Abstract
OBJECTIVE
To investigate whether the dipeptidyl peptidase-4 inhibitor vildagliptin improves endothelium-dependent vasodilatation in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Sixteen subjects with type 2 diabetes (age 59.8 ± 6.8 years, BMI 29.1 ± 4.8 kg/m2, HbA1c 6.97 ± 0.61) on oral blood glucose–lowering treatment were included. Participants received vildagliptin 50 mg b.i.d. or acarbose 100 mg t.i.d. for four consecutive weeks in a randomized, double-blind, cross-over design. At the end of each treatment period, we measured forearm vasodilator responses to intra-arterially administered acetylcholine (endothelium-dependent vasodilator) and sodium nitroprusside (endothelium-independent vasodilator).
RESULTS
Infusion of acetylcholine induced a dose-dependent increase in forearm blood flow in the experimental arm, which was higher during vildagliptin (3.1 ± 0.7, 7.9 ± 1.1, and 12.6 ± 1.4 mL ⋅ dL−1 ⋅ min−1 in response to three increasing dosages of acetylcholine) than during acarbose (2.0 ± 0.7, 5.0 ± 1.2, and 11.7 ± 1.6 mL ⋅ dL−1 ⋅ min−1, respectively; P = 0.01 by two-way ANOVA). Treatment with vildagliptin did not significantly change the vascular responses to sodium nitroprusside.
CONCLUSIONS
Four weeks’ treatment with vildagliptin improves endothelium-dependent vasodilatation in subjects with type 2 diabetes. This observation might have favorable cardiovascular implications.
Worldwide, almost 200 million people suffer from type 2 diabetes, and the prevalence is rapidly increasing (1). Type 2 diabetes causes a twofold excess risk for cardiovascular disease, partly independent from other risk factors (2). Ideally, pharmacotherapy for type 2 diabetes not only lowers blood glucose levels but also has glucose-independent beneficial cardiovascular effects.
Endothelial dysfunction is considered an early marker of vascular complications, also in type 2 diabetes (3). Endothelial dysfunction comprises a number of functional alternations in the vascular endothelium, such as impaired endothelium-dependent vasodilatation, impaired barrier function, inflammatory activation, and stimulated coagulation (4). Endothelium-dependent vasodilatation, which is impaired in endothelial dysfunction, can be assessed by measuring responses to endothelium-dependent vasodilators such as acetylcholine (5).
Recently, incretin-based therapy has been approved for the treatment of type 2 diabetes. Incretins are a group of gastrointestinal hormones, predominantly glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are secreted in response to food ingestion and stimulate insulin secretion (6). Dipeptidyl peptidase-4 (DPP-4) rapidly degrades the incretin hormones to inactive metabolites (7). In addition to incretins, DPP-4 also inactivates chemokines, cytokines, and neuropeptides (8). In type 2 diabetes DPP-4 inhibitors reduce the breakdown of GLP-1 and improve metabolic control by increasing insulin secretion, improving β-cell function, and decreasing glucagon secretion (9).
Apart from glycemic actions, GLP-1 has beneficial cardiovascular effects. GLP-1 has vasodilatory actions, which are believed to be mediated through a specific GLP-1 receptor in the vascular endothelium, and improves endothelial function in animals and humans (10,11). In addition, GLP-1 may have GLP-1 receptor-independent cardiovascular effects, which appear to be mediated by metabolites of GLP-1 (12). It is not known whether a pharmacological approach of increasing GLP-1 levels by inhibiting DPP-4, which changes the ratio between GLP-1 and its degradation product, exhibits the same vascular profile.
DPP-4 inhibition affects not only GLP-1 levels, but also GIP breakdown, and perhaps also other substrates, such as chemokines and cytokines. Whether intervening in the breakdown of these other potential substrates affects endothelial function is unknown. Indirect evidence for a potential beneficial effect on endothelial function comes from a study demonstrating that the DPP-4 inhibitor sitagliptin increases endothelial progenitor cells in type 2 diabetes by inhibiting degradation of the chemokine stromal-derived factor 1-α (13).
The fact is that meta-analyses summarizing the effects of DPP-4 inhibitors on major cardiovascular events in phase 2 and 3 studies all suggest a lower relative risk compared with placebo or other medication (14,15). These observations need to be confirmed in large cardiovascular outcome studies. The current study therefore aims to determine whether the DPP-4 inhibitor vildagliptin can improve endothelial function in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study population
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The local ethics committee (Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands) approved the study, and all subjects gave written informed consent before participation. The trial was registered at clinicaltrials.gov (number NCT01000688).
The study population consisted of 16 patients with type 2 diabetes, recruited through advertisements in a local newspaper and through the outpatient clinic of the Radboud University Nijmegen Medical Centre.
Included were subjects with type 2 diabetes who were treated with metformin with or without sulphonylurea or thiazolidinediones, aged 35–75 years, and had an HbA1c <8.0%. Exclusion criteria were use of acetylsalicylic acid or vitamin K antagonists, heart failure (New York Heart Association class III or IV), smoking, pregnancy or breastfeeding, a serum alanine aminotransferase or aspartate aminotransferase level of more than three times the upper limit of the normal range, a serum creatinine level higher than 130 μmol/L, and inability to give informed consent.
Protocol
After inclusion in the study, subjects received either vildagliptin 50 mg b.i.d. for 28 days or acarbose 50 mg t.i.d. for 7 days followed by acarbose 100 mg t.i.d. for the remaining 21 days. Participants were randomized into treatment with vildagliptin in the first treatment period and acarbose in the second treatment period or vice versa. Investigational medication was given on top of their other medication in a randomized, double-blind, cross-over design. Blinding of the study medication was performed by a double dummy technique.
Between the two treatment periods there was a wash-out period of 1 week.
Participants were contacted by telephone halfway each treatment period to check for compliance and possible side effects.
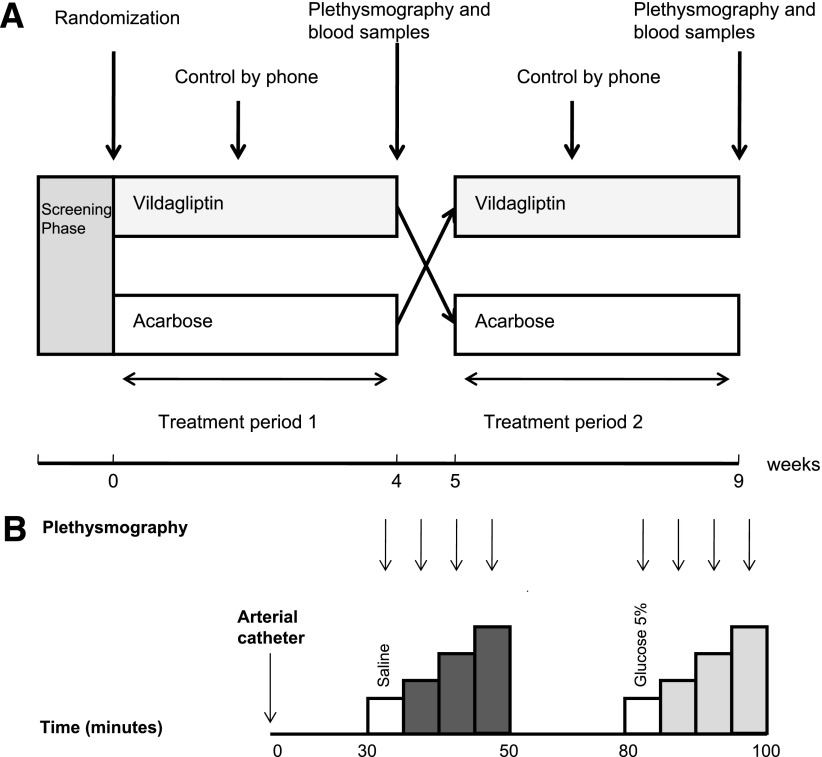
At the end of each treatment period (days 25–28) endothelial function was measured, body weight and potential side effects were determined, and arterial blood was collected for serum hematological and chemical safety profiles. A schedule of the study protocol is shown in Fig. 1A. Compliance was monitored by pill counts.
Figure 1.
The study protocol (A) in which 4 weeks’ treatment with vildagliptin and acarbose are administered in a cross-over design. The protocol of venous occlusion plethysmography (B) started with insertion of arterial catheter at t = 0, after which a 30-min equilibration period followed. Baseline FBF measurements and subsequently FBF measurements during the infusion of acetylcholine (dark gray; 0.5, 2.0, and 8.0 µg ⋅ dL−1 ⋅ min−1) are performed. Again, after a 30-min equilibration period, baseline FBF and FBF during infusion of sodium nitroprusside (light gray; 0.06, 0.20, and 0.60 µg ⋅ dL−1 ⋅ min−1) are assessed.
Experimental procedures
Endothelial function.
The experiments started at 8.30 a.m. after an overnight fast in a quiet, temperature-controlled room (23–24°C). Study medication was ingested the day of the experiments. The subjects abstained from caffeine for 24 h before the experiment. The brachial artery of the nondominant arm (experimental arm) was cannulated (Angiocath, 20-gauge; Becton Dickinson, Sandy, UT) under local anesthesia (Xylocaine 2%) for infusion of vasodilators and collection of blood samples. The brachial artery catheter was connected with an arterial pressure-monitoring line for continuous blood pressure monitoring (Hewlett Packard, Böblingen, Germany).
Forearm blood flow (FBF) was measured using mercury-in-silastic strain gauge, venous occlusion plethysmography as previously described (16). Forearm volume was measured with the water displacement method, and all drugs were dosed per 100 mL forearm tissue with an infusion rate of 100 mL ⋅ dL−1 ⋅ min−1.
After complete instrumentation, a 30 min equilibration period was included, after which baseline measurements were performed with infusion of saline. Subsequently, three increasing doses of acetylcholine (0.5, 2.0, and 8.0 µg ⋅ dL−1 ⋅ min−1, 10 mg/mL dry powder, dissolved to its final concentration with saline, Novartis, Greece) were infused into the brachial artery. Acetylcholine stimulates endothelial muscarinic receptors thereby activating nitric oxide (NO) synthase. This results in the endothelial release of NO causing vasodilatation (17). Each dose was infused for 5 min and FBF was measured during the last 3 min of the 5 min period. After the infusion of all three doses of acetylcholine a 30 min equilibration period followed. Subsequently, baseline measurements were performed with the infusion of 5% glucose solution. Subsequently, three increasing doses of sodium nitroprusside (0.06, 0.20, and 0.60 µg ⋅ dL−1 ⋅ min−1, 25 mg/mL, dissolved to its final concentration with 5% glucose solution; Clinical Pharmacy, Radboud University Medical Centre), endothelium-independent vasodilators (18) were infused in the brachial artery. Again, each dose was infused for 5 min, and FBF was measured during the last 3 min of the 5-min period. FBF registrations of the last 2 min of each dosage of vasodilator were averaged to a single value for data analysis. An overview of the measurements is shown in Fig. 1B.
Calculations and statistical analysis
Sample size calculation.
We considered a 30% change in primary end point between treatment groups, acetylcholine-induced vasodilatation, as clinically relevant. Assuming a test-to-test correlation coefficient of 0.5 and a mean FBF in response to acetylcholine of 19 mL ⋅ dL−1 ⋅ min−1 would require a total of 15 subjects to detect a 30% change in FBF with a power of 80% at a significance level of 0.05 (Zα = 1.96).
Before each set of drug infusion, baseline FBF measurements were performed. Repeated-measures ANOVA was used to assess differences in increments in FBF between groups. We used the factors treatment (vildagliptin vs. acarbose) and time (three increasing dosages of vasodilator), and subjects were included as matched variables. Differences in means of laboratory results and hemodynamic parameters were tested by paired Student t test or Wilcoxon signed rank test for nonnormally distributed data. Statistical analyses were performed using Graphpad 5.0. Results in tables and figures are expressed as mean ± SEM, unless otherwise indicated. Significance was set at a P value of less than 0.05.
RESULTS
Seventeen of the 20 initially screened subjects underwent randomization and were enrolled in the study. One participant withdrew because of nausea and upper abdominal discomfort during the first week of vildagliptin treatment. The mean age of the remaining 16 participants was 59.8 ± 6.8 years. The mean duration of diabetes was 7.9 ± 5.3 years with an HbA1c of 6.97 ± 0.61%. Ten participants (62%) were treated with metformin and six were treated (38%) with metformin in combination with a sulfonylurea. Of these six participants, three used glimepiride, two used tolbutamide, and one used glibenclamide.
Table 1 shows the baseline characteristics of all subjects that completed the study.
Table 1.
Baseline characteristics
| Characteristic | |
|---|---|
| Age (years) | 59.8 ± 6.8 |
| Sex (men:women) | 12:4 |
| Weight (kg) | 88.5 ± 14.6 |
| BMI (kg/m2) | 29.1 ± 4.8 |
| Blood pressure systolic (mmHg) | 141 ± 8 |
| Blood pressure diastolic (mmHg) | 83 ± 7 |
| Nonfasting glucose (mmol/L) | 9.55 ± 3.91 |
| HbA1c (%) | 6.97 ± 0.61 |
| Duration diabetes (years) | 7.9 ± 5.3 |
| Antihypertensives (%) | 44 |
| Statins (%) | 68 |
| Diabetes medication: metformin monotherapy (%)/metformin + sulfonylurea (%) | 62/38 |
Data are mean ± SD.
After completion of the trial but before deblinding one subject was excluded from statistical analyses because of unreliable measurement results, probably the result of a technical problem with one of the strain gauges.
Vascular responses to acetylcholine
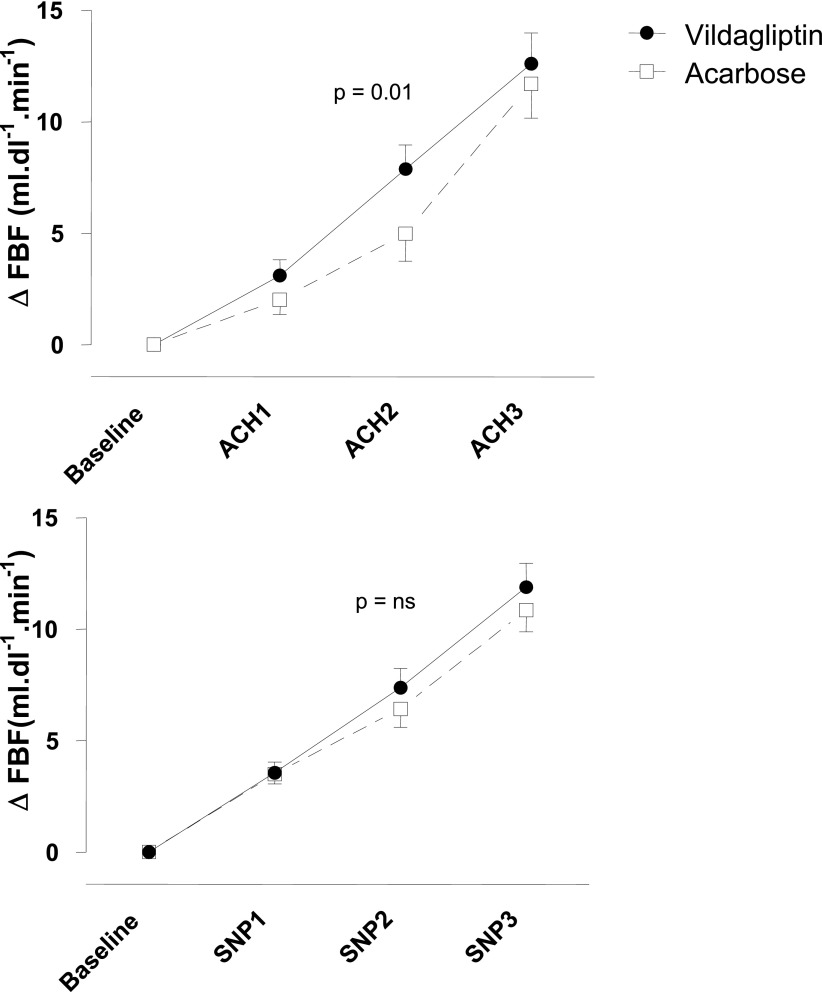
Baseline FBF in the experimental arm in the vildagliptin group was higher than in the acarbose group. Baseline values for FBF in the experimental arm were 3.3 ± 0.3 mL ⋅ dL−1 ⋅ min−1 in the vildagliptin group and 2.5 ± 0.2 mL ⋅ dL−1 ⋅ min−1 in the acarbose group (P = 0.02). Corresponding values in the nonexperimental arm were 2.7 ± 0.3 and 2.2 ± 0.3 mL ⋅ dL−1 ⋅ min−1 (P = 0.07). To compensate for differences in baseline FBF, responses to drugs were expressed as absolute change in FBF (ΔFBF) above baseline. Infusion of acetylcholine induced a dose-dependent increase in FBF in the experimental arm, whereas FBF did not change in the nonexperimental arm. Vildagliptin treatment augmented the absolute increase in FBF in response to acetylcholine from baseline compared with acarbose (P = 0.01 by two-way ANOVA). Changes in FBF from baseline with vildagliptin were 3.1 ± 0.7, 7.9 ± 1.1, and 12.6 ± 1.4 mL ⋅ dL−1 ⋅ min−1 in response to acetylcholine 0.5, 2.0, and 8.0 µg ⋅ dL−1 ⋅ min−1, respectively, compared with 2.0 ± 0.7, 5.0 ± 1.2, and 11.7 ± 1.6 mL ⋅ dL−1 ⋅ min−1 with acarbose. Results of the repeated-measures two-way ANOVA for acetylcholine were as follows: P < 0.0001 for changes over time, P = 0.002 for differences between treatment, and P = 0.01 for their interaction. Figure 2 shows the change in FBF from baseline in response to the different vasodilators.
Figure 2.
Change in FBF in response to acetylcholine (ACH) in the experimental arm in response to acetylcholine (top panel; dosage 0.5, 2.0, and 8.0 µg ⋅ dL−1 ⋅ min−1) and sodium nitroprusside (lower panel; dosage 0.06, 0.20, and 0.60 µg ⋅ dL−1 ⋅ min−1), during vildagliptin (filled circles, solid lines) or acarbose (open squares, dashed lines). P = 0.01 by two-way repeated-measures ANOVA.
Comparable results were obtained when data were expressed as relative changes in FBF from baseline. When data were expressed as mean FBF (without correction for baseline differences) the repeated-measures two-way ANOVA was still borderline significant. We did not find a carry-over effect.
Vascular responses to sodium nitroprusside
Infusion of sodium nitroprusside induced a dose-dependent increase in FBF in the experimental arm. FBF did not change in the nonexperimental arm. Treatment with vildagliptin did not affect vascular responses to sodium nitroprusside. Changes in FBF from baseline when treated with vildagliptin were 3.6 ± 0.5, 7.4 ± 0.9, and 11.9 ± 1.0 mL ⋅ dL−1 ⋅ min−1 in response to sodium nitroprusside 0.06, 0.20, and 0.60 µg ⋅ dL−1 ⋅ min−1, respectively, compared with 3.5 ± 0.4, 6.4 ± 0.8, and 10.7 ± 1.0 mL ⋅ dL−1 ⋅ min−1 with acarbose. Results of the repeated-measures two-way ANOVA were as follows: P < 0.0001 for changes over time, P = 0.06 for differences between treatment, and P = 0.36 for their interaction. Again, we did not find a carry-over effect.
Hemodynamic and metabolic parameters
Both systolic and diastolic blood pressure (measured intra-arterially during the venous occlusion plethysmography) tended to be lower when treated with vildagliptin compared with acarbose (143.1 ± 4.7 vs. 145.2 ± 3.6 mmHg [P = 0.37] and 73.3 ± 2.4 vs. 74.9 ± 2.3 mmHg [P = 0.22]). Mean arterial pressure was 100.7 ± 3.2 during vildagliptin compared with 102.4 ± 2.8 mmHg during acarbose treatment (P = 0.27). Heart rate was 67.9 ± 2.1 bpm when treated with vildagliptin and 66.3 ± 2.5 bpm when treated acarbose (P = 0.41).
Fasting plasma glucose (measured intra-arterially) was lower during vildagliptin than during acarbose treatment (6.62 ± 0.27 vs. 7.59 ± 0.28 mmol/L; P < 0.0001). Fasting insulin levels were 14.5 ± 2.0 mE/L after vildagliptin and 15.6 ± 2.4 mE/L after acarbose treatment (P = not significant [NS]). HbA1c levels tended to be lower during with vildagliptin compared with acarbose treatment (6.73 ± 0.12 vs. 6.84 ± 0.12%, respectively; P = 0.07).
There was no change in body weight and lipid profile (Supplementary Table 1).
Side effects and safety
Most reported side effects during vildagliptin treatment were flatulence (n = 4) and nausea (n = 3). One subject withdrew during the first week of vildagliptin treatment because of upper abdominal pain and nausea. Flatulence (n = 15) and diarrhea (n = 5) were the most frequent side effects during acarbose treatment. Fortunately, no hypoglycemic events occurred.
No changes in blood count, renal function, and liver function were observed during vildagliptin treatment.
CONCLUSIONS
The main finding of the current study is that 4-week treatment with vildagliptin improves endothelial function in patients with type 2 diabetes. This conclusion is based on the observation that vildagliptin augments the vasodilator response in the forearm vascular bed to acetylcholine, an endothelium-dependent vasodilator, whereas the vascular response to sodium nitroprusside, an endothelium-independent vasodilator, remains unaffected. Baseline blood flow tended to be higher during vildagliptin, accompanied by slightly lower blood pressure values, but these changes did not attain statistical significance.
A number of mechanisms may underlie these results. Given that GLP-1 is a physiological substrate of DPP-4, DPP-4 inhibition by vildagliptin may be expected to increase circulating levels of GLP-1 (19). Several studies have reported beneficial effects of GLP-1 on the cardiovascular system. In humans, Nikolaidis et al. (20) have shown that 72-h infusion of GLP-1 improved left ventricular function in patients with acute myocardial infarction and systolic dysfunction after successful reperfusion therapy, an effect that was observed in both diabetic and nondiabetic patients. They discussed that this observation might be explained by the insulinotropic and insulinomimetic properties of GLP-1, but alternatively GLP-1 might also improve endothelial function. Studies have shown that GLP-1 improves endothelium-dependent vascular responses in the brachial artery while leaving endothelium-independent responses unaffected in healthy humans and patients with type 2 diabetes (11,21). The cardiovascular actions of GLP-1 may occur either directly through the GLP-1 receptor or alternatively by a GLP-1 receptor-independent effect of the degradation product of GLP-1, GLP-1(9–36) (12).
Apart from GLP-1, DPP-4 also degrades GIP, and perhaps cytokines and certain chemokines (including stromal-derived factor 1-α), thus other substrates of DPP-4 may be responsible for the improvement in endothelial function. Alternatively, vildagliptin might improve endothelial function by influencing insulin levels and glucose level. Insulin causes vasodilatation by increasing endothelial production of NO (22). However, we did not find changes in fasting insulin levels when treated with vildagliptin. This is in correspondence with literature, in which DPP-4 inhibitors do not influence fasting insulin levels (19).
Although we aimed for similar glucose levels during both treatments, this was not completely accomplished since the fasting glucose levels were slightly higher in the acarbose group (6.62 ± 0.27 for vildagliptin vs. 7.59 ± 0.28 mmol/L for acarbose). It seems unlikely that this small difference of 1.0 mmol/L causes the observed difference in vascular response. This is supported by the absence of any correlation between change in glucose and change in increase in FBF from baseline in response to acetylcholine (r2 = 0.05, data not shown).
Finally, vildagliptin might have a direct pharmacological, yet unidentified, effect on the endothelium.
The improvement in endothelium-dependent vasodilatation in the forearm observed after 4-week treatment with vildagliptin might translate into a decrease in cardiovascular events. Endothelial dysfunction is a marker of cardiovascular complications, and a close relation exists between endothelial function in the human coronary circulation and peripheral circulation (23,24). This possibility is supported by a recent meta-analysis showing that treatment with vildagliptin 50 mg b.i.d. may decrease the relative risk (RR) of cardio-cerebrovascular events (RR 0.84, CI 0.62–1.14) (14). Of course these findings need to be confirmed by a long-term cardiovascular outcome study.
As secondary findings, we found slightly lower diastolic and systolic blood pressure with a slightly higher heart rate after vildagliptin treatment. Furthermore, baseline measurement of FBF was also higher after vildagliptin than after acarbose treatment (3.3 ± 0.3 vs. 2.5 ± 0.2 mL ⋅ dL−1 ⋅ min−1 in the experimental arm and 2.7 ± 0.3 vs. 2.2 ± 0.3 mL ⋅ dL−1 ⋅ min−1 in the nonexperimental arm). Basal vascular tone can be affected by the NO pathway as well as by the sympathetic nervous system (24). The lower blood pressure and increased basal FBF might implicate that vildagliptin is a systemic vasodilator. This observation can be a result of an enhanced availability of NO as a result of improved endothelial function or alteration in the activity of the sympathetic nervous system. However, 26-week treatment with DPP-4 inhibitor alogliptin did not cause clinical meaningful changes in either systolic or diastolic blood pressure (25).
Our study has limitations. First of all, the duration of treatment with vildagliptin was relatively short. This 4-week period was enough to show improved endothelial function but larger outcome trials are needed to evaluate a potential beneficial effect on cardiovascular outcome. Second, although this study was double-blinded, more subjects experienced flatulence during acarbose (15 vs. 4), which might compromise this. However, the investigator analyzed the FBF results of plethysmography of each treatment period separately and the flow measurements are objective. Third, the forearm vasodilator response to acetylcholine is only one of the methods to study endothelial function. Nevertheless, these functional measurements are one of the most representative indexes of endothelial function. Fourth, acarbose in an α-glucosidase inhibitor might increase GLP-1 levels. The reason for using an active comparator drug in our study was to aim for equal glucose levels. Our study design does not allow us to conclude that the effect of vildagliptin on endothelial function is GLP-1 dependent. But if this were true, the observed difference in FBF would have been diminished by acarbose and larger differences would have been found using placebo. However, when using placebo our results might have been confounded by a larger change in fasting glucose. Furthermore, acarbose is the optimal active comparator drug in this trial, since it is not absorbed into the circulation and thus cannot influence endothelial function directly.
The study has also strengths; the effects of vildagliptin were studied in a relevant population—metformin-treated patients with type 2 diabetes—and is thus applicable to clinical practice. In addition, an appropriate control treatment arm was used to avoid major differences in glycemic control. In conclusion, 4 weeks’ treatment with vildagliptin improves endothelium-dependent vasodilatation. These findings may be relevant in the context of future cardiovascular complications and provide a basis for further cardiovascular outcome studies.
Supplementary Material
Acknowledgments
This study was supported by an unrestricted grant from Novartis, which also kindly provided the vildagliptin and placebo medication used in this study. No other potential conflicts of interest relevant to this article were reported.
P.C.M.v.P. wrote the study protocol, researched data, and wrote the manuscript. M.G.N. reviewed and edited the manuscript. P.S. contributed to study design and reviewed and edited the manuscript. C.J.T. contributed to study design, researched data, contributed to discussion, and reviewed and edited the manuscript.
The authors thank research nurses A. Rasing, K. Saini, and M. Verstegen, Radboud University Medical Centre, for assistance during the experiments.
Footnotes
Clinical trial reg. no. NCT01000688, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2421/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev 2001;22:36–52 [DOI] [PubMed] [Google Scholar]
- 4.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 2009;335:165–189 [DOI] [PubMed] [Google Scholar]
- 5.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285–1295 [DOI] [PubMed] [Google Scholar]
- 6.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876–913 [DOI] [PubMed] [Google Scholar]
- 7.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995;136:3585–3596 [DOI] [PubMed] [Google Scholar]
- 8.Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept 1999;85:9–24 [DOI] [PubMed] [Google Scholar]
- 9.Ahrén B. Emerging dipeptidyl peptidase-4 inhibitors for the treatment of diabetes. Expert Opin Emerg Drugs 2008;13:593–607 [DOI] [PubMed] [Google Scholar]
- 10.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 2003;21:1125–1135 [DOI] [PubMed] [Google Scholar]
- 11.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 12.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 2010;33:1607–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large phase III type 2 diabetes population. Diabetes Obes Metab 2010;12:485–494 [DOI] [PubMed] [Google Scholar]
- 15.Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med 2010;122:16–27 [DOI] [PubMed] [Google Scholar]
- 16.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 1995;25:918–923 [DOI] [PubMed] [Google Scholar]
- 17.Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol 1997;83:1785–1796 [DOI] [PubMed] [Google Scholar]
- 18.Ignarro LJ, Ross G, Tillisch J. Pharmacology of endothelium-derived nitric oxide and nitrovasodilators. West J Med 1991;154:51–62 [PMC free article] [PubMed] [Google Scholar]
- 19.Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89:2078–2084 [DOI] [PubMed] [Google Scholar]
- 20.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004;109:962–965 [DOI] [PubMed] [Google Scholar]
- 21.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007;293:E1289–E1295 [DOI] [PubMed] [Google Scholar]
- 22.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 2007;28:463–491 [DOI] [PubMed] [Google Scholar]
- 23.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995;26:1235–1241 [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 2001;52:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q; Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract 2009;63:46–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.