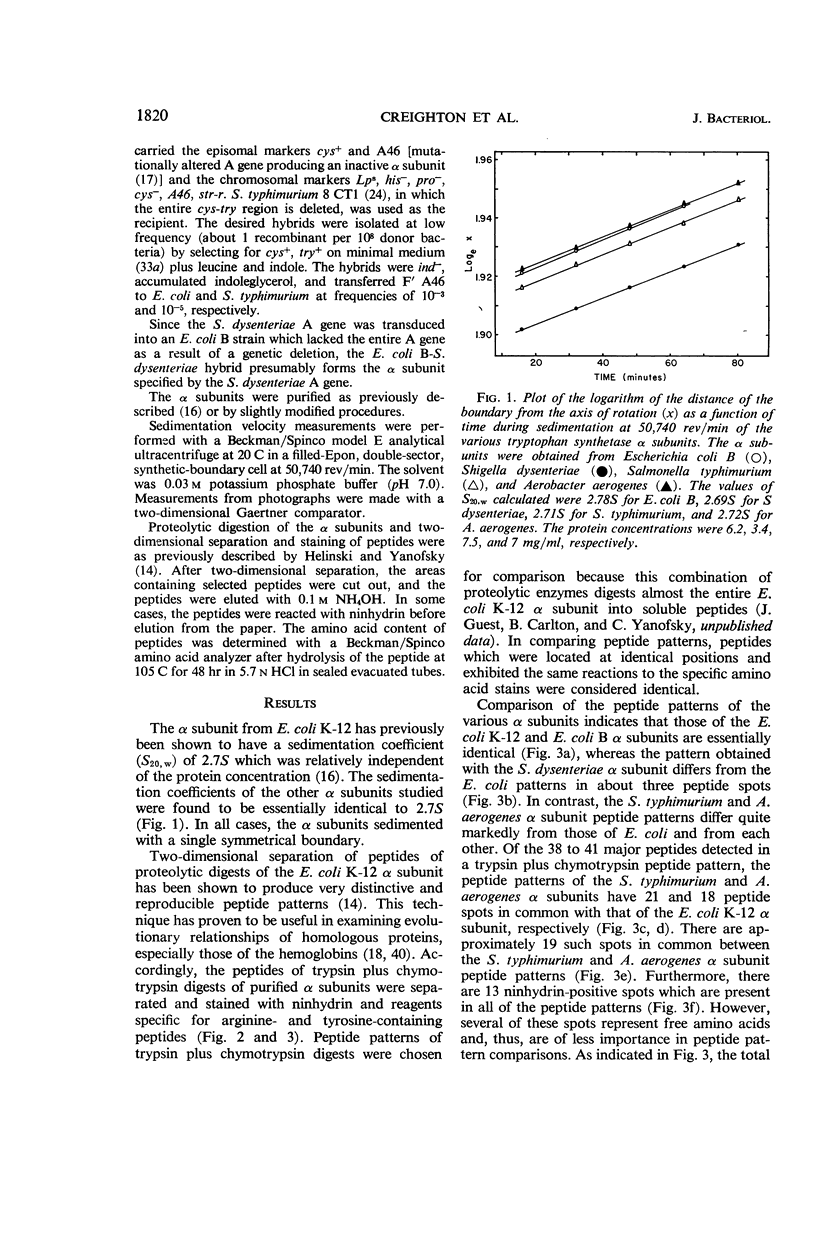
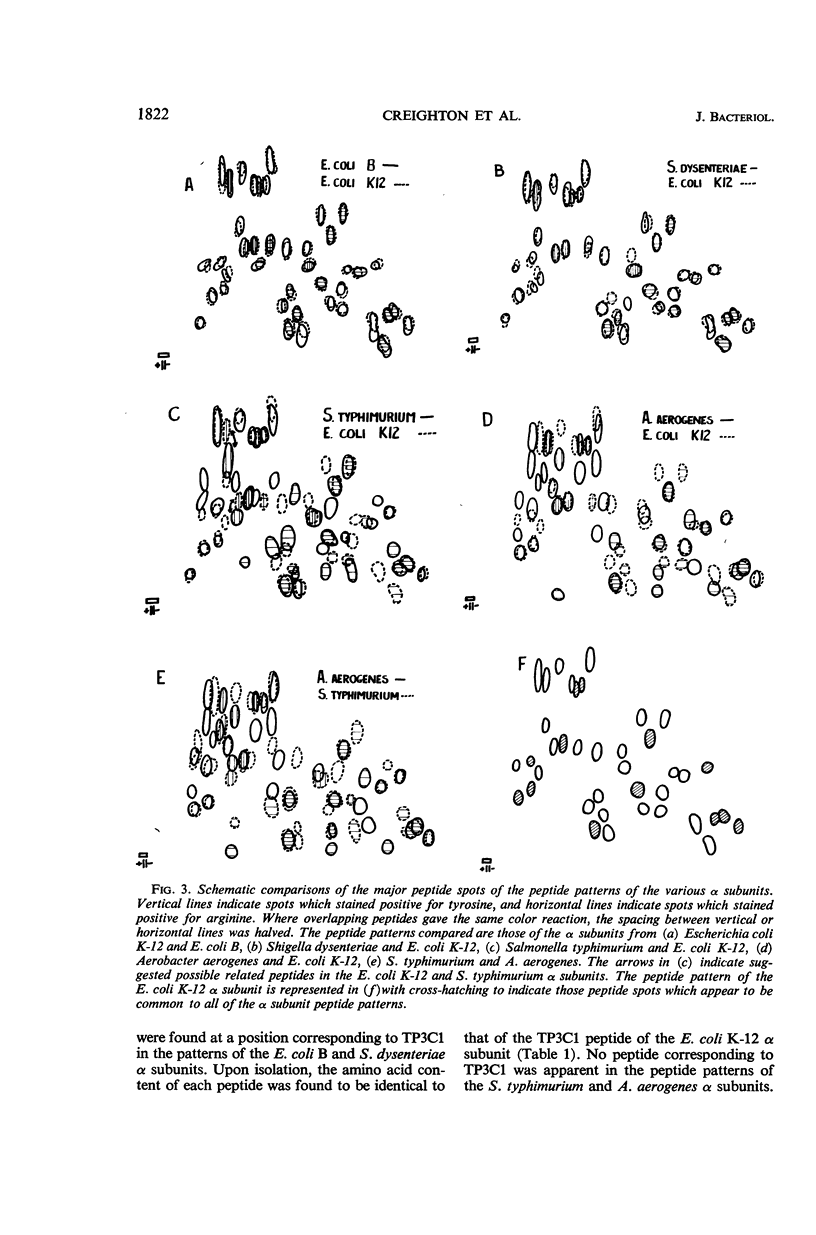
Abstract
Creighton, T. E. (Stanford University, Stanford), D. R. Helinski, R. L. Somerville, and C. Yanofsky. Comparison of the tryptophan synthetase α subunits of several species of Enterobacteriaceae. J. Bacteriol. 91:1819–1826. 1966.—The tryptophan synthetase α subunits of Escherichia coli K-12, E. coli B, Shigella dysenteriae, Salmonella typhimurium, and Aerobacter aerogenes have been purified and their structures compared. Each of these α subunits exhibits a sedimentation coefficient of about 2.7S. Peptide patterns of trypsin plus chymotrypsin digests of the α subunits have indicated that all of the α subunits have peptide regions in common. The patterns of E. coli K-12, E. coli B, and S. dysenteriae α subunits appear to be nearly identical, whereas the α subunits from S. typhimurium and A. aerogenes differ from those of E. coli and from each other. It has also been shown that the E. coli structural gene for the α subunit is translated identically in E. coli and S. typhimurium.
Full text
PDF

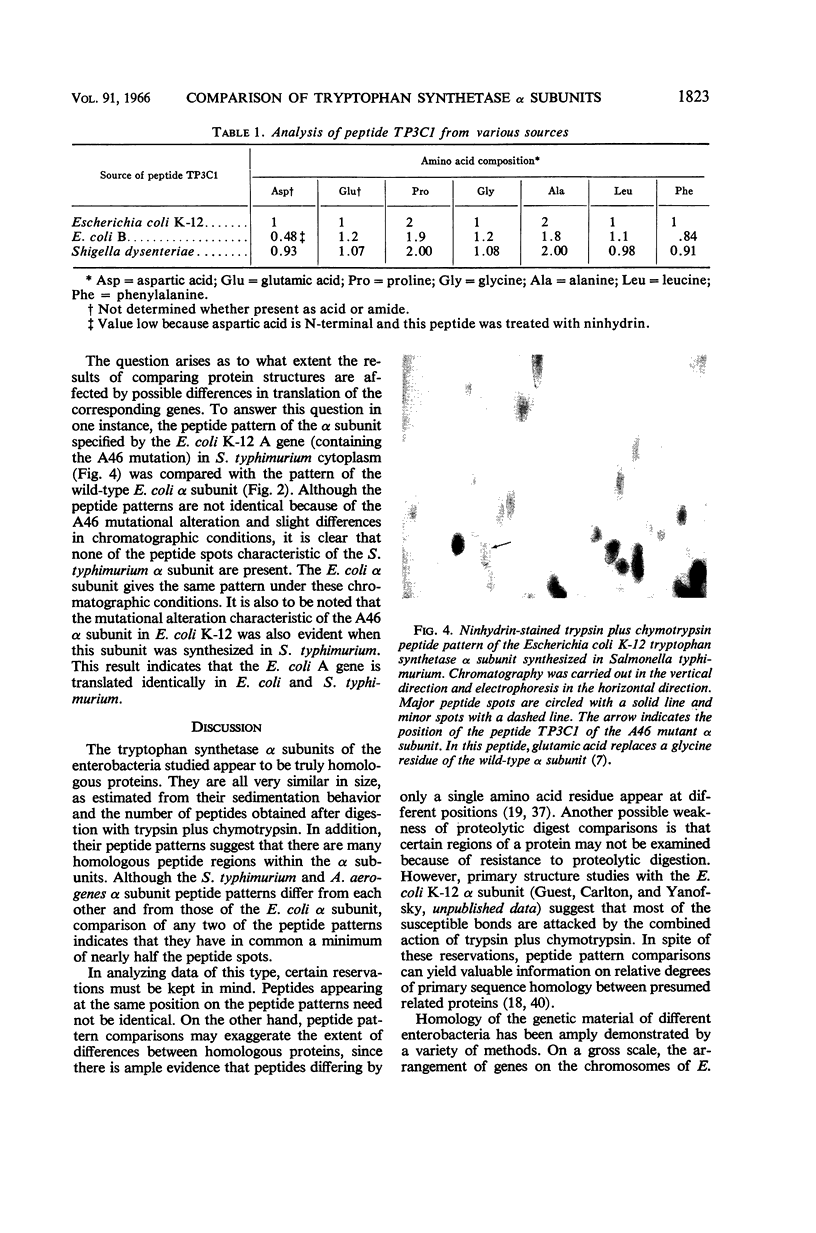



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARLTON B. C., YANOFSKY C. Studies on the position of six amino acid substitutions in the tryptophan synthetase A protein. J Biol Chem. 1963 Jul;238:2390–2392. [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., OHTA N. GENETIC ANALYSES OF SALMONELLA TYPHIMURIUM X ESCHERICHIA COLI HYBRIDS. Proc Natl Acad Sci U S A. 1964 Aug;52:317–323. doi: 10.1073/pnas.52.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTEIN R. B., YANOFSKY C. Tryptophan synthetase levels in Escherichia coli, Shigella dysenteriae, and transduction hybrids. J Bacteriol. 1962 Jan;83:193–204. doi: 10.1128/jb.83.1.193-204.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDMAN J. K., YANOFSKY C. STUDIES ON THE ACTIVE SITE OF THE A PROTEIN SUBUNIT OF THE ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. J Biol Chem. 1965 Feb;240:725–732. [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- HELINSKI D. R., YANOFSKY C. A genetic and biochemical analysis of second site reversion. J Biol Chem. 1963 Mar;238:1043–1048. [PubMed] [Google Scholar]
- HELINSKI D. R., YANOFSKY C. Peptide pattern studies on the wild-type A protein of the tryptophan synthetase of Escherichia coli. Biochim Biophys Acta. 1962 Sep 10;63:10–19. doi: 10.1016/0006-3002(62)90333-5. [DOI] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- HENNING U., YANOFSKY C. An alteration in the primary structure of a protein predicted on the basis of genetic recombination data. Proc Natl Acad Sci U S A. 1962 Feb;48:183–190. doi: 10.1073/pnas.48.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. L., BUETTNER-JANUSCH J., BUETTNER-JANUSCH V. EVOLUTION OF HEMOGLOBIN IN PRIMATES. Proc Natl Acad Sci U S A. 1963 Nov;50:885–893. doi: 10.1073/pnas.50.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. III. The chemical difference between normal and sickle cell haemoglobins. Biochim Biophys Acta. 1959 Dec;36:402–411. doi: 10.1016/0006-3002(59)90183-0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E. PRIMARY STRUCTURE AND EVOLUTION OF CYTOCHROME C. Proc Natl Acad Sci U S A. 1963 Oct;50:672–679. doi: 10.1073/pnas.50.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIN P. BIPOLARITY OF INFORMATION TRANSFER FROM THE SALMONELLA TYPHIMURIUM CHROMOSOME. Science. 1965 Mar 19;147(3664):1456–1458. doi: 10.1126/science.147.3664.1456. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. An approach to the measurement of genetic relatedness among organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:156–164. doi: 10.1073/pnas.50.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M., Leder P., Bernfield M., Brimacombe R., Trupin J., Rottman F., O'Neal C. RNA codewords and protein synthesis, VII. On the general nature of the RNA code. Proc Natl Acad Sci U S A. 1965 May;53(5):1161–1168. doi: 10.1073/pnas.53.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERHAM R. N., HARRIS J. I. AMINO ACID SEQUENCES AROUND THE REACTIVE CYSTEINE RESIDUES IN GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASES. J Mol Biol. 1963 Sep;7:316–320. doi: 10.1016/s0022-2836(63)80011-x. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. The formation of hybrid DNA molecules and their use in studies of DNA homologies. J Mol Biol. 1961 Oct;3:595–617. doi: 10.1016/s0022-2836(61)80024-7. [DOI] [PubMed] [Google Scholar]
- SIGNER E. R. GENE EXPRESSION IN FOREIGN CYTOPLASM. J Mol Biol. 1965 May;12:1–8. doi: 10.1016/s0022-2836(65)80276-5. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C., HELINSKI D. R., MALING B. D. The effects of mutation on the composition and properties of the A protein of Escherichia coli tryptohan synthetase. Cold Spring Harb Symp Quant Biol. 1961;26:11–24. doi: 10.1101/sqb.1961.026.01.006. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., HORN V., THORPE D. PROTEIN STRUCTURE RELATIONSHIPS REVEALED BY MUTATIONAL ANALYSIS. Science. 1964 Dec 18;146(3651):1593–1594. doi: 10.1126/science.146.3651.1593. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Hybrids of Escherichia and Salmonella. Science. 1960 Mar 18;131(3403):813–815. doi: 10.1126/science.131.3403.813. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E., Jones R. T., Pauling L. A COMPARISON OF ANIMAL HEMOGLOBINS BY TRYPTIC PEPTIDE PATTERN ANALYSIS. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1349–1360. doi: 10.1073/pnas.46.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]