Abstract
OBJECTIVE
To evaluate a sensor-augmented insulin pump with a low glucose suspend (LGS) feature that automatically suspends basal insulin delivery for up to 2 h in response to sensor-detected hypoglycemia.
RESEARCH DESIGN AND METHODS
The LGS feature of the Paradigm Veo insulin pump (Medtronic, Inc., Northridge, CA) was tested for 3 weeks in 31 adults with type 1 diabetes.
RESULTS
There were 166 episodes of LGS: 66% of daytime LGS episodes were terminated within 10 min, and 20 episodes lasted the maximum 2 h. LGS use was associated with reduced nocturnal duration ≤2.2 mmol/L in those in the highest quartile of nocturnal hypoglycemia at baseline (median 46.2 vs. 1.8 min/day, P = 0.02 [LGS-OFF vs. LGS-ON]). Median sensor glucose was 3.9 mmol/L after 2-h LGS and 8.2 mmol/L at 2 h after basal restart.
CONCLUSIONS
Use of an insulin pump with LGS was associated with reduced nocturnal hypoglycemia in those at greatest risk and was well accepted by patients.
Continuous glucose monitoring (CGM) can reduce HbA1c in type 1 diabetes (1–3). Despite the use of hypoglycemia alarms, most studies have not demonstrated a significant reduction in hypoglycemia, and prolonged nocturnal hypoglycemia occurs frequently (4). This may be because patients sleep through many of the alarms (5) and insulin delivery continues during hypoglycemia.
We report a user evaluation of the Paradigm Veo insulin pump (Medtronic, Inc., Northridge, CA), which can automatically suspend basal insulin delivery for up to 2 h in the event of CGM-detected hypoglycemia, thus reducing the duration of hypoglycemia.
RESEARCH DESIGN AND METHODS
The Veo was evaluated by 31 patients (10 men) with type 1 diabetes (mean age, 41.9 ± 10.6 years) from six U.K. centers. Regional ethics committees approved the study, and patients provided informed consent. The Veo system has alarms for hypoglycemia, predicted hypoglycemia, rate-of-change of glucose, and, uniquely, a low glucose suspend (LGS) feature that is activated when sensor glucose reaches a glucose threshold set by the user. An alarm sounds, and if the user does not respond, basal insulin delivery is suspended for a maximum of 2 h, after which basal insulin delivery is resumed at the programmed rate. The patient may resume basal insulin delivery at any point.
During a 2-week run-in, CGM was used with only predictive, rate-of-change, high and low alerts active (LGS-OFF). LGS was then activated for 3 weeks (LGS-ON). We evaluated the response to LGS and compared hypoglycemia exposure and mean blood glucose during LGS-OFF and LGS-ON. Patients were divided into four equal groups (quartiles) by the duration of hypoglycemia during the run-in period, because we wished to test the hypothesis that those with the most hypoglycemia at baseline (without LGS) would have the greatest benefit with LGS. Hypoglycemia was defined as the lower limit of detection of the sensor (2.2 mmol/L) (6). The glucose threshold to trigger LGS was individualized (median 2.4 [range 2.2–3.5] mmol/L). Night was defined as 0000–0800 h. Treatment satisfaction questionnaires were completed at study end.
Data were compared using the Student t test, except for skewed data (hypoglycemia duration), which were compared with the Wilcoxon test. Values are mean ± SD or median (range).
RESULTS
Two subjects withdrew during run-in due to difficulties using sensors, and one subject failed to activate the LGS. There were 166 LGS episodes in 25 of 28 (89%) completers (mean 1.9 LGS events/week), of which 76% occurred during daytime, and 55% were terminated within 10 min. LGS continued for the maximum 2 h in 20 episodes (12%), 75% of which were nocturnal. Of 20 completed 2-h suspends, 7 (35%) had no patient response throughout. In the remaining 13 (65%), patients responded to the alarm but elected to continue LGS for 2 h. Mean response time to the LGS alarm was longer at night compared with day (63.2 ± 8.2 vs. 17.4 ± 2.7 min, P < 0.001).
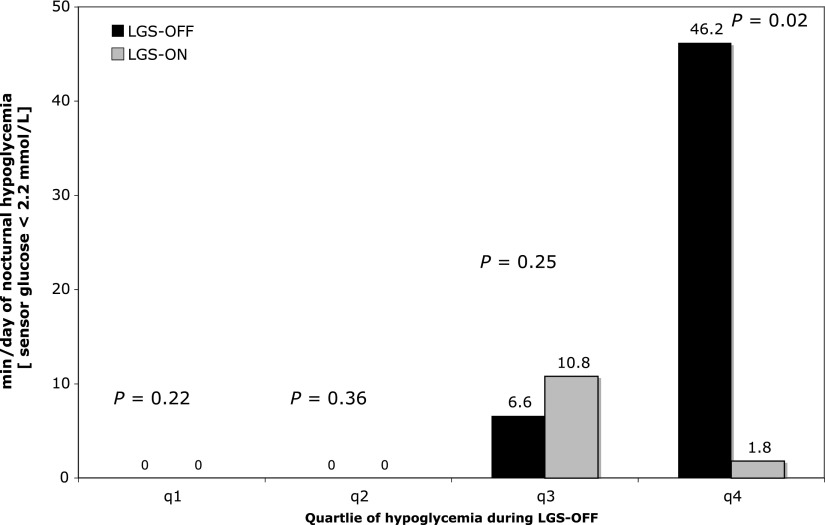
LGS use was associated with significant reduction in the duration of nocturnal hypoglycemia (≤2.2 mmol/L) in those in the highest quartile of hypoglycemia duration at baseline: median 46.2 (36.6–191.4) vs. 1.8 (0.0–45) min/day (P = 0.02; LGS-OFF vs. LGS-ON) (Fig. 1) and mean 75.1 ± 54 vs. 10.2 ± 18 min/day (P = 0.02). Mean sensor glucose was not different with LGS-OFF or LGS-ON (6.4 ± 1.3 vs. 6.6 ± 1.1 mmol/L, P = 0.26). After the 20 complete 2-h LGS episodes, median sensor glucose was 3.9 (2.4–14.2) mmol/L at the restart of basal insulin and was 8.2 (3.3–17.3) mmol/L 2 h after restart. Carbohydrate ingestion was not recorded.
Figure 1.
Duration of nocturnal hypoglycemia (sensor glucose < 2.2 mmol/L) with and without LGS. The bars show median duration of hypoglycemia at night with LGS-OFF (black bars) and LGS-ON (gray bars) by quartile (q) of nocturnal hypoglycemia exposure at baseline.
Concomitant (within 15 min of LGS) capillary glucose values were available for 43 of 166 episodes (25.9%) of LGS. These were >5 mmol/L in 13 episodes and >10 mmol/L in 4, although we do not know if any carbohydrate was ingested before testing. LGS was terminated within 2 min in all four episodes with capillary glucose >10 mmol/L, with sensor error alerts in two of these.
All subjects reported finding LGS “useful,” and 93% reported feeling more secure at night, with reduced anxiety, and wanted to continue using it.
CONCLUSIONS
These data suggest that LGS has the potential to reduce nocturnal hypoglycemia in patients with type 1 diabetes at the highest risk. This is similar to results with insulin pump therapy providing the greatest reduction in hypoglycemia in those with the most hypoglycemia at baseline (7).
The risk of ketosis and hyperglycemia after the 2-h suspension of insulin delivery is low (8,9). In our study, median sensor glucose after 2 h of LGS was 3.9 (2.4–14.2) mmol/L. We could not determine if carbohydrate had been consumed during the LGS. There was no evidence of deterioration of overall glucose control with LGS activated.
Patients took longer to respond to the LGS alarm at night, and 75% of completed 2-h LGS events occurred overnight. This may relate to the combined effects of sleep and hypoglycemia on alertness/arousability, and reduced counter-regulatory responses during sleep (10). Most daytime LGS episodes were terminated by users within 10 min.
The sensor may under-report glucose, particularly during nocturnal hypoglycemia and in view of the lag between interstitial and capillary glucose (11). Although the lowest displayed sensor value is 2.2 mmol/L, the Veo algorithm has improved hypoglycemia detection compared with previous algorithms, with a mean absolute relative difference between 2.2 and 4.4 mmol/L reduced from 24.8 to19.5% (12). We set the LGS threshold low (mean, 2.4 mmol/L), and a higher threshold may have led to greater reduction in hypoglycemia. Our study could not determine rates of false-positive LGS: 4 of 43 LGS had a capillary glucose reading within 15 min >10 mmol/L, and 2 of these were preceded by sensor error alerts. However, these capillary readings may be biased toward episodes when the patient thought the LGS was erroneous.
LGS reduces anxiety about nocturnal hypoglycemia. Randomized controlled trials that evaluate hypoglycemia and quality-of-life in type 1 diabetes using LGS pumps compared with insulin pump therapy alone, with or without CGM, are now needed. This is the first system that modulates insulin delivery in response to glucose levels without human intervention and is an important step toward clinically available closed-loop systems.
Acknowledgments
This study was funded by Medtronic, Inc. (Northridge, CA). P.C., M.L.E., P.J.H., D.K., J.A.M.S., J.C.P., and S.A.A. have received speaker fees and/or travel support and/or serve on advisory boards from or for Medtronic, Inc. J.S. and Y.W. are employees of Medtronic, Inc. No other potential conflicts of interest relevant to this article were reported.
P.C. collected data, completed the analyses, wrote the manuscript, and reviewed the manuscript. J.S. and Y.W. completed the analyses. M.L.E., P.J.H., D.K., and J.A.M.S. collected data and reviewed the manuscript. J.C.P. and S.A.A. collected data and reviewed the manuscript.
The authors acknowledge the help of Dr. Reman McDonagh and Brenda Perry of Medtronic Diabetes, Ltd., in conducting this study and the help of the research and clinical teams at each of the centers.
Footnotes
Clinical trial reg. no. NCT01267175, clinicaltrials.gov.
See accompanying editorial, p. 2136.
References
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 2.Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: The RealTrend study. Diabetes Care 2009;32:2245–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 [DOI] [PubMed] [Google Scholar]
- 4.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010;35:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckingham B, Block J, Burdick J, et al. ; Diabetes Research in Children Network. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther 2005;7:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–1147 [DOI] [PubMed] [Google Scholar]
- 7.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 8.Guerci B, Meyer L, Sallé A, et al. Comparison of metabolic deterioration between insulin analog and regular insulin after a 5-hour interruption of a continuous subcutaneous insulin infusion in type 1 diabetic patients. J Clin Endocrinol Metab 1999;84:2673–2678 [DOI] [PubMed] [Google Scholar]
- 9.Pickup JC, Viberti GC, Bilous RW, et al. Safety of continuous subcutaneous insulin infusion: metabolic deterioration and glycaemic autoregulation after deliberate cessation of infusion. Diabetologia 1982;22:175–179 [DOI] [PubMed] [Google Scholar]
- 10.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998;338:1657–1662 [DOI] [PubMed] [Google Scholar]
- 11.Monsod TP, Flanagan DE, Rife F, et al. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care 2002;25:889–893 [DOI] [PubMed] [Google Scholar]
- 12.Keenan DB, Cartaya R, Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol 2010;4:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]