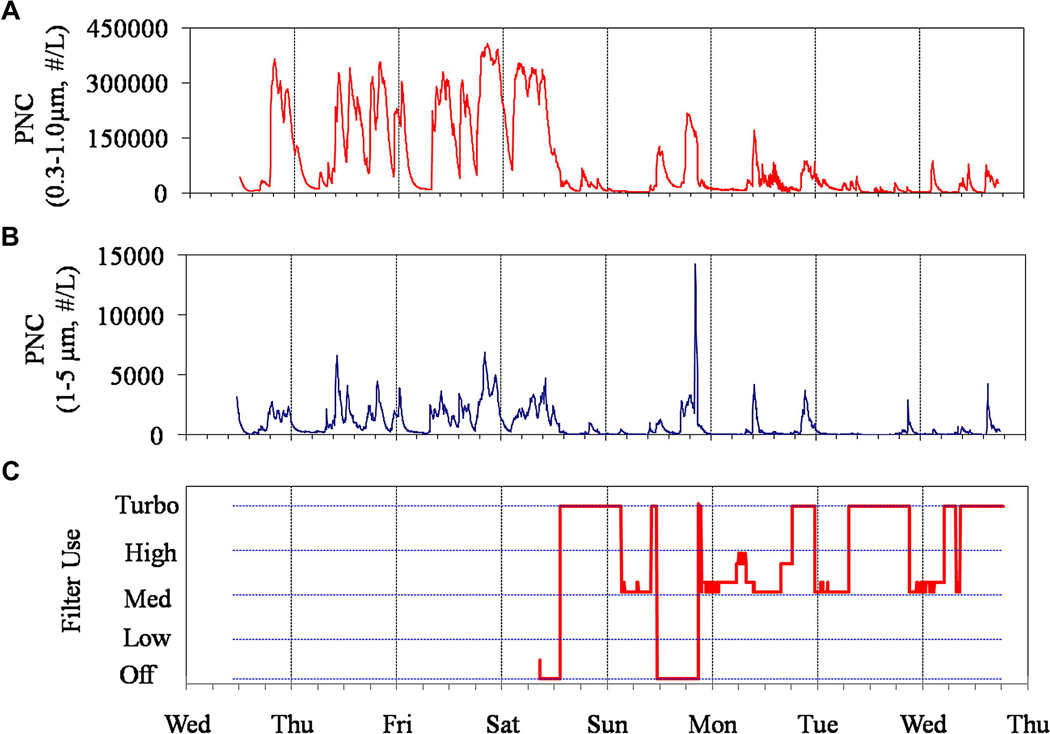
PM, CO2 and AER measurements prior to installation of the HEPA filters are given in Table 3; cumulative distributions of PM and CO2 are shown in Figure 1. PM levels in bedrooms averaged 28 ± 23 µg m−3 (n=115). The highest level, 96 µg m−3, was reached in a home that also experienced very high CO2 levels (median = 2,056 ppm, essentially the instrument’s maximum); no ETS was detected in this home. Measurements in Table 3 are multiday averages, typically 3 to 6 days in duration. Across all homes, PNCs averaged 70,777 ± 60,890 and 1,471 ±1,386 # L−1 in the 0.3–1.0 and 1–5 µm size fractions, respectively. As expected, concentration measurements were right-skewed. (Additional statistics, including medians, are provided in Tables 3 and 4). PNCs and PM concentrations were significantly correlated, e.g., 24-hr PM concentrations had Spearman rank correlation coefficients of 0.58 and 0.62 for PNC in 0.3–1.0 and 1–5 µm size ranges, respectively. The PNC traces were diagnostic of conditions in the home, e.g., peaks indicated smoking or cooking events, the subsequent removal of PM associated with the event, and the effect of the filter. As an example, Figure 2 trends PNCs and filter use for a typical home over one week. The traces suggest PM generating events during the day and evening, and peaks in the two size fractions were often not always coincident, indicating different emission sources. When the filter was operating (mid-Saturday onward, except for Sunday afternoon), both fine and coarse PNCs decreased considerably, and levels after a pollution event declined much faster.
Table 3.
IAQ measurements prior to air filter installation, showing particulate matter (PM) concentrations, particle number concentrations (PNC) in 0.3–1.0 µm and 1–5 µm sizes, CO2 concentrations, and air exchange rates (AERs). CHW=community health worker "control" case; AF=air filter "intervention" case.
| Statistic | N | Average | SD | Median | 90th | Max | p-valuea | |
|---|---|---|---|---|---|---|---|---|
| PM (µg/m3) | CHW | 36 | 31.8 | 16.4 | 28.9 | 51.8 | 79.7 | 0.007 |
| AF | 79 | 26.5 | 25.3 | 14.2 | 66.5 | 95.6 | ||
| All | 115 | 28.1 | 22.9 | 20.0 | 61.2 | 95.6 | ||
| 0.3–1.0 µm PNC (#/liter) | CHW | 35 | 83,240 | 57,696 | 69,626 | 179,899 | 241,743 | 0.014 |
| AF | 78 | 65,184 | 61,811 | 40,328 | 153,191 | 324,299 | ||
| All | 113 | 70,777 | 60,890 | 50,279 | 163,299 | 324,299 | ||
| 1–5 µm PNC (#/liter) | CHW | 35 | 1,688 | 1,492 | 1,175 | 3,285 | 7,528 | 0.102 |
| AF | 78 | 1,374 | 1,334 | 868 | 3,630 | 5,599 | ||
| All | 113 | 1,471 | 1,386 | 955 | 3,553 | 7,528 | ||
| CO2 (ppm) | CHW | 36 | 1,054 | 429 | 892 | 1,662 | 2,194 | 0.157 |
| AF | 87 | 1,185 | 478 | 1,051 | 1,900 | 1,724 | ||
| All | 123 | 1,147 | 466 | 1,018 | 1,849 | 1,724 | ||
| AER LR (h−1) | CHW | 29 | 0.78 | 0.55 | 0.57 | 1.50 | 2.38 | 0.964 |
| AF | 71 | 0.75 | 0.52 | 0.66 | 1.34 | 3.06 | ||
| All | 102 | 0.76 | 0.53 | 0.65 | 1.36 | 3.06 | ||
| AER BR (h−1) | CHW | 28 | 1.84 | 1.40 | 1.38 | 3.85 | 5.38 | 0.248 |
| AF | 72 | 1.61 | 1.50 | 1.14 | 3.04 | 9.52 | ||
| All | 100 | 1.67 | 1.47 | 1.24 | 3.34 | 9.52 | ||
p-value from Mann Whitney test.
Figure 1.
Cumulative distributions of IAQ parameters prior to filter use: A) PM; B) 0.3–1.0 µm PNC; C) 1–5 µm PNC; and D) CO2.
Table 4.
IAQ measurements on a seasonal basis for combined control and treatment groups. Otherwise as Table 3.
| Statistic | N | Ave | SD | Med | p-valuea | |
|---|---|---|---|---|---|---|
| PM (µg/m3) | Spring | 14 | 34.2 | 30.3 | 20.1 | <0.001 |
| Summer | 22 | 39.3 | 19.4 | 40.6 | ||
| Fall | 35 | 30.3 | 24.5 | 20.3 | ||
| Winter | 44 | 18.9 | 17.0 | 11.9 | ||
| 0.3–1.0 µm PNC (#/liter) | Spring | 14 | 64,643 | 49,408 | 48,003 | <0.001 |
| Summer | 19 | 109,315 | 75,134 | 84,037 | ||
| Fall | 35 | 80,433 | 55,350 | 69,176 | ||
| Winter | 45 | 48,903 | 53,129 | 32,143 | ||
| 1–5 µm PNC (#/liter) | Spring | 14 | 1,252 | 1,480 | 702 | 0.019 |
| Summer | 19 | 1,771 | 1,822 | 1,458 | ||
| Fall | 35 | 1,797 | 1,243 | 1,474 | ||
| Winter | 45 | 1,159 | 1,204 | 590 | ||
| CO2 (ppm) | Spring | 14 | 1,096 | 387 | 995 | 0.112 |
| Summer | 21 | 1,085 | 427 | 929 | ||
| Fall | 41 | 1,297 | 501 | 1,269 | ||
| Winter | 47 | 1,058 | 455 | 920 | ||
| AER LR (h−1) | Spring | 11 | 0.48 | 0.36 | 0.40 | 0.270 |
| Summer | 21 | 0.88 | 0.60 | 0.84 | ||
| Fall | 33 | 0.71 | 0.55 | 0.60 | ||
| Winter | 38 | 0.79 | 0.49 | 0.73 | ||
| AER BR (h−1) | Spring | 8 | 1.38 | 0.91 | 1.19 | 0.949 |
| Summer | 20 | 1.94 | 2.12 | 1.30 | ||
| Fall | 34 | 1.74 | 1.59 | 1.22 | ||
| Winter | 38 | 1.53 | 0.99 | 1.23 | ||
p-value from Mann Whitney test.
Figure 2.
PNC and air filter trends is case study home: A) 0.3–1.0 PNC; B) 1–5 µm PNC; and C) filter fan speed.
The average pre-intervention PM level in the Detroit homes, 28 µg m−3, exactly matched average levels found in 294 homes in 7 U.S. inner cities as determined using optical measurements [16]. Outdoor concentrations were not measured in the present study, however, long term (2007–2009) PM2.5 measurements at Detroit sites averaged 11 to 14 µg m−3 [47]. Thus, indoor PM levels in the Detroit homes considerably exceeded average ambient PM2.5 levels, a result of internal PM sources such as ETS (see below), cooking, burning of incense and candles, movement of people, vacuuming and sweeping. In the study cited previously, outdoor PM accounted for 25% of indoor concentrations, and smoking was the main PM source [16].
The median CO2 concentration was 1,018 ppm (n=123). Because short-term CO2 levels frequently exceeded the instrument's range (about 2,100 ppm), average, standard deviation and maximum statistics for CO2 are underestimated, however, medians are accurate. CO2 levels more than 1,000 ppm above ambient levels (about 380 ppm) have long been used to indicate inadequate ventilation and/or crowding (e.g., ventilation rate <7 L s−1 occupant−1) [41]. By this benchmark, over 25% of the study homes had inadequate ventilation air in the bedroom. Because this multiday (typically 6 day) average includes periods with low occupancy, e.g., when children are at school, CO2 levels during the occupied period are certainly higher. Low ventilation rates can increase concentrations from building emission sources, and also may indicate excess humidity, a concern for biological contaminants. (As discussed later, CO2 levels increased slightly, but not significantly, after filter deployment.)
Baseline pollutant levels in control and treatment homes are compared in Table 3 and Figure 1. Control homes had higher concentrations of PM and 0.3–1.0 µm PNCs (p<0.05); differences in other parameters, e.g., 1–5 µm PNCs and CO2, were not statistically significant. These differences are partly attributable to seasonal effects (see below). However, the analysis of filter performance is largely insensitive to case-to-control or house-to-house differences since the paired analysis compares pollutant levels before and after filter installation in the same home and same season. (The following section shows that filter performance, expressed as percentage of PM removed, does not vary by season.)
We also investigated whether concentrations varied by the sampling schedule. In control homes, no significant differences were seen between sampling days 2–3 and 5–7 for PM, 0.3–1.0 µm and 1–5 µm PNCs, and CO2 concentrations (p = 0.56, 0.06, 0.67 and 0.88, respectively), suggesting that no changes occurred in occupant behavior or some other (unknown) factor during the study week. Thus, the CO2 results suggest that that occupancy and ventilation did not depend on grouping or period. Overall, the randomization scheme did not show large differences in IAQ parameters. Some day-of-week effects were noticed in indoor pollutant levels prior to filter deployment, although these were not statistically significant (p=0.21, Kruskal-Wallis H test): typically Mondays and Tuesdays had the lowest concentrations (median=16 µg m−3), while Friday through Sunday had the highest concentrations (22 to 23 µg m−3). This should not bias the performance evaluation since filters were deployed randomly and nearly same number of measurements were obtained on each day of the week.
Table 4 shows a seasonal analysis of IAQ parameters. Levels of both PM and 0.3–1.0 µm PNC differed by season, with summer levels approximately twice winter levels. Fall and spring had intermediate levels. No significant trends were seen for 1–5 µm PNC and CO2 concentrations. These results suggest that smaller particles (<1 µm) account for a large share of PM, however, because different homes (and different numbers of homes) were tested in different seasons, this analysis may combine "house" and "seasonal" effects. It does indicate the importance of accounting for seasonal effects in evaluating filter effectiveness.
4.3 Environmental tobacco smoke
Caregiver surveys indicated that 76 of the households (60%) contained smokers. In most homes, respondents stated that actions were taken to limit indoor exposure, such as smoking outdoors (Table 2), but 38 (30%) of the homes contained smokers and did not have rules prohibiting indoor smoking. In half (52%) of these cases, smoking was limited to one room. We detected ETS tracers in 23 homes, most commonly in both the living room and the bedroom, and the concordance between ETS tracer detections and survey data was 70%. It should be noted that the ETS tracer measurements were week-long measurements which indicate neither whether the child was present during smoking events nor the temporal pattern of smoking. While PNC traces often showed strong and repetitive peaks suggestive of smoking events, this is not a unique identifier of ETS. In 8 homes, levels of the tracer were higher in the child's bedroom than in the living room.
The impact of smoking was estimated by stratifying the concentration data before the filters were installed using both the ETS tracer and the caregiver survey response. Using the tracer, PM levels in homes with and without smoking averaged 40 ± 31 and 25 ±20 µg m−3, respectively, representing an increase of 1.6 times, and 0.3–1.0 and 1–5 µm PNCs increased by 2.5 and 1.7 times, respectively (Table 5). Differences were somewhat smaller, e.g., 10 µg m−3, if survey data alone were used to stratify results. Most ETS is less than 2 µm in dia [25, 48], thus larger changes in smaller PM are expected, as reflected by the PNC data. In a few cases, PNCs may have been underestimated due to coincident (“saturation”) error affecting the PNC detector, which occurs at about 600,000 # L−1 for the instrument used. CO2 concentrations did not differ by smoking status (p=0.31), suggesting that occupancy and ventilation did not vary among smoking and non-smoking homes.
Table 5.
PM, PNC and CO2 concentrations in bedrooms with and without ETS. "None" is based on household survey; "Yes-Tracer" indicates ETS tracer was detected; "Yes-Survey" indicates indoor smoking may have occurred based on caregiver survey.
| ETS Present | N | Ave | SD | Med | 90th | p-valuea | |
|---|---|---|---|---|---|---|---|
| PM (µg/m3) | None | 93 | 25.3 | 19.8 | 18.8 | 53.6 | |
| Yes - Tracer | 22 | 40.3 | 30.8 | 30.6 | 86.0 | 0.005 | |
| Yes - Survey | 34 | 34.2 | 22.9 | 32.4 | 58.1 | 0.068 | |
| 0.3–1.0 µm PNC (#/liter) | None | 91 | 55,006 | 42,234 | 40,384 | 115,354 | |
| Yes - Tracer | 22 | 136,009 | 81,200 | 117,121 | 239,492 | <0.001 | |
| Yes - Survey | 31 | 111,563 | 80,016 | 84,612 | 197,548 | <0.001 | |
| 1–5 µm PNC (#/liter) | None | 91 | 1,317 | 1,315 | 907 | 2,549 | |
| Yes - Tracer | 22 | 2,109 | 1,518 | 1,693 | 3,960 | 0.010 | |
| Yes - Survey | 31 | 1,710 | 1,274 | 1,357 | 3,660 | 0.241 | |
| CO2 (ppm) | None | 100 | 1,126 | 458 | 995 | 1,789 | |
| Yes - Tracer | 23 | 1,237 | 500 | 1,180 | 1,874 | 0.305 | |
| Yes - Survey | 34 | 1,160 | 453 | 1,099 | 1,822 | 0.844 | |
p-value from independent-samples t-test.
ETS is a well known contributor of indoor PM. For example, smoking elevated concentrations by 37 µg m−3 in inner city homes in the 7 city study mentioned earlier [16], and by 33 to 54 µg m−3 in children’s bedrooms in a Baltimore study [7], while PNCs in living rooms with smokers averaged 291,000 ± 111,000 and 1,980 ± 1,300 # L−1 in 0.3–1.0 and 1–5 µm sizes, respectively [19]. In the present study, PM concentrations in the child’s bedrooms attributable to smoking increased by 15 µg m−3, and 0.3–1.0 µm PNCs increased by 81,000 # L−1. The increases reported here are smaller than seen in other studies, several of which measured air in the home’s living area, generally the room where smoking occurs. In contrast, our sampling was conducted in the child’s bedroom, and the attenuation of concentrations likely reflects effects of pollutant migration, dilution and other losses. Still, the ETS exposures in bedrooms were sizable.
4.4 Filter effectiveness
Table 6 summarizes IAQ measurements with and without the filter. While 89 homes had filters installed, for this paired analysis, filters had to run for at least 75% on at least one day to be included, which excluded 16 homes; occasional sampler failures also removed homes. (Relaxing the running criterion to 50% added only 2 more homes.) PM concentrations with the filter operating averaged 8.7 ± 13.1 µg m−3 (n=47), and the average and median PM removal rates were 69 ± 24% and 75%, respectively. PNC removals were slightly higher, averaging 76 ± 20% and 80 ± 19% (n=56) for 0.3–1.0 and 1–5 µm sizes. Removal rates were unaffected by the presence of ETS (p=0.91, 0.27 and 0.90 for PM, 0.3–1.0 and 1–5 µm PNCs, respectively, independent-sample t-tests, based on ETS tracer detection); season (p = 0.08, 0.19 and 0.46 for the same PM measures); or averaging time of the measurements (p=0.28 and 0.84 for differences in concentrations without and with filters, respectively, as stratified by 1, 2 and 3 day averaging times, Kruskal-Wallis H test).
Table 6.
PM, PNC and CO2 concentrations with and without air filters, and removal rates (R).
| Statistic | N | Ave | SD | Med | 90th | p-valuea | |
|---|---|---|---|---|---|---|---|
| PM (µg/m3) | No AF | 47 | 26.0 | 23.8 | 16.5 | 59.8 | <0.001 |
| With AF | 47 | 8.4 | 13.1 | 4.1 | 18.4 | ||
| R (%) | 47 | 69 | 24 | 75 | 94 | ||
| 0.3–1.0 µm PNC (#/liter) | No AF | 56 | 72,393 | 68,353 | 42,283 | 162,695 | <0.001 |
| With AF | 56 | 14,986 | 17,986 | 8,933 | 44,148 | ||
| R (%) | 56 | 76 | 20 | 82 | 94 | ||
| 1–5 µm PNC (#/liter) | No AF | 56 | 1,382 | 1,336 | 868 | 3,639 | <0.001 |
| With AF | 56 | 187 | 167 | 121 | 433 | ||
| R (%) | 56 | 80 | 19 | 86 | 99 | ||
| CO2 (ppm) | No AF | 80 | 1,159 | 458 | 1,037 | 1,865 | <0.001 |
| With AF | 80 | 1,115 | 472 | 993 | 1,861 | ||
p-value from paired sample t-test.
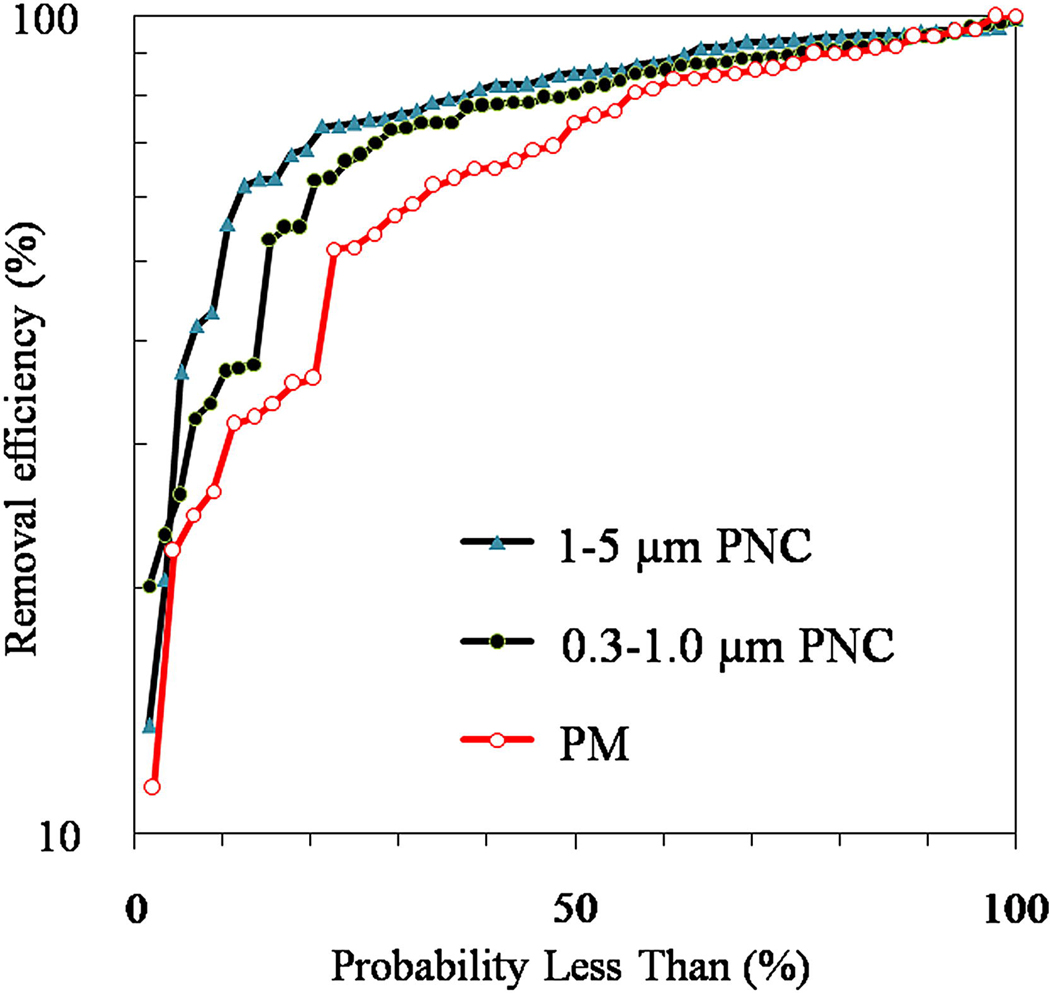
Figure 3 shows cumulative distributions of the removal rates across the study homes. Filters were highly effective in most (>80%) homes where PM removal rates of 70 to 80% were achieved. Removal rates fell below 50% for either PM, 0.3–1.0 µm PNC, or 1–5 µm PNCs in 15 homes (17%). Considering only the PM measures, 8 homes had low (<50%) removal rates, but five of these had very low PM concentrations (3 to 7 µg m−3) before filter installation, and four of these achieved normal removal rates (74–86%) based on PNCs. For these four homes, PM removal rates appear erroneous, a result of experimental errors at low concentrations of the gravimetric PM measurements (MDL = about 2 µg m−3). Based on 0.3–1.0 µm PNCs, 5 homes had low removal rates (<50%), and based on 1–5 µm PNCs, 6 homes had low removals (<50%). Three homes had low removal rates (<50%) in two of the three measures, and one home had low removal for all three measures. In one case, only one day's worth of measurements was available. Other than experimental errors (which should be negligible for the PNC measurements), the low removal rates might be explained by increases in PM emissions during the period when the filter was installed that exceeded the filter’s ability to remove PM. A daily query regarding occupant activities would have been useful to identify and confirm such emission events, but was felt too burdensome. In a few cases, it is possible that the filter was moved to a different room. In sum, filters were highly effective in the vast majority of homes.
Figure 3.
Cumulative distributions of PM and PNC removal rates.
Of the study homes, 42 homes had air conditioners when the study started, but only 4 of these were studied in the summer. While we expected that air conditioners would decrease AERs and in turn increase the filter’s effectiveness [19, 25, 30, 31], the number of homes with air conditioners in use was too small to see any differences, and effects may be confounded by seasonal differences in AERs (p=0.07) and differences between AERs in living and bed rooms (p=0.05).
Filter use was not associated with CO2 concentrations (p=0.29, Table 6), suggesting that ventilation and occupancy was unaltered by filter operation. However, CO2 levels increased modestly (but not statistically) after filter deployment in a subset of the homes, e.g., 23 homes had >50 ppm increases, and 17 had >100 ppm increases. Several participants stated that children preferred to sleep in the room with the air filter, which may have increased occupancy and CO2 levels. Additionally, we instructed caregivers to close the bedroom door as much as practicable to increase filter effectiveness, which would tend to increase CO2 levels. Still, the change in CO2 levels were negligible.
4.5 Sensitivity analyses
Nominal, upper- and lower-bound values were selected for each parameter in the sensitivity analysis. Generally, the nominal value was set to the median value among the study homes. Filter flow rate Qr depends on the filter's fan speed, which was previously measured in new units at four speed settings: 400 (lowest) to 745 (highest) m3 h−1 [19]; in-use daily-averaged flow rates also depend on the frequency of use. For the nominal case, the lowest speed and continuous use were assumed (Qr = 675 m3 h−1); for the lower bound, 75% usage at the lowest speed were assumed (318 m3 h−1); and the highest speed and continuous use were assumed for the best case (745 m3 h−1). PM capture rate ηr depends on the filter, PM characteristics, Qr, and filter loading. For the HEPA filters, we assumed ηr = 1.0 for both nominal and upper bound cases; the lower bound used 0.95. Kp depends on PM properties (e.g., particle size and density) and room characteristics (e.g., volume to surface area ratio), and values from 0 to 3.6 h−1 have been proposed based on theoretical and experimental values [19, 25, 49]. We used 0.5 h−1, 0.1 h−1 and 1.0 h−1 for nominal, lower and upper bound cases, respectively. For room volume V, the nominal case used the median bedroom volume (25 m3); lower and upper bound cases used the 25th and 75th percentile measurements (22 and 30 m3). The effective volume may differ since windows and door(s) are not always closed, and measured volumes excluded furniture, closets, etc. AERs can be defined in several ways. Considering only outside air, the conventional definition and relevant in the sense that outside air dilutes pollutants, the median, 25th and 75th percentile AERs were 2.1, 0.7 and 3.1 h−1, respectively. However, air also enters the child's bedroom from the main part of the home; this air must also be treated by the filter. Considering both outside air and interzonal flows, the median, 25th and 75th AERs were 2.9, 2.0 and 4.2 h−1. These values were utilized in the sensitivity analysis.
We investigated model responses for a “nominal” case (based on mainly median values), a "best case" (lower-bound values of AER and V, and upper-bound values for Qr, ηr and Kp), and a "worst case" (the opposite for the five parameters). Predicted PM removals for the nominal, worst and best cases ranged from 64 to 94% respectively (Table 7), quite similar to rates found in the field (55 to 95%, depending on the measurement, Table 6, Figure 3). With respect to individual parameters, predicted removals were about equally sensitive to AER, V, Qr and ηr, and largely insensitive to Kp. In the best case, removal rates were largely insensitive to all parameters, since the filter flow rate was high relative to the incoming flows. Sensitivity increased for the worst case, e.g., the relative sensitivity of most parameters was 0.4 or −0.4, e.g., doubling the AER or V, or halving Qr or ηr, would reduce PM removal rates by about 40%.
Table 7.
Relative sensitivity (RS) to reductions of 10% in each parameter.
| Parameter | Units | Median Value | Best Case | Worst Case | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | R (%) | RS | Value | R (%) | RS | Value | R (%) | RS | ||
| AER | h−1 | 2.9 | 89.6 | 0.10 | 2.0 | 94.7 | 0.06 | 4.2 | 65.6 | 0.27 |
| Qr | m3 h−1 | 675 | 87.6 | −0.12 | 745 | 93.6 | −0.06 | 318 | 61.4 | −0.39 |
| ηr | % | 100 | 87.6 | −0.12 | 100 | 93.6 | −0.06 | 95 | 61.4 | −0.39 |
| V | m3 | 25 | 89.7 | 0.11 | 22 | 94.7 | 0.06 | 30 | 66.3 | 0.38 |
| Kp | h−1 | 0.5 | 88.9 | 0.02 | 0.1 | 94.2 | 0.00 | 1.5 | 64.5 | 0.10 |
| Nominal case | - | 88.7 | - | - | 94.2 | - | - | 63.9 | - | |
From eq. 3, the performance of stand-alone filters depends on the filter’s PM removal, given by Qr ηr/V, relative to the removal attained by deposition/settling and ventilation, Kp + AER. Generally, deposition and settling processes have limited impact. Thus, when filter flow rate Qr is high relative to the AER flow (AER V), the filter will treat most of the air and PM removal rates will be high. Removals can be maximized by keeping the filter air flow rate high (e.g., using a high fan setting) and by minimizing the AER, e.g., closing windows and doors. This was our motivation in asking participants to keep the bedroom door closed (as practical) and by providing air conditioners in the summer (allowing windows to be kept closed).
4.6 Strengths and limitations
This study evaluated a large number of occupied homes, and its design used a powerful paired analysis that controlled for seasonal and cross-sectional changes in emission rates, AERs and other potential confounders. We conducted both integrated and continuous measurements over a week's time, and obtained ancillary measurements such as filter usage, which turned out to be important since many of the households did not use filters as instructed. We used a community-based participatory research approach to enhance the relevance of the intervention to community residents, and to help recruit and retain participants. The results appear robust, match theoretical predictions, and most measurements are comparable to those obtained in other urban studies, suggesting that results are generalizable.
The study has several limitations. Each home was tested in a single season, and the number of homes tested in each season was unbalanced, which limited our ability to make seasonal analyses. (Subsequent papers will report on seasonal levels of PM and other parameters.) Only limited information is available regarding occupant activity, window opening and other factors that generate or affect PM levels. While three types of PM measurements were utilized, our gravimetric measurements were not size selective, although in most cases, PM appears to be dominated by small (<1 µm dia) particles based on correlations with 0.3–1.0 µm PNCs, seasonal variations, the literature, and visual inspection of filters. The filter performance measures assume that indoor and outdoor emissions and concentrations (and human activities causing them) were independent of filter operation; this could only be partly confirmed by the CO2 measurements. Still, we have some concern that the filter’s noise and drafts could either drive children away, or possibly attract children (and maybe smokers). PM levels outside of the study homes, which would account for a portion of the indoor PM, were not monitored. However, based on Detroit area monitoring, the systematic day-of-week variation in ambient PM2.5 levels is too small to affect results; additionally, this variation is uncorrelated with the filter deployment or sampling schedule and thus would not affect the performance evaluation.
We did not evaluate mixing and the other model assumptions, however, complete mixing can be justified in the small bedrooms, especially since the filter discharged air at a moderate velocity that would promote mixing. With this assumption, performance does not depend on the filter’s location in the space, or whether pollutants arise from indoor or outdoor sources. Finally, steady-state conditions (time-invariant parameters) were assumed. This could be only partially confirmed, e.g., control homes did not show significant changes.
5 Conclusions
Understanding levels of indoor pollutants and the potential for air filters to improve air quality has special relevance for children with asthma. In 126 households with an asthmatic child in Detroit, Michigan, PM concentrations in the child's bedroom averaged 28 ± 23 µg m−3 (maximum of 96 µg m−3), and the median CO2 concentration was 1,018 ppm. Environmental tobacco smoke (ETS) was detected in most homes where smoking was unrestricted and occupants included smokers, which elevated PM levels in bedrooms by an average of 15 ± 11 µg m−3. The use of a stand-alone HEPA filter placed in the child's sleeping area reduced PM concentrations by an average of 69 to 80%, depending on the PM measure. Filter air flow rates, room volumes and AERs are the key parameters affecting PM removal, especially when filter flow rates are low and room volumes and AERs are high. These results resemble findings of other indoor exposure studies, however, monitoring emphasized the child's bedroom, where most exposure is likely to occur, and filter performance was characterized in a robust manner. While both PM and ETS exposures are high in a substantial fraction of urban homes with asthmatic children, filters can dramatically reduce PM levels. Thus, broad-scale interventions using filters can be an effective strategy to lower PM exposures, especially if indoor and outdoor PM sources cannot be reduced or eliminated.
Acknowledgements
We thank our Detroit participants, our Detroit and Ann Arbor staff including Sonya Grant, Leonard Brakefield, Dennis Fair, Ricardo de Majo, Xiaodan Ren, Huda Elasaad and Andrew Ekstrom, and our CAAA Steering Committee members (Arab Community Center for Economic and Social Services (ACCESS); Community Health & Social Services Center (CHASS); Detroit Hispanic Development Corporation (DHDC); Detroiters Working for Environmental Justice (DWEJ); Friends of Parkside (FOP); Latino Family Services (LFS); Warren/Conner Development Coalition; City of Detroit Dept of Health and Wellness Promotion, and the University of Michigan Schools of Public Health and Medicine). This study was conducted as part of NIEHS grant R01-ESO14566-01A1, "A Community Based Participatory Research Intervention for Childhood Asthma Using Air Filters and Air Conditioners." Liuliu Du was supported in part by the China Scholarship Council (CSC).
References
- 1.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. In-Home Particle Concentrations and Childhood Asthma Morbidity. Environmental Health Perspectives. 2009;117(2):294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: literature review. Journal of Advanced Nursing. 2005;52(3):328–339. doi: 10.1111/j.1365-2648.2005.03591.x. [DOI] [PubMed] [Google Scholar]
- 3.Sublett JL, Seltzer J, Burkhead R, Williams PB, Wedner HJ, Phipatanakul W. Air filters and air cleaners: Rostrum by the American Academy of Allergy, Asthma & Immunology Indoor Allergen Committee. Journal of Allergy and Clinical Immunology. 2010;125(1):32–38. doi: 10.1016/j.jaci.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz J, Neas LM. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology. 2000;11(1):6–10. doi: 10.1097/00001648-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environmental Health Perspectives. 2000;108(10):941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. Journal of Urban Health-Bulletin of the New York Academy of Medicine. 2007;84(4):577–590. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environmental Research. 2005;98(2):167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner-city children with asthma: The National Cooperative Inner-City Asthma Study. Pediatric Pulmonology. 1997;24(4):253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Thomson NC. The role of environmental tobacco smoke in the origins and progression of asthma. Current Allergy and Asthma Reports. 2007;7(4):303–309. doi: 10.1007/s11882-007-0045-8. [DOI] [PubMed] [Google Scholar]
- 10.Cook DG, Strachan DP. Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–365. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Analysis and Environmental Epidemiology. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 12.Abt E, Suh HH, Allen G, Koutrakis P. Characterization of indoor particle sources: A study conducted in the metropolitan Boston area. Environmental Health Perspectives. 2000;108(1):35–44. doi: 10.1289/ehp.0010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macintosh DL, Myatt TA, Ludwig JF, Baker BJ, Suh HH, Spengler JD. Whole House Particle Removal and Clean Air Delivery Rates for In-Duct and Portable Ventilation Systems. Journal of the Air & Waste Management Association. 2008;58(11):1474–1482. doi: 10.3155/1047-3289.58.11.1474. [DOI] [PubMed] [Google Scholar]
- 14.Hussein T, Korhonen H, Herrmann E, Hameri KH, Lehtinen KEJ, Kulmala M. Emission rates due to indoor activities: Indoor aerosol model development, evaluation, and applications. Aerosol Science and Technology. 2005;39(11):1111–1127. [Google Scholar]
- 15.Monn C, Fuchs A, Hogger D, Junker M, Kogelschatz D, Roth N, et al. Particulate matter less than 10 µm (PM10) and fine particles less than 2.5 µm (PM2.5): relationships between indoor, outdoor and personal concentrations. Science of the Total Environment. 1997;208(1–2):15–21. doi: 10.1016/s0048-9697(97)00271-4. [DOI] [PubMed] [Google Scholar]
- 16.Wallace LA, Mitchell H, O'Connor GT, Neas L, Lippmann M, Kattan M, et al. Particle concentrations in inner-city homes of children with asthma: The effect of smoking, cooking, and outdoor pollution. Environmental Health Perspectives. 2003;111(9):1265–1272. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environmental Research. 2008;106(2):148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crain EF, Walter M, O'Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven US urban communities and design of an environmental intervention: The Inner-City Asthma Study. Environmental Health Perspectives. 2002;110(9):939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batterman S, Godwin C, Jia CR. Long duration tests of room air filters in cigarette smokers' homes. Environmental Science & Technology. 2005;39(18):7260–7268. doi: 10.1021/es048951q. [DOI] [PubMed] [Google Scholar]
- 20.Zhao B, Guan P. Modeling particle dispersion in personalized ventilated room. Building and Environment. 2007;42(3):1099–1109. [Google Scholar]
- 21.Lai ACK, Wong SL. Experimental Investigation of Exhaled Aerosol Transport Under Two Ventilation Systems. Aerosol Science and Technology. 2010;44(6):444–452. [Google Scholar]
- 22.Xu Y, Raja S, Ferro AR, Jaques PA, Hopke PK, Gressani C, et al. Effectiveness of heating, ventilation and air conditioning system with HEPA filter unit on indoor air quality and asthmatic children's health. Building and Environment. 2010;45(2):330–337. [Google Scholar]
- 23.Myatt TA, Minegishi T, Allen JG, MacIntosh DL. Control of asthma triggers in indoor air with air cleaners: a modeling analysis. Environmental Health. 2008;7 doi: 10.1186/1476-069X-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis H, Fletcher G, Anthony C, Pickering C, Oldham L, Hadley E, et al. Clinical effects of air filters in homes of asthmatic adults sensitized and exposed to pet allergens. Clinical and Experimental Allergy. 2003;33(1):101–105. doi: 10.1046/j.1365-2222.2003.01570.x. [DOI] [PubMed] [Google Scholar]
- 25.Fisk WJ, Faulkner D, Palonen J, Seppanen O. Performance and costs of particle air filtration technologies. Indoor Air. 2002;12(4):223–234. doi: 10.1034/j.1600-0668.2002.01136.x. [DOI] [PubMed] [Google Scholar]
- 26.Howard-Reed C, Wallace LA, Emmerich SJ. Effect of ventilation systems and air filters on decay rates of particles produced by indoor sources in an occupied townhouse. Atmospheric Environment. 2003;37(38):5295–5306. [Google Scholar]
- 27.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Annals of Allergy Asthma & Immunology. 2005;95(6):518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 28.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, Demasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. Journal of Allergy and Clinical Immunology. 1990;85(6):1050–1057. doi: 10.1016/0091-6749(90)90050-e. [DOI] [PubMed] [Google Scholar]
- 29.Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. American Journal of Respiratory and Critical Care Medicine. 1998;158(1):115–120. doi: 10.1164/ajrccm.158.1.9712110. [DOI] [PubMed] [Google Scholar]
- 30.Cheng YS, Lu JC, Chen TR. Efficiency of a portable indoor air cleaner in removing pollens and fungal spores. Aerosol Science and Technology. 1998;29(2):92–101. [Google Scholar]
- 31.Green R, Simpson A, Custovic A, Faragher B, Chapman M, Woodcock A. The effect of air filtration on airborne dog allergen. Allergy. 1999;54(5):484–488. doi: 10.1034/j.1398-9995.1999.00029.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Heide S, van Aalderen WMC, Kauffman HF, Dubois AEJ, de Monchy JGR. Clinical effects of air cleaners in homes of asthmatic children sensitized to pet allergens. Journal of Allergy and Clinical Immunology. 1999;104(2):447–451. doi: 10.1016/s0091-6749(99)70391-x. [DOI] [PubMed] [Google Scholar]
- 33.vanderHeide S, Kauffman HF, Dubois AEJ, deMonchy JGR. Allergen reduction measures in houses of allergic asthmatic patients: Effects of air-cleaners and allergen-impermeable mattress covers. European Respiratory Journal. 1997;10(6):1217–1223. doi: 10.1183/09031936.97.10061217. [DOI] [PubMed] [Google Scholar]
- 34.Sulser C, Schulz G, Wagner P, Sommerfeld C, Keil T, Reich A, et al. Can the Use of HEPA Cleaners in Homes of Asthmatic Children and Adolescents Sensitized to Cat and Dog Allergens Decrease Bronchial Hyperresponsiveness and Allergen Contents in Solid Dust? International Archives of Allergy and Immunology. 2009;148(1):23–30. doi: 10.1159/000151502. [DOI] [PubMed] [Google Scholar]
- 35.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans RI, et al. Results of a home-based environmental intervention among urban children with asthma. New England Journal of Medicine. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AL, Colome SD, Tian Y, Becker EW, Baker PE, Behrens DW, et al. California residential air exchange rates and residence volumes. Journal of Exposure Analysis and Environmental Epidemiology. 1996;6(3):311–326. [PubMed] [Google Scholar]
- 37.Howard-Reed C, Wallace LA, Ott WR. The effect of opening windows on air change rates in two homes. Journal of the Air & Waste Management Association. 2002;52(2):147–159. doi: 10.1080/10473289.2002.10470775. [DOI] [PubMed] [Google Scholar]
- 38.Luoma M, Batterman SA. Autocorrelation and variability of indoor air quality measurements. Aihaj. 2000;61(5):658–668. doi: 10.1080/15298660008984575. [DOI] [PubMed] [Google Scholar]
- 39.Chao CYH, Tung TCW, Burnett J. Influence of different indoor activities on the indoor particulate levels in residential buildings. Indoor Built Environ. 1998;7(2):110–121. [Google Scholar]
- 40.The Center for Urban Studies. WSU. 2000 Census Demographic Profile of City of Detroit. 2001 http://www.cus.wayne.edu/census/censuspubs.aspx.
- 41.Morey PR, Crawfird GB, Rottersman RB. Indoor Air Quality in NonIndustrial Occupational Environments. In: Rose VE, Chohrssen B, editors. Patty's Industrial Hygiene. Sixth Edition. New York: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 42.Jia C, Batterman S, Godwin C. Continuous, intermittent and passive sampling of airborne VOCs. Journal of Environmental Monitoring. 2007;9(11):1220–1230. doi: 10.1039/b708119g. [DOI] [PubMed] [Google Scholar]
- 43.Charles SM, Jia C, Batterman SA, Godwin C. VOC and particulate emissions from commercial cigarettes: Analysis of 2,5-DMF as an ETS tracer. Environmental Science & Technology. 2008;42(4):1324–1331. doi: 10.1021/es072062w. [DOI] [PubMed] [Google Scholar]
- 44.Batterman S, Metts T, Kalliokoski P, Barnett E. Low-flow active and passive sampling of VOCs using thermal desorption tubes: theory and application at an offset printing facility. Journal of Environmental Monitoring. 2002;4(3):361–370. doi: 10.1039/b203289a. [DOI] [PubMed] [Google Scholar]
- 45.Batterman S, Jia CR, Hatzivasilis G, Godwin C. Simultaneous measurement of ventilation using tracer gas techniques and VOC concentrations in homes, garages and vehicles. Journal of Environmental Monitoring. 2006;8(2):249–256. doi: 10.1039/b514899e. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Shendell DG, Winer AM, Zhang J. Residential air exchange rates in three major US metropolitan areas: results from the Relationship Among Indoor, Outdoor, and Personal Air Study 1999–2001. Indoor Air. 20(1):85–90. doi: 10.1111/j.1600-0668.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 47.Michigan Department of Natural Rsources and Environment. Lansing, MI: [Accessed 3/11/2011]. Michigan 2009 Air Quality Report. http://michigan.gov/documents/deq/AQD-aqe-2009-AIR-QUALITY-REPORT_322419_7.pdf. [Google Scholar]
- 48.Nazaroff WW, Hung WY, Sasse A, Gadgil AJ. Predicting regional lung deposition of environmental tobacco-smoke particles. Aerosol Science and Technology. 1993;19(3):243–254. [Google Scholar]
- 49.Zhao B, Wu J. Effect of particle spatial distribution on particle deposition in ventilation rooms. Journal of Hazardous Materials. 2009;170(1):449–456. doi: 10.1016/j.jhazmat.2009.04.079. [DOI] [PubMed] [Google Scholar]