Abstract
OBJECTIVE
Understanding the relationship between age and islet autoantibody (Ab) seroconversion can establish the optimal screening interval(s) to assess risk for type 1 diabetes, identify subjects who can participate in prevention trials, and determine associated costs. This study assessed the rates of seroconversion to glutamic acid decarboxylase positive (GAD65+), insulin positive (mIAA+), and insulinoma-associated protein 2 positive (ICA512+) in a large cohort of relatives of type 1 diabetes probands undergoing Ab rescreening in the TrialNet Natural History Study.
RESEARCH DESIGN AND METHODS
Of 32,845 children aged <18 years screened for Abs, 1,287 (3.9%) were GAD65+, 778 (2.4%) were mIAA+, 677 (2.1%) were ICA512+, and 31,038 were Ab-negative. Ab-negative children were offered annual rescreening up to 18 years of age. Cox regression was used to estimate the risk for GAD65, mIAA, and ICA512 seroconversion.
RESULTS
There were 205 children who seroconverted to GAD65+, 155 who seroconverted to mIAA+, and 53 who seroconverted to ICA512+ over 5.8 years of follow-up. The risk of mIAA (hazard ratio 0.89 [95% CI 0.85–0.92]) and GAD65 (0.96 [0.93–0.99]) seroconversion significantly decreased with increasing age (i.e., for each 1-year increase in age, the risk of seroconversion decreased by 11% [P < 0.0001] for mIAA and 4% [P = 0.04] for GAD65) across all ages. The cumulative Ab seroconversion was 2% for those <10 years of age versus 0.7% for those ≥10 years of age.
CONCLUSIONS
The risk of development of islet Abs declines with increasing age in type 1 diabetes relatives. These data support annual screening for children <10 years of age and one additional screening in adolescence.
The incidence of type 1 diabetes varies by geography with 1 in 300 affected worldwide. Generally, pancreatic islet autoantibodies (Abs) precede type 1 diabetes and can identify those at increased risk of developing type 1 diabetes (1). The most important Abs to date are glutamic acid decarboxylase (GAD65), insulinoma-associated protein 2 (ICA512), and insulin (mIAA). The frequency of these Abs in a high-risk type 1 diabetes population (i.e., high genetic risk and/or relative of a proband) is 4% for GAD65, 2% for ICA512, and 3% for mIAA (2), whereas in the general population, the frequency is 1% for GAD65 (3), 0.6% for ICA512 (3), and 1.1% for mIAA (4).
The timing of Ab-positive seroconversion is not clearly understood. Some studies have reported that the majority of seroconversions occur before the age of 6 years (5,6); however, these populations were limited to those <10 years of age. In contrast, two recent reports have shown that seroconversion can occur throughout childhood and adolescence (3,7). Knip et al. (3) reported that, in the general population in Finland, β-cell autoimmunity can be induced at any age. In the Diabetes Prevention Trial–Type 1 (DPT-1) study involving relatives with type 1 diabetes, we recently reported that seroconversion declines with age and extends throughout childhood and early adulthood (2). We found that GAD65 and ICA had the highest 2-year seroconversion risks, with GAD65 having the greatest risk in the very young (≤6 years of age). The DPT-1 study results suggested that Ab screening should be started in early childhood and conducted annually through early adolescence to identify those with the greatest risk of type 1 diabetes.
A better understanding of the timing of Ab seroconversion can help identify potential environmental triggers of autoimmunity. It is also of value to developing an optimal screening strategy in at-risk populations and more cost-effective approaches to identify participants for type 1 diabetes prevention trials. We therefore sought to extend the findings in the DPT-1 study by assessing the risk of Ab (GAD65, ICA512, and mIAA) development by age in the TrialNet Natural History Study (NHS), which extended screening for mIAA on its entire population at a uniform annual screen.
RESEARCH DESIGN AND METHODS
TrialNet, an offshoot of the DPT-1 study group, is composed of 18 clinical centers and 170 affiliates across nine countries, a coordinating center, and a central core laboratory. The NHS is a prospective cohort study that is identifying potential participants for prevention trials and assembling a large cohort to provide new natural history information about preclinical type 1 diabetes (8). The protocol was approved by institutional review boards at participating centers, and all participants provided written informed consent before participation in the screening phase.
All subjects in this analysis were screened for GAD65, ICA512, and mIAA between February 2004 and April 2010. Annual rescreening was offered to Ab-negative children <18 years of age. Criteria for inclusion in this analysis are indicated as follows: age 1–17 years; first-, second-, or third-degree relative of type 1 diabetes proband (onset of diabetes before age 40 years and use of insulin within 1 year of diagnosis); Ab-negative on the first screening test; absence of diabetes; and at least one Ab rescreening test. The cost for Ab screening of $134 was based on a weighted average across clinical centers and affiliates and included the cost of the blood draw (phlebotomist wages and fringe benefits), sample preparation, laboratory equipment and reagents, supplies, data reporting, and testing for autoimmune markers. Shipping costs were not included given that the relative cost is determinant on the specific site costs (i.e., geography, bulk shipping, and interval). Costs per seroconversion were calculated by multiplying the weighted average cost of an Ab screen by the total number of those initially screened or rescreened and then dividing by the total number of seroconversions. Race/ethnicity was determined by self-report, and sex and family history of diabetes were obtained at the initial screening.
Laboratory methods
Ab samples were processed following standard procedures as outlined by the 2009 Diabetes Antibody Standardization Program. All Ab assays and the cut points to define Ab positivity used established methods (9) and were performed at the Barbara Davis Center for Childhood Diabetes (Denver, CO). The Ab laboratory had sensitivities and specificities of 90/99% for GAD65 (coefficient of variation [CV]: intra-assay 5%, interassay 10%), 74/100% (CV: intra-assay 5%, interassay 7.7%) for ICA512, and 66/99% (CV: intra-assay 12%, interassay 16%) for mIAA. Ab positivity was defined using threshold indexes of GAD65 ≥0.032, ICA512 ≥0.049, and mIAA ≥0.01.
Statistical analysis
Data were analyzed using SAS Software (version 9.2; SAS Institute, Cary, NC). Categorical variables were analyzed using Pearson χ2 tests. Continuous variables were tested using the t test for differences in means or Wilcoxon rank sum test for differences in medians. Medians and quartiles of 25 and 75% are presented as the median (Q1–Q3). Ab seroconversion was defined as the first positive sample for a specific Ab. Cox proportional hazard models for an open cohort design were used to estimate 2- and 3-year risks for seroconversion and to determine significant predictors of seroconversion. The 2- and 3-year risk estimates were chosen based on median follow-up time in this cohort. Multivariable analyses were adjusted for sex, race/ethnicity, and relation to type 1 diabetes proband. Efron’s method for tied survival times was used in the Cox analysis. Selection of variables for the final model was accomplished by removing nonsignificant effects in a stepwise fashion. Entry and exit levels were at 5%. The assumption of constant hazard function was assessed by examining the log-cumulative survival plots.
RESULTS
There were 32,845 children aged 1–17 years screened for Abs. Of these, 31,038 were Ab-negative and 1,807 were Ab-positive at the initial screen. Demographic characteristics of the study population by positivity for any Ab at screening are presented in Table 1. Distributions of race/ethnicity and relation to index type 1 diabetes proband were significantly different (P < 0.0001). The Ab-positive children were more likely to be non-Hispanic white (75 vs. 72%) and siblings of the type 1 diabetes proband (64 vs. 56%) versus Ab-negative children. The median age at initial screen and sex were similar between the groups (Ab-positive: 9.1 years of age, 49% female subjects; Ab-negative: 9.2 years of age, 50% female subjects).
Table 1.
Characteristics of the TrialNet cohort at time of initial Ab screen
| Characteristic | Ab (−) at initial screening | Ab (+) at initial screening | P value |
|---|---|---|---|
| Total (n) | 31,038 | 1,807 | |
| Age at initial screen (median [Q1–Q3]) | 9.2 (5.4–12.8) | 9.1 (6.0–12.4) | 0.5511 |
| Sex (% female) | 50 | 49 | 0.1985 |
| Race/ethnicity (%) | <0.0001 | ||
| White | 72 | 75 | |
| Hispanic | 16 | 11 | |
| Black | 2 | 3 | |
| Other | 3 | 3 | |
| Unknown | 7 | 8 | |
| Relationship to index patient with type 1 diabetes (%) | <0.0001 | ||
| Sibling | 56 | 64 | |
| Offspring | 23 | 23 | |
| Other relative | 21 | 13 |
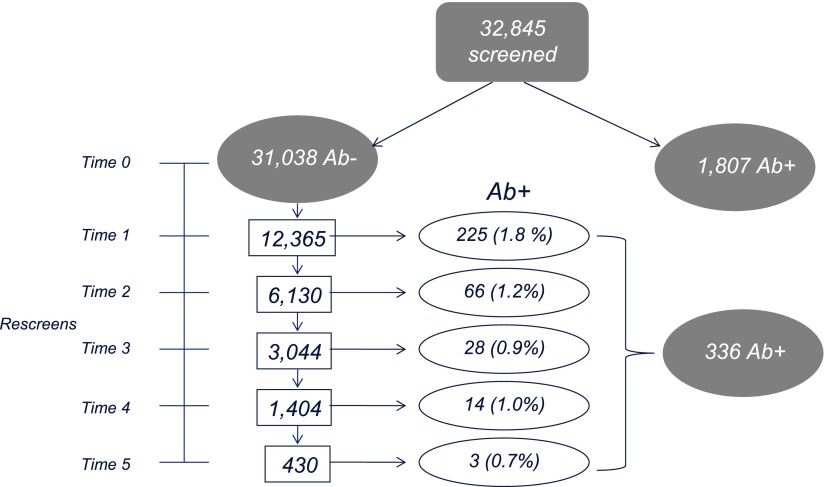
The prevalence of Ab positivity at the initial screen was 5.5% (n = 1,807) for any antibody, 3.9% (n = 1,287) for GAD65, 2.4% (n = 778) for mIAA, and 2.1% (n = 677) for ICA512. Figure 1 shows the onset of Ab positivity at the screen and rescreen tests. Of 31,038 Ab-negative children, 39% (n = 12,365) returned for at least one rescreening over 5.8 years of follow-up (median duration, 24 months [Q1–Q3: 13–36 months]). Overall, 2.7% (n = 336) seroconverted for at least one Ab as follows: 205 (1.7%) developed GAD65, 155 (1.3%) developed mIAA, and 53 (0.4%) developed ICA512. Children who seroconverted were significantly younger at the initial screen compared with children who did not seroconvert (median age [Q1–Q3] 7.4 years [3.8–10.5] vs. 8.6 years [5.2–12.1]; P < 0.0001). They were also more likely to be an offspring or sibling of a type 1 diabetes relative (seroconverters 84% vs. nonconverters 80%, P = 0.05). There were no differences in race (P = 0.78) and sex (P = 0.99) among children who seroconverted compared with those who did not.
Figure 1.
Flow diagram of study screens and Ab seroconversion (screen time points shown on rescreen scale from Time 0 [initial screen] to Time 5 [5 years of follow-up]).
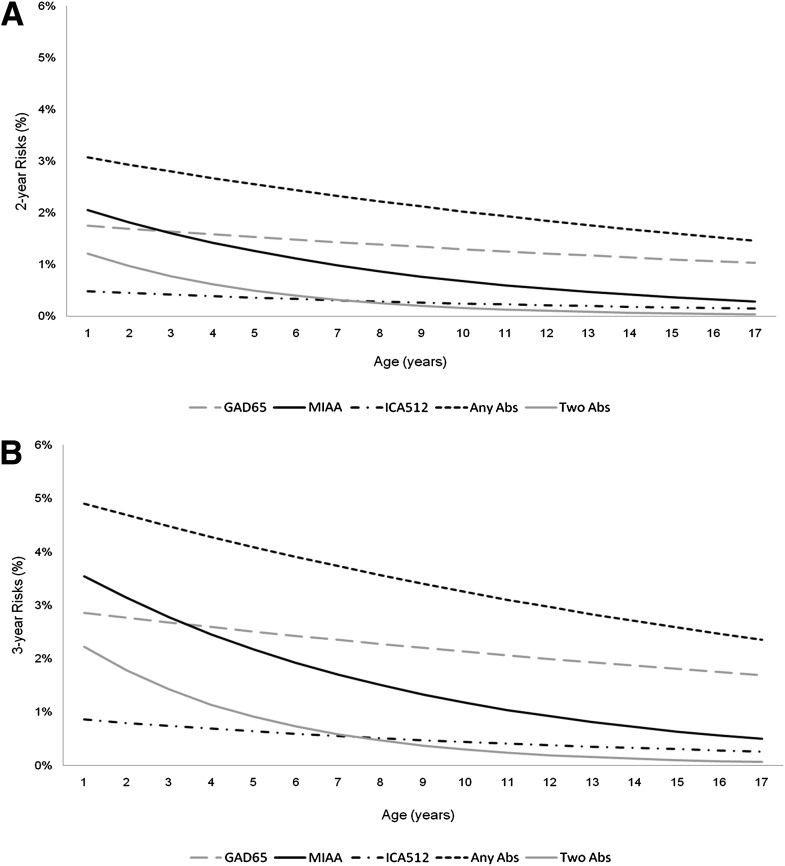
The estimated 2- and 3-year risks for Ab seroconversion by age using Cox regression are shown in Figure 2. Figure 2A shows the age-specific 2-year risks by Ab. Across all ages, there were significant decreases in seroconversion risks to GAD65+ (P = 0.02), mIAA+ (P < 0.0001), any Ab positivity (P < 0.0001), and development of any two Abs (P < 0.0001). The 3-year risks (Fig. 2B) followed a similar pattern. Although the risk was higher in the youngest ages, mIAA seroconversion was most likely in children ≤3 years of age, whereas GAD65 seroconversion was most likely in children and adolescents >3 years of age.
Figure 2.
The 2-year risks (A) and 3-year risks (B) of Ab seroconversion by Ab and development of any Ab(s) by age (years).
The age at initial screening was a significant predictor for risk of Ab seroconversion for mIAA (P < 0.0001) and GAD65 (P = 0.04) across all ages. Univariate analysis showed that the relation to type 1 diabetes proband (P = 0.61), race/ethnicity (P = 0.76), and sex (P = 0.74) were not significant predictors of Ab seroconversion. The age at first screening remained an independent predictor of seroconversion to any Ab in multivariable models that adjusted for race/ethnicity, sex, and relation to proband (P = 0.0007). Overall, the risk of Ab seroconversion significantly decreased with increasing age for GAD65 (hazard ratio [HR] 0.96 [95% CI 0.93–0.99]; P = 0.04) and mIAA (0.89 [0.85–0.92]; P < 0.0001). For each 1-year increase in age, the risk of any Ab seroconversion decreased by 5% (0.95 [0.93–0.98]; P = 0.0007), and risk for seroconversion to any two Abs decreased by 20% (0.80 [0.74–0.86]; P < 0.0001).
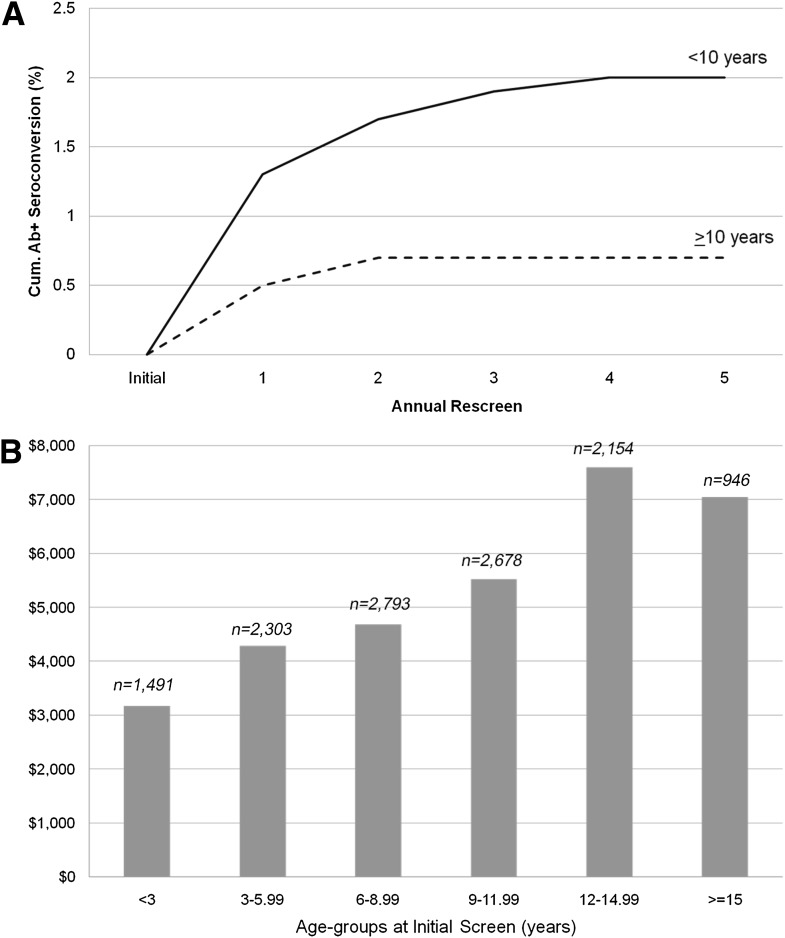
Figure 3A shows the cumulative proportion of seroconversion for any Ab by rescreen number stratified by age (<10 and ≥10 years of age). For both age-groups, most if not all of the detectable Ab seroconversion occurred by the third rescreen test. However, a greater proportion of children <10 years of age seroconverted compared with children ≥10 years of age. Using Cox regression to assess the relationship between age-subgroups (<6 vs. ≥6 and <10 vs. ≥10 years of age) and risk for seroconversion to specific Abs, children <6 years of age had a greater risk of ICA512 and mIAA seroconversion with a 2-fold increase in ICA512 risk (HR 1.98 [95% CI 1.16–3.41]; P = 0.01) and 2.5-fold increased risk of mIAA (2.57 [1.87–3.52]; P < 0.0001). Children <10 years of age had greater risks of GAD65 and mIAA seroconversion, with a 1.5-fold increased risk for GAD65 (1.42 [1.05–1.93]; P = 0.02) and 2.8-fold increased risk for mIAA (2.77 [1.82–4.22]; P < 0.0001). These age-group findings remained statistically significant after adjustment for sex, race/ethnicity, and relationship to type 1 diabetes proband. In addition, children <6 years of age had a 3.5-fold (3.71 [CI 2.20–6.26]; P < 0.0001) increase, and those <10 years of age had a 6-fold (6.19 [2.48–15.47]; P < 0.0001) increase in seroconversion risk to any two Abs. No difference was noted for GAD65 seroconversion (P = 0.30) for those <6 years of age or ICA512 seroconversion (P = 0.12) for those <10 years of age.
Figure 3.
Cumulative Ab seroconversion by annual rescreen number by age (A) and cost of rescreening by age at initial screen (B).
Overall, 1,807 of 31,038 (5.5%) Ab-positive children were found on the first screening test, and rescreening identified a further 2.7% (n = 336) who seroconverted. Statistically significant differences were not seen in sex, age at initial screen, race/ethnicity, or relation to type 1 diabetes proband among those who returned for rescreening (n = 12,365) versus those who did not return (n = 18,718). Applying the same absolute seroconversion rate of 2.7% to the latter group would yield an additional 505 Ab-positive children. Thus, the expected total number of Ab-positive children that could be identified in this population is 2,648 (summation of 1,807, 336, and 505), of whom 68% were identified on the initial screen. At an average cost of screen and rescreen test of $134, the cost to identify one Ab-positive participant on screening was $2,436 and to identify one Ab-positive participant on rescreening was $4,931. Figure 3B shows the average cost per detection of Ab seroconversion at screening or rescreening by age at the time of the initial screen. The cost to detect one case of Ab seroconversion was directly associated with increasing age, ranging from $3,200 among children <3 years of age to >$7,000 in children older than 12 years of age.
CONCLUSIONS
This study in the NHS cohort substantiated findings of its predecessor, the DPT-1 study, that Ab seroconversion declines with age and the greatest risk is in the very young. The DPT-1 study showed that GAD65, ICA, and ICA512 seroconversion significantly declined with age. The NHS study validated this finding for GAD65 across all ages and for ICA512 in <6 years of age. In addition, the NHS assessed mIAA rescreening (which was not done in the DPT-1) and found that the risk of seroconversion also significantly declines with age with those <6 years of age having the greatest risk. This study confirms that diabetes-associated autoimmunity is age-dependent, being greatest in childhood but still extending into early adulthood.
The majority (68%) of the Ab-positive children were identified at the initial screen at a cost of $2,436 per seroconversion, whereas rescreening identified 13% of children who subsequently became Ab-positive at a cost of $4,931 per seroconversion. A single screen to identify the majority of those Ab-positive is lower in cost at a loss of timing of Ab seroconversion. Accordingly, the screening strategy is dependent on the motive. A previous study assessing the cost of predicting type 1 diabetes in Finland (10) concluded that a genetic and immunologic approach would be most cost-effective and improve compliance in the general population where knowledge of the type 1 diabetes burden is limited. For example, a public health approach may focus on identifying those at the highest risk to reduce associated complications (i.e., diabetic ketoacidosis), whereas a research study may need to identify high-risk children prior to seroconversion to assess potential triggers of autoimmunity. Hence, annual screening starting in very early childhood would identify a higher proportion of those at risk for Ab seroconversion at a lower cost, whereas research focused on treatment and prevention of type 1 diabetes onset may need to screen only once to identify those with Abs regardless of time of seroconversion. The best approach would be to screen annually throughout childhood and adolescence in the genetically at-risk population. However, economic realities limit the ability to continually rescreen. This study showed that the rescreen cost to identify one case increases with age, and the majority of those likely to seroconvert are identified at ages <10 years. This group is also reported to have the highest annual incidence: 5.4% (4.8–6.1) for children age 0–4 years, 4.3% (3.8–4.8) for children age 5–9 years, and 2.9% (2.5–3.3) for children age 10–14 years (11). It is crucial to identify every subject eligible for prevention studies so that TrialNet can work toward its primary goal—prevention of type 1 diabetes.
Many studies have evaluated the appearance of diabetes-associated autoimmunity in high-risk populations; however, few have been able to fully evaluate rescreening because of small sample size and study design. The strengths of the NHS study include its size, uniform annual screening up to age 18 years, and population not chosen by genetic risk as defined by HLA typing. In addition, the NHS screens for all biochemical Abs. This differs from the DPT-1 study in which ICA was used as the screening and rescreening Ab and GAD65 and ICA512 testing were done only if ICA was positive. The NHS measures GAD65, mIAA, and ICA512 at the same time, whereas the DPT-1 study measured ICA first and, if positive, went back years later to test for the other antibodies.
Our study has limitations. First, we could not assess the contribution of HLA because of lack of informed consent for such collection in the screening phase, which did not allow for the assessment of seroconversion rates by HLA, especially for the high-risk haplotypes. Thus, our rates represent the average rate in an at-risk population. Second, lack of follow-up on nonresponders could affect our estimates of seroconversion by underestimating the true rate (i.e., ∼40% of those initially tested returning for a rescreen). Given the difficulty in getting subjects to return for screening, we defined seroconversion as presence of first positive Ab, while recognizing that it is a surrogate indicator of Ab positivity that is more clearly linked to future type 1 diabetes. This can be limiting because of type 1 error, and we agree that a more conservative measure, such as confirmed autoimmunity or multiple confirmed Abs, would be a better predicator of type 1 diabetes risk. However, in light of limited information and the current data on screening strategies (including repeat screening), it is practical to evaluate whether a positive test, in addition to basic risk information (age, relation to proband, race/ethnicity, and sex), can be used in developing a screening strategy and assessing the relationship between age and Ab seroconversion. Lastly, we did not assess the zinc transporter 8 Ab (ZnT8A) because it was recently added to the NHS protocol. Our results therefore underestimate seroconversion as defined by the onset of the first positive Ab test, where the underestimate depends on ZnT8A positivity as the first manifestation of autoimmunity. In that respect, isolated ZnT8A positivity occurred in 0.2% (3 of 1,505) of children with a family history of type 1 diabetes (11), whereas recent unpublished NHS data found that 8 of 811 (1%) Ab-negative relatives were ZnT8A-positive (L. Yu, J.L.M., D. Boulware, et al., unpublished data).
This study validates the DPT-1 study findings that the rate of Ab seroconversion declines with age and occurs throughout childhood into early adulthood. These findings showed that the risk of ICA512 and mIAA seroconversion significantly declines with increasing age in early childhood, and children <10 years of age are at a significantly higher risk of seroconversion for GAD65 and mIAA. On the basis of economic and risk realities, our finding suggests that annual Ab screening should be conducted very early in life, continued up to the age of 10 years, and performed once in early adolescence to identify the majority of those at risk for preclinical type 1 diabetes. A better understanding of the etiology of preclinical type 1 diabetes by determining the time of Ab seroconversion may assist in identifying potential environmental triggers and their contribution at different times in the life course to the risk of type 1 diabetes.
Acknowledgments
This work was supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Center for Complementary and Alternative Medicine, and the General Clinical Research Centers Program; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
K.V. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. C.A.B. researched data. J.L.M. and M.J.H. contributed to discussion and reviewed and edited the manuscript. D.A.S. reviewed and edited the manuscript. J.M.S., J.S.S., and J.P.K. reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
References
- 1.Krischer JP, Cuthbertson DD, Yu L, et al. Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003;88:103–108 [DOI] [PubMed] [Google Scholar]
- 2.Vehik K, Haller MJ, Beam CA, et al. ; DPT-1 Study Group. Islet autoantibody seroconversion in the DPT-1 study: justification for repeat screening throughout childhood. Diabetes Care 2011;34:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care 2010;33:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schatz D, Krischer J, Horne G, et al. Islet cell antibodies predict insulin-dependent diabetes in United States school age children as powerfully as in unaffected relatives. J Clin Invest 1994;93:2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler AG, Hillebrand B, Rabl W, et al. On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia 1993;36:402–408 [DOI] [PubMed] [Google Scholar]
- 6.Leslie RD, Elliott RB. Early environmental events as a cause of IDDM. Evidence and implications. Diabetes 1994;43:843–850 [DOI] [PubMed] [Google Scholar]
- 7.Colman PG, McNair PD, Gellert S, et al. Development of autoantibodies to islet antigens during childhood: implications for preclinical type 1 diabetes screening. Pediatr Diabetes 2002;3:144–148 [DOI] [PubMed] [Google Scholar]
- 8.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. ; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Miao D, Babu S, et al. Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007;92:88–92 [DOI] [PubMed] [Google Scholar]
- 10.Hahl J, Simell T, Ilonen J, Knip M, Simell O. Costs of predicting IDDM. Diabetologia 1998;41:79–85 [DOI] [PubMed] [Google Scholar]
- 11.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]