Abstract
BACKGROUND:
Cognitive impairment, from mild forms to dementia, is an important social and health concern, principally among older individuals. Elderly patients are usually followed by general internists, who may overlook this condition.
OBJECTIVE:
Our aim was to determine whether cognitive impairment diagnosed by specialists had been previously detected by general internists.
SUBJECTS AND METHODS:
A total of 248 elderly individuals randomly selected from a list of outpatients seen by general internists in a public university hospital in São Paulo, Brazil, were evaluated by a geriatrician. Patients were then classified as having probable cognitive impairment or not, based on their performance on the Mini-Mental State Examination and the Informant Questionnaire on Cognitive Decline in the Elderly. Cases of probable impairment were submitted to routine laboratory investigation, brain computed tomography, and neuropsychological evaluation. The final diagnoses were established by a consensus panel comprising two neurologists and the geriatrician who evaluated the patients using all available data. General internists' files for all cognitively impaired cases and for a selected sample of individuals without cognitive impairment were checked for any record of cognitive complaints or decline.
RESULTS:
Forty-three patients were classified as demented (n = 21) or as cognitively impaired but not demented (n = 22). The evaluation of the general internists' files revealed that information on cognitive complaints or decline was recorded for seven (16.3%) of the 43 patients with dementia or cognitive impairment without dementia.
CONCLUSIONS:
General internists seldom detected cognitive decline in elderly patients in Brazil. Further studies should be conducted to elucidate the reasons for this low rate of detection.
Keywords: Aged, Cognition disorders, Dementia, Diagnosis, Primary care
INTRODUCTION
Cognitive impairment, which ranges from mild cognitive decline to dementia, represents an important social and health concern, principally among the elderly.1 In Brazil, most older individuals are followed by general internists (GIs) in a range of settings, including public basic healthcare units, university-based tertiary hospitals, and private practices.2
Two population-based studies performed in the state of São Paulo, Brazil, found a prevalence of dementia from 7.1% to 8.8% in patients aged 65 years and older.3,4 Furthermore, the prevalence of potentially reversible dementia was found to be 8% in a study conducted at the neurology outpatient clinic of a university-based Brazilian tertiary hospital.5
Several studies in the medical literature have shown that in the elderly, cognitive impairment and other neuropsychiatric disorders, such as depression, are frequently undiagnosed in primary care settings in multiple countries.6-10 Other studies have also found GI expertise on dementia to be lacking.11,12
The aim of this study was to determine whether GIs are effectively diagnosing cognitive impairment and dementia in Brazil.
SUBJECTS AND METHODS
The study was conducted at two outpatient services, the General Teaching Outpatient Service (GTOS) and the Internal Medicine Outpatient Service (IMOS), both run by the Internal Medicine Department of the Hospital das Clínicas of the University of São Paulo Medical School, a public university hospital in Brazil. In the GTOS, patients who are referred by physicians from the Emergency Unit are seen and treated in up to three medical consultations. In the GTOS, interns and medical residents examine patients and discuss all clinical cases with experienced GIs. In the IMOS, patients are followed for longer periods because they usually harbor more chronic conditions. Experienced GIs and residents of the Internal Medicine Department care for these patients.
We assumed that the prevalence of cognitive impairment in the elderly within hospital settings is approximately 20%. Thus, to evaluate 50 individuals with cognitive impairment, we estimated that 250 patients should be screened. The inclusion criteria were as follows: to have been followed at one of the two outpatient services, aged 65 or older, and contactable by telephone. Patients were contacted by telephone and invited to undergo a cognitive evaluation. To assure the randomness of the sample, a list randomizer was used. We initially invited patients selected from a list of 1,480 elderly individuals seen by GIs during a three-month period (November 1, 2003 to January 31, 2004). Of 296 patients contacted by telephone, 118 patients agreed to be evaluated by the geriatrician. These evaluations were conducted up to May 2005. To provide a shorter interval between the evaluations by the GI and the geriatrician, a second list of 1637 elderly patients seen by GIs from a different three-month period (June 1 to August 31, 2005) was used. Of the 204 contacted patients from this second list, 130 patients were evaluated up to May 2006. Thus, the final sample comprised 248 elderly patients who underwent evaluation by the geriatrician. It was not possible to compare the included subjects with the patients who refused evaluation.
The patients' informants were invited to accompany the patients during the first evaluation. When this was not possible, informants were later contacted by telephone to gather further information on the patient's cognitive status. All of the participants signed a written consent form before taking part in the study.
The assessment consisted of recording subjective memory complaints, medical antecedents, and use of medications as well as the application of the following tests and questionnaires: the Mini-Mental State Examination (MMSE),13,14 the short version of the Informant Questionnaire on Cognitive Decline in the Elderly (Short-IQCODE),15 the Brief Screening Cognitive Battery,16-18 the Functional Activities Questionnaire (FAQ),19 the Forward and Backward Digit Span, and the 15-item Geriatric Depression Scale (GDS).20 Initially, Short-IQCODE and MMSE scores were used to classify patients as having probable cognitive impairment or otherwise, as recommended by previous Brazilian studies.14,21 These two screening instruments were chosen because they are frequently used in Brazil.16 The MMSE is a brief neuropsychological screening instrument consisting of questions on temporal and spatial orientation, immediate memory, attention/concentration, delayed recall, and language as well as a task involving the copy of a drawing. The maximum score is 30. The short version of the IQCODE is a 16-item questionnaire, in which an informant compares the patient's current versus previous (ten years ago) cognitive performance. Scores higher than three are suggestive of decline, and the worst possible score is five. Patients with either a below-normal score on the MMSE or an above-normal score on the Short-IQCODE were classified as probable cases (suspected cognitive impairment). A group of patients with normal scores on both tests was randomly selected (non-probable case group) to serve as the control for comparison. The probable cases underwent neuropsychological evaluation, including the Dementia Rating Scale,22,23 laboratory tests (blood count, thyroid hormones, syphilis serology, liver function, kidney function, vitamin B12, and folic acid levels), and a brain computed tomography (CT) scan.24 Non-probable cases were not submitted to this evaluation.
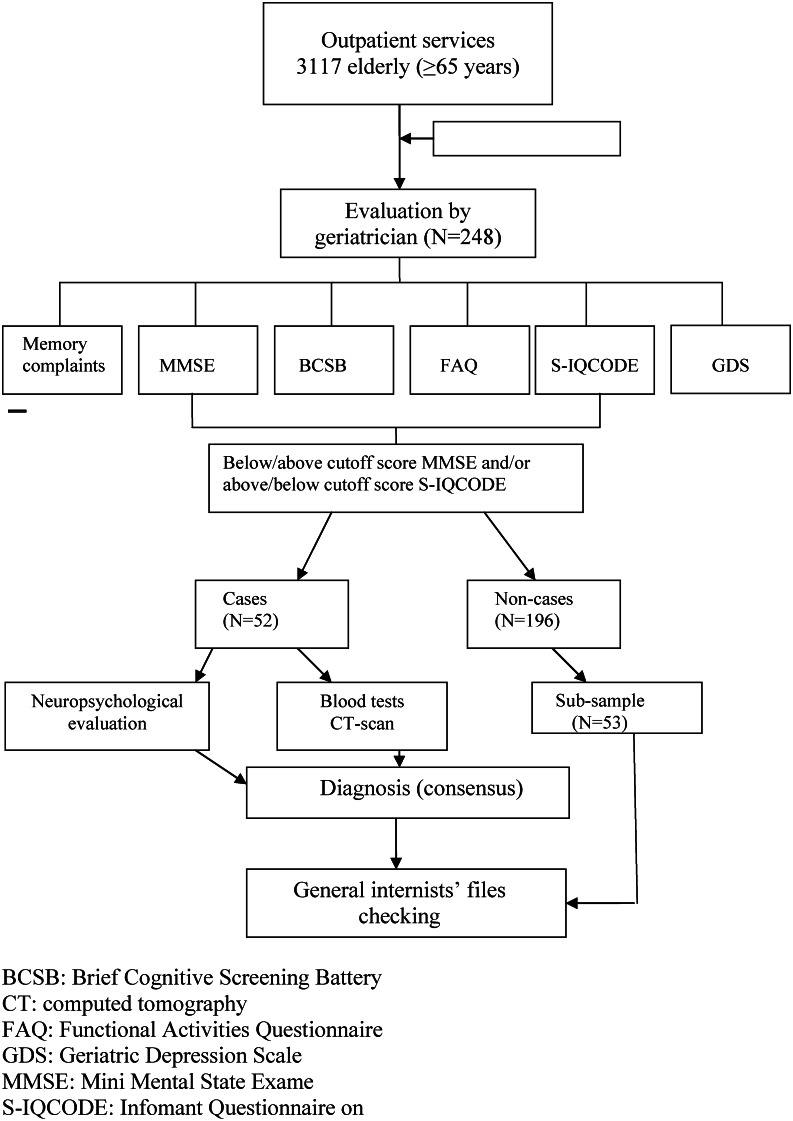
The final diagnoses were established in a consensus meeting of two neurologists specializing in dementia and the geriatrician who evaluated the patients using all available data. The probable cases and the control group were evaluated on the basis of clinical data, performances on neuropsychological tests and questionnaires, and the probable cases were also diagnosed using their laboratory and CT results. Patients were classified as having dementia,25 being cognitively impaired but not demented (CIND)26 or being without cognitive impairment. The GIs' files were checked for any record of cognitive impairment in both the probable and non-probable cases. All GIs' notes until the patients' last appointment (prior to their evaluation by the geriatrician) were checked. Figure 1 shows the diagnosis flowchart.
Figure 1.
Flow diagram from outpatient services to study completion. BCSB: Brief Cognitive Screening Battery. CT: Computed Tomography. FAQ: Functional Activities Questionnaire. GDS: Geriatric Depression Scale. MMSE: Mini-Mental State Exam. Short-IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly (short version).
The Ethics Committee of the Hospital das Clínicas of the University of São Paulo Medical School in Brazil approved this study.
Statistical analysis
Statistical analysis was used to compare the three groups: “dementia”, “cognitive impairment with no dementia” (CIND), and “without cognitive impairment”. Continuous variables were compared using the non-parametric Kruskal-Wallis test followed by a multiple comparisons test (Dunn's post-hoc test). Frequencies of categorical variables were compared using the Chi-square test. Data were analyzed using SPSS (Statistical Package for Social Sciences) version 11.5 for Windows. The accepted level of significance was set at 0.05.
RESULTS
Of the 248 evaluated elderly patients, 52 were classified as probable cases of cognitive impairment and 196 as without cognitive impairment, based on the screening interview. For the control group, 53 individuals were randomly selected from the 196 subjects without cognitive impairment. Although many patients were undergoing treatment for chronic disorders at the time of the interview, in no case did these disorders hamper the cognitive evaluation. The median age and level of education of these subjects are shown in Table 1. A difference in education was found among the three groups (p = 0.02), with the dementia group that had a lower level of education than the group of healthy individuals.
Table 1.
Age, education level and gender of the subjects with and without cognitive impairment.
| NCI(n = 53) | CIND(n = 22) | Dementia(n = 21) | p-value | Multiple comparisons | |
| Age,median(IQI) | 70(67-74) | 69.5(67-73) | 72(67-75) | 0.28* | Dementia = CIND = NCI |
| Years of EducationMedian(IQI) | 4(3-10) | 4(2-8) | 2(2-4) | 0.02* | Dementia = CIND†CIND = NCIDementia<WCI |
| Gender(Female) | 37(69.8%) | 20(90.9%) | 18(85.7%) | 0.7§ | Dementia = CIND = NCI |
Kruskal-Wallis test; †Dunn's post hoc test; IQI: interquartile interval; §Chi-Square Test; CIND: cognitive impairment with no dementia; NCI: no cognitive impairment.
Of the 52 probable cases, 33 were seen by GIs at the IMOS and 19 at the GTOS unit. Eighteen individuals had scores below the cutoff on the MMSE; 12 had above-cutoff scores on the IQCODE; and 22 were impaired according to both questionnaires. The mean time interval between the GI evaluation and the geriatrician evaluation was 5.0 (±1.7) months.
Forty-three probable-case patients underwent neuropsychological evaluation, and six subjects were not submitted to further neuropsychological evaluation due to severe cognitive impairment. Two individuals refused further evaluation after the first appointment, and one moved to another town.
All 52 probable cases and the 53 control cases without cognitive impairment were evaluated at the consensus meeting. Of the 52 probable cases, 21 were given a final diagnosis of dementia, and 22 were diagnosed with CIND, producing a total prevalence of cognitive impairment of 17.3%. None of the cases without cognitive impairment were diagnosed as having dementia or CIND at the consensus meeting. Table 2 shows the nosological diagnoses of the probable and non-probable case groups.
Table 2.
Nosological diagnoses of individuals in the probable case group and in a subsample of the non-probable case group.
| Diagnosis | Probable cases (%)(n = 52) | Sample ofnon-probable cases (%)(n = 53) |
| Probable AD | 12 (23.0) | 0 |
| AD+cerebrovascular disease | 4 (7.7) | 0 |
| Vascular dementia | 2 (3.8) | 0 |
| Dementia due to intracranial mass lesions | 1 (1.9) | 0 |
| Parkinson's disease dementia | 1 (1.9) | 0 |
| Alcoholic dementia | 1 (1.9) | 0 |
| CIND | 22 (42.3) | 0 |
| Depression | 0 | 7 (13.2) |
| Not cognitively impaired (only subjective memory complaint) | 2 (3.8) | 17 (32.0) |
| No subjective or objective cognitive impairment | 4 (7.7) | 29 (54.7) |
| No diagnosis (insufficient data) | 3 (5.8) | 0 |
AD: Alzheimer's disease; CIND: cognitive impairment with no dementia.
Information related to cognitive impairment was found in the outpatient service medical records of seven (16.3%) of the 43 patients diagnosed with dementia or CIND. Six of these seven cases had moderate or severe dementia based on MMSE and Short-IQCODE scores, while one case had CIND (Table 3). These seven patients had been seen at either the GTOS (4 patients) or the IMOS (3 patients). All of the information related to cognitive impairment recorded in the outpatient service files is shown in Table 3. No record of cognitive impairment was present in the GI files for the 53 cases without cognitive impairment.
Table 3.
Patient demographic data, diagnoses, MMSE and Short-IQCODE scores, and records from general internists' files.
| Case | Age | Gender | Education | Diagnosis | MMSE | Short-IQCODE | GIs' file notes |
| 1 | 82 | Female | 0 | Probable AD | 18 | 3.83 | Subjective memory complaint |
| 2 | 88 | Female | 5 | Probable AD | 11 | 4.84 | AD diagnosis |
| 3 | 73 | Female | 0 | Probable AD | 13 | 4.37 | AD diagnosis |
| 4 | 79 | Female | 4 | Probable AD | 17 | 3.9 | Subjective memory complaint and impairment in daily life activities |
| 5 | 70 | Female | 1 | Probable AD | 0 | 5.0 | AD diagnosis |
| 6 | 85 | Female | 4 | AD plus cerebrovascular disease | 18 | 3.96 | Impairment in daily life activities |
| 7 | 70 | Female | 4 | CIND | 23 | 3.76 | Subjective memory complaint |
AD: Alzheimer's disease; CIND: cognitively impaired but not demented; GI: general internist; MMSE: Mini-Mental State Exam; Short-IQCODE: short version of the Informant Questionnaire on Cognitive Decline in the Elderly.
DISCUSSION
GIs had previously identified 16.3% of the cases that were later diagnosed with dementia (6 cases) or CIND (1 case) by specialists. Each of these seven patients had been seen by a different GI; four were seen at one outpatient service, whereas three were seen in the other service, indicating that the lack of recognition of cognitive impairment did not come from a specific professional or outpatient service. It should be emphasized that there was no record of cognitive or functional decline in the files of 15 patients diagnosed with dementia, despite the fact that this study was conducted in a university-based tertiary hospital where professionals are highly qualified and kept abreast of recent scientific developments.
Two studies adopting a similar methodology (analyzing GIs' files after specialists had diagnosed cognitive impairment in elderly patients) have also been published. Valcour et al. reported that 65% of the dementia cases diagnosed by geriatricians in their study had previously been identified by GIs who had followed these patients routinely.6 Another study using a similar methodology was conducted in Chicago, Illinois (USA) and showed that less than 20% of the cognitively impaired cases had been previously recorded as such in the GIs' files.8
In 2000, Renshaw et al. demonstrated that doctors in the United Kingdom believed that knowledge about the symptoms of cognitive impairment in the elderly is not important because there is no effective treatment for these conditions. The same study also revealed that these professionals felt they had received insufficient training on dementia issues in their medical graduate programs.11
In Canada and Australia, Lorentz et al. showed that GIs felt that applying cognitive tests in their work settings (a large number of patients and a short period to attend to them) was not feasible because these instruments are very complex and time consuming.7
The present study did not investigate knowledge about dementia and its respective diagnostic tools among the GIs. The medical literature shows GIs' knowledge about dementia to be limited, and this might explain the findings of our study.11,12 In Brazil, Bertolucci et al. gave a questionnaire about dementia knowledge to specialists (geriatricians, psychiatrists, and neurologists) and GIs. The results revealed that GIs had less knowledge about dementia than did neurologists, psychiatrists, and geriatricians.12 Other factors that may be causally related to the low diagnostic rate of cognitive impairment in our study are the difficulties that GIs face in the public healthcare system, where there is limited time available for each appointment and where patients often present with multiple co-morbidities. Another factor contributing to the under-reporting of the condition could be the low educational level of the patients and caregivers in this study, who consequently reported few or no complaints of cognitive impairment. A limitation of this study might be the fact that it was conducted at one specific location (University Hospital in São Paulo), so these findings cannot be generalized to all of Brazilian society.
Problems associated with an aging population were faced by economically developed countries almost one century ago. Brazil is now having to deal with the rapid aging of its population and should begin developing strategies in the near future to avoid serious repercussions. In Brazil, despite the efforts of public officials, there has been little reform of the public healthcare system to cater to an aging population, and the elderly encounter difficulties when seeking public healthcare services. Access to services equipped to receive older patients remains very limited. Basic healthcare units and even some large tertiary hospitals (university-associated or otherwise) are inadequately prepared for the growing demands placed on them by the elderly population.27
Advanced medical and scientific resources allow the early detection of diseases; cancer, diabetes, arterial hypertension, osteoporosis, and dyslipidemia are examples of diseases commonly screened for in public health programs on a preventive basis. However, the different types of dementia are often excluded from such programs despite their high prevalence.28,29
Dementia is the largest contributor to disability in elderly people in countries with low and middle incomes,30 and the societal costs of dementia have been increasing in recent years.31 Early diagnosis of cognitive impairment is important for several reasons. Ruling out potentially reversible conditions is the most relevant of these reasons, but providing more detailed instructions on how to take medications is another significant reason. The safety of the patient and of others may be safeguarded by preventing car accidents and disease complications arising from patients' inability to take care of themselves.32-34 For the patient and his/her family, a diagnosis of cognitive impairment has an impact on their plans for the future, including financial and legal matters.35,36
Mild cognitive decline that does not significantly affect patients' daily living activities may be more frequently overlooked by GIs. In this study, only one case classified as CIND contained a report of cognitive impairment in the GI file. Considering that patients with CIND may have higher rates of progression to dementia than non-impaired subjects, this diagnosis may be relevant for the patient.37
A note of caution should be made regarding the 17.3% prevalence of cognitive impairment found in this study. In spite of the random selection of cases selected for evaluation, many of them could not participate, and only a small sample was interviewed. It is possible that those who came for the interview had greater concerns about their cognitive functions.
Further studies should be conducted to elucidate the reasons underlying the low detection of cognitive impairment by GIs and to investigate the advantages and pitfalls of early detection of dementia.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues MA, Facchini LA, Piccini RX, Tomasi E, Thumé E, Silveira DS, et al. Use of outpatient services by the elderly in the South and Northeast of Brazil. Cad Saude Publica. 2008;24:2267–78. doi: 10.1590/s0102-311x2008001000008. 10.1590/S0102-311X2008001000008 [DOI] [PubMed] [Google Scholar]
- 3.Herrera E, Caramelli P, Silveira ASB, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16:103–8. doi: 10.1097/00002093-200204000-00007. 10.1097/00002093-200204000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Bottino CM, Azevedo D, Jr, Tatsch M, Hototian SR, Moscoso MA, Folquitto J, et al. Estimate of dementia prevalence in a community sample from São Paulo, Brazil. Dement Geriatr Cogn Disord. 2008;26:291–9. doi: 10.1159/000161053. 10.1159/000161053 [DOI] [PubMed] [Google Scholar]
- 5.Takada LT, Caramelli P, Radanovic M, Anghinah R, Hartmann AP, Guariglia CC, et al. Prevalence of potentially reversible dementias in a dementia outpatient clinic of tertiary university-affiliated hospital in Brazil. Arq Neuropsiquiatr. 2003;6:925–9. doi: 10.1590/s0004-282x2003000600007. 10.1590/S0004-282X2003000600007 [DOI] [PubMed] [Google Scholar]
- 6.Valcour VG, Masaki H, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Int Med. 2000;160:2964–8. doi: 10.1001/archinte.160.19.2964. 10.1001/archinte.160.19.2964 [DOI] [PubMed] [Google Scholar]
- 7.Lorentz WJ, Scanian JM, Borson S. Brief screening tests for dementia. Can J Psychiatry. 2002;47:723–33. doi: 10.1177/070674370204700803. [DOI] [PubMed] [Google Scholar]
- 8.Finkel SI. Cognitive screening in the primary care setting: the role of physicians at the first point entry. Geriatrics. 2003;58:43–4. [PubMed] [Google Scholar]
- 9.Barret JJ, Haley WE, Harrel LE, Powers RE. Knowledge about Alzheimer's disease among primary care physicians, psychologists, nurses and social workers. Alzheimer Dis Assoc Disord. 1997;11:99–106. doi: 10.1097/00002093-199706000-00006. 10.1097/00002093-199706000-00006 [DOI] [PubMed] [Google Scholar]
- 10.Henriques SG, Fráguas R, Iosifescu DV, Menezes PR, Lucia MC, Gattaz WF, Martins MA. Recognition of depressive symptoms by physicians. Clinics. 2009;64:629–35. doi: 10.1590/S1807-59322009000700004. 10.1590/S1807-59322009000700004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renshaw J, Scurfield LC, Cloke L, Orrel M. General practitioner's views on the early diagnosis of dementia. Br J Gen Pract. 2001;51:37–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolucci PHF, Ferretti CEL, Naylor FGM. Knowledge of dementia among doctors in Brazil. Neurobiol Aging. 2002;23((Suppl 1)):S168. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14.Brucki SMD, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini-mental state examination in Brazil. Arq Neuropsiquiatr. 2003;61:777–81. doi: 10.1590/s0004-282x2003000500014. 10.1590/S0004-282X2003000500014 [DOI] [PubMed] [Google Scholar]
- 15.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–53. doi: 10.1017/s003329170002691x. 10.1017/S003329170002691X [DOI] [PubMed] [Google Scholar]
- 16.Nitrini R, Caramelli P, Bottino CM, Damasceno BP, Brucki SM, Anghinah R Academia Brasileira de Neurologia. [Diagnosis of Alzheimer's disease in Brazil: cognitive and functional evaluation. Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology] Arq Neuropsiquiatr. 2005;63:720–7. doi: 10.1590/s0004-282x2005000400034. 10.1590/S0004-282X2005000400034 [DOI] [PubMed] [Google Scholar]
- 17.Nitrini R, Caramelli P, Herrera Júnior E, Porto CS, Charchat-Fichman H, Carthery MT, et al. Performance of illiterate and literate nondemented elderly subjects in two tests of long-term memory. J Int Neuropsychol Soc. 2004;10:634–8. doi: 10.1017/S1355617704104062. 10.1017/S1355617704104062 [DOI] [PubMed] [Google Scholar]
- 18.Takada LT, Caramelli P, Fichman HC, Porto CS, Bahia VS, Anghinah R, et al. Comparison between two tests of delayed recall for the diagnosis of dementia. Arq Neuropsiquiatr. 2006;64:35–40. doi: 10.1590/s0004-282x2006000100008. 10.1590/S0004-282X2006000100008 [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. 10.1300/J018v05n01_09 [Google Scholar]
- 21.Bustamante SE, Bottino CM, Lopes MA, Azevedo D, Hototian SR, Litvoc J, et al. Combined instruments on the evaluation of dementia in the elderly: preliminary results. Arq Neuropsiquiatr. 2003;61:601–6. doi: 10.1590/s0004-282x2003000400014. 10.1590/S0004-282X2003000400014 [DOI] [PubMed] [Google Scholar]
- 22.Mattis S. Professional Manual. Florida: Psychological Assessment Resources; 1998. Dementia Rating Scale. [Google Scholar]
- 23.Porto CS, Fichman HC, Caramelli P, Bahia VS, Nitrini R. Brazilian version of the Mattis dementia rating scale: diagnosis of mild dementia in Alzheimer's disease. Arq Neuropsiquiatr. 2003;61:339–45. doi: 10.1590/s0004-282x2003000300004. 10.1590/S0004-282X2003000300004 [DOI] [PubMed] [Google Scholar]
- 24.Nitrini R, Caramelli P, Bottino CM, Damasceno BP, Brucki SM, Anghinah R. Diagnosis of Alzheimer's disease in Brazil: diagnostic criteria and auxiliary tests. Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Arq Neuropsiquiatr. 2005;63:713–9. doi: 10.1590/s0004-282x2005000400033. 10.1590/S0004-282X2005000400033 [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatry Association. Washington, DC: American Psychiatric Press; 1994. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. [Google Scholar]
- 26.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly: results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–9. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 27.Chaimowicz F. Health of Brazilian elderly just before of the 21st century: current problems, forecasts and alternatives. Rev Saude Publica. 1997;31:184–200. doi: 10.1590/s0034-89101997000200014. 10.1590/S0034-89101997000200014 [DOI] [PubMed] [Google Scholar]
- 28.Larson EB. Recognition of dementia: discovering the silent epidemic. J Am Geriatr Soc. 1998;46:1576–7. doi: 10.1111/j.1532-5415.1998.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 29.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21:349–55. doi: 10.1002/gps.1470. 10.1002/gps.1470 [DOI] [PubMed] [Google Scholar]
- 30.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–30. doi: 10.1016/S0140-6736(09)61829-8. 10.1016/S0140-6736(09)61829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wimo A, Winblad B, Jönsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. 10.1016/j.jalz.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in the primary care: a summary of the evidence for the US preventive services task force. Ann Intern Med. 2003;138:927–37. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 33.Clarfield AM. Reversible dementia: the implications of a fall in prevalence. Age Ageing. 2005;34:544–5. doi: 10.1093/ageing/afi203. 10.1093/ageing/afi203 [DOI] [PubMed] [Google Scholar]
- 34.Tierney MC, Charles J, Naglie G, Jaglal S, Kiss A, Fisher RH. Risk factors for harm in cognitively impaired seniors who live alone: a prospective study. J Am Geriatr Soc. 2004;52:1435–41. doi: 10.1111/j.0002-8614.2004.52404.x. 10.1111/j.0002-8614.2004.52404.x [DOI] [PubMed] [Google Scholar]
- 35.Ashford JW, Borson S, O'Hara R, Dash P, Frank L, Robert P, et al. Should older adults be screened for dementia. Alzheimer's and Dementia. 2006;2:76–85. doi: 10.1016/j.jalz.2006.02.005. 10.1016/j.jalz.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 36.Raicher I, Shimizu MM, Takahashi DY, Nitrini R, Caramelli P. Alzheimer's disease diagnosis disclosure in Brazil: a survey of specialized physicians' current practices and attitudes. Int Psychogeriatr. 2008;20:471–81. doi: 10.1017/S1041610207005819. 10.1017/S1041610207005819 [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]