Abstract
OBJECTIVE
Interferon therapy can trigger induction of several autoimmune diseases, including type 1 diabetes. To assess the clinical, immunologic, and genetic characteristics of type 1 diabetes induced by interferon therapy, we conducted a nationwide cross-sectional survey.
RESEARCH DESIGN AND METHODS
Clinical characteristics, anti-islet autoantibodies, and HLA-DR typing were examined in 91 patients for whom type 1 diabetes developed during or shortly after interferon therapy.
RESULTS
Median age at the onset of type 1 diabetes was 56 (interquartile range 48–63) years and mean ± SD BMI was 20.8 ± 2.7 kg/m2. The time period from the initiation of interferon therapy to type 1 diabetes onset in patients receiving pegylated interferon and ribavirin was significantly shorter than that in patients with nonpegylated interferon single therapy (P < 0.05). Anti-islet autoantibodies were detected in 94.5% of patients at diabetes onset. Type 1 diabetes susceptibility HLA-DRs in the Japanese population, DR4 and DR9, were also associated with interferon treatment–related type 1 diabetes. Furthermore, the prevalence of HLA-DR13 was significantly higher in interferon treatment–related type 1 diabetes than in healthy control subjects (odds ratio 3.80 [95% CI 2.20–7.55]; P < 0.0001) and classical type 1 diabetes (2.15 [1.17–3.93]; P < 0.05).
CONCLUSIONS
Anti-islet autoantibodies should be investigated before and during interferon therapy to identify subjects at high risk of type 1 diabetes. Stronger antiviral treatment may induce earlier development of type 1 diabetes. Furthermore, patients who develop interferon-induced type 1 diabetes are genetically susceptible.
Interferons are ubiquitous cytokines produced by all mononuclear cell types as part of an immune response to a viral infection or other immune trigger; they induce antiviral proteins and activate natural killer cells (1). Interferon-based treatment is currently used in a number of conditions, including chronic viral hepatitis, hematological malignancies such as chronic myelogenous leukemia, renal cell carcinoma, and melanoma. On the other hand, the development of autoimmune diseases, such as autoimmune thyroid disease (AITD), autoimmune hemolytic anemia, and Behçet disease, has been reported as a side effect during or after treatment with interferon (2); type 1 diabetes has also been reported sporadically (3–8). Conversely, there has been a report that islet autoantibodies did not appear in patients with interferon treatment (9). Also, there is a single case report in which diabetes was resolved when interferon treatment stopped (10). Because the number of reports on interferon treatment–related type 1 diabetes have increased in recent years, it is important for preventative purposes to investigate the clinical, immunologic, and genetic characteristics of such cases to identify the predictive markers. In the current study, we conducted a nationwide survey of patients with type 1 diabetes that developed during or after interferon therapy under the auspices of the Japan Diabetes Society.
RESEARCH DESIGN AND METHODS
We asked all the members of the Japan Diabetes Society through direct mail and the readership of the Journal of the Japan Diabetes Society whether they knew of candidates who had developed type 1 diabetes during or after interferon treatment. We received 48 positive responses. To those who responded positively, we sent questionnaires asking for a description of their characteristics, including the disease for which interferon-based treatment was administered, the type of interferon used, the use of ribavirin combination therapy, the co-occurrence of AITD, and the number of patients with type 1 diabetes and interferon-treated patients in their institutions. We received data on 62 cases. In addition, we searched the PubMed/Medline and Ichushi-Web (Japan Medical Abstract Society) databases for the period 1983 to 2010 using the keywords interferon, type 1 diabetes, and Japanese and identified 29 additional cases that did not overlap with the previous 62 cases. Therefore, a total of 91 patients (male, 48; female, 43) of Japanese origin were investigated in this study. Patients were reported from regions throughout Japan, from Hokkaido Island to Kyushu Island, and although the majority of cases were clustered in highly populated areas they were not restricted to any specific region or prefecture in Japan.
Type 1 diabetic patients were divided into three groups according to the mode of diabetes onset (acute onset, slow onset, and fulminant). Among the following five criteria, patients who met either criteria 1–4 or criteria 1–3 plus criterion 5 were placed in the acute-onset type 1 diabetes group: 1) the presence of ketosis or ketoacidosis at the onset of diabetes; 2) the presence of hyperglycemic symptoms for <3 months before the commencement of insulin therapy; 3) the requirement of insulin replacement therapy at both onset and 6 months after onset; 4) the presence of at least one anti-islet autoantibody (GAD autoantibody [GADAb], islet cell antibody [ICA], insulin autoantibody [IAA], or insulinoma-associated antigen-2 autoantibody [IA-2Ab]); and 5) decreased insulin-secreting capacity (urinary C-peptide excretion <20 µg/day, fasting serum C-peptide level <0.4 ng/mL, or peak serum C-peptide level <1.0 ng/mL after glucagon injection or meal load). Diagnosis of slow-onset type 1 diabetes was based on the following criteria: 1) originally diagnosed as type 2 diabetes and no sign of ketosis at diabetes onset; 2) proven anti-islet autoantibody positivity; and 3) insulin treatment started ≥12 months after the diagnosis. The patients who met the following criteria were placed in the fulminant type 1 diabetes group: 1) ketosis or ketoacidosis within a week after the onset of hyperglycemic symptoms; 2) plasma glucose levels ≥16 mmol/L and HbA1c <8.9% at the first visit, and 3) urinary C-peptide level <10 µg/day, fasting serum C-peptide level <0.3 ng/mL, or serum C-peptide <0.5 ng/mL after glucagon or a meal load (11).
AITD was defined as Graves disease, Hashimoto thyroiditis, or the presence of autoantibodies to thyroid peroxidase, thyroglobulin, or thyrotropin receptor.
Autoantibodies
GADAb, ICA, IA-2Ab, and IAA were determined at the onset of type 1 diabetes. GADAb, IA-2Ab, and IAA were measured by radioimmunoassay or radioligand-binding assay, and ICAs were measured by immunohistochemical methods (12). The disease sensitivity/specificity for GADAb, IA-2Ab, and IAA was 82.6/93.6, 66.0/98.9, and 32.0/98.9%, respectively, in the Diabetes Autoantibody Standardization Program 2009.
HLA typing
The serological subtype of HLA-DR or DRB1 genotype was determined (13,14). Allele frequencies were obtained by direct counting. As a control for the analysis of HLA-DR, we also studied 304 unrelated healthy individuals (115 females; median age 45.0 years [range 20.0–74.0]) and 192 patients with classical type 1 diabetes (122 females; age at onset 27.0 years [1.0–75.0]).
Statistical analysis
The results are given as median (interquartile range) or mean ± SD unless otherwise indicated. Statistical analysis was performed with a χ2 test and the Mann-Whitney U test. The multiple comparison test was used to compare the groups with regard to the duration from initiation of interferon therapy to type 1 diabetes onset. The cumulative free incidence rate of type 1 diabetes was compared using the Kaplan-Meier method with log-rank test. The significance of differences in the distribution of HLA-DR alleles between case and control subjects was determined by a χ2 test. Odds ratios (ORs) (95% CI) were also calculated. StatView (version 5.0; SAS Institute, Cary, NC) were used for these tests. A P value < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics at type 1 diabetes onset
Table 1 summarizes the clinical characteristics of 91 patients with type 1 diabetes developed during or after interferon therapy at diabetes onset. The female-to-male ratio was 0.90, which is lower than that for classical type 1 diabetes in the Japanese population (1.4–1.5) (11). Median age at onset of type 1 diabetes was 56 years (interquartile range 48–63). Fourteen (15.4%) case subjects had been diagnosed as having type 2 diabetes many years before the diagnosis of type 1 diabetes. Thirst and body weight loss were observed in 75.8 and 61.5% of patients, respectively, and the mean weight loss rate was 15.8 ± 25.4%. Interferon had been administered for chronic hepatitis C virus (HCV) in 85, for chronic hepatitis B in 1, for chronic myelogenic leukemia in 2, and for renal cell carcinoma in 3 patients. HCV genotypes were analyzed in 32 cases. Genotypes 1a, 1b, 2a, and 2b were detected in 0, 26 (81.3%), 3 (9.4%), and 3 patients (9.4%), respectively.
Table 1.
Clinical characteristics of 91 patients with interferon treatment–related type 1 diabetes
| Sex (male) | 48 (53.4) |
| Age at onset of type 1 diabetes (years) | 56 (48–63) |
| Family history of type 1 diabetes | 0 (0) |
| Family history of type 2 diabetes | 23 (25.3) |
| Past history of type 2 diabetes | 14 (15.4) |
| Thirst | 69 (75.8) |
| Body weight loss | 56 (61.5) |
| Indication for IFN therapy | |
| Chronic HCV | 85 (93.4) |
| Chronic hepatitis B | 1 (1.1) |
| Chronic myelogenic leukemia | 2 (2.2) |
| Renal cell carcinoma | 3 (3.3) |
| HCV genotype (n = 32) | |
| 1a | 0 (0) |
| 1b | 26 (81.3) |
| 2a | 3 (9.4) |
| 2b | 3 (9.4) |
| Type of IFN | |
| IFNα | 10 (9.9) |
| IFNα-2a | 11 (12.1) |
| PegIFNα-2a | 8 (8.8) |
| IFNα-2b | 21 (23.1) |
| PegIFNα-2b | 38 (41.8) |
| IFNβ | 3 (3.3) |
| Combination of ribavirin (from 2002) | 52 (72.2) |
| Mode of type 1 diabetes | |
| Fulminant onset | 5 (5.5) |
| Acute onset | 74 (81.3) |
| Slow onset | 7 (7.7) |
| Others | 5 (5.5) |
| Period from recent IFN treatment to type 1 diabetes onset (years) | 0.68 (0.38–1.75) |
| Co-occurrence of autoimmune thyroid disease | 25 (27.5) |
Data are n (%) or median (interquartile range). Family history of type 1 or type 2 diabetes was given for first-degree relatives. Body weight loss was defined as losing at least 5% of usual body weight within a few months. IFN, interferon; IFNα, natural IFNα.
There are at least three subtypes of type 1 diabetes based on their mode of onset, i.e., acute onset, slow onset, and fulminant onset, in Japanese patients. Seventy-four of 91 patients (81.3%) were classified as having the acute onset form, 7 (7.7%) the slow onset form, and 5 (5.5%) the fulminant onset form, with the remaining 5 unclassified. The median period from recent interferon treatment to type 1 diabetes onset was 0.68 years (interquartile range 0.38–1.75; range 0.02–9.62). The co-occurrence of AITD was observed in 25 cases (27.5%).
The total number of patients with type 1 diabetes and of interferon-treated patients in the institutions from which questionnaires had been returned were 5,264 and 18,110, respectively. Therefore, the proportion of cases with interferon treatment–related diabetes among patients with type 1 diabetes was estimated to be 1.18%. Furthermore, the prevalence of interferon treatment–related type 1 diabetes among interferon-treated patients was estimated to be 0.34%, which is 10 times higher than that in the general population (∼0.03%) (11). These results suggest that interferon treatment is associated with the development of type 1 diabetes.
Period from initiation or termination of interferon treatment to type 1 diabetes onset
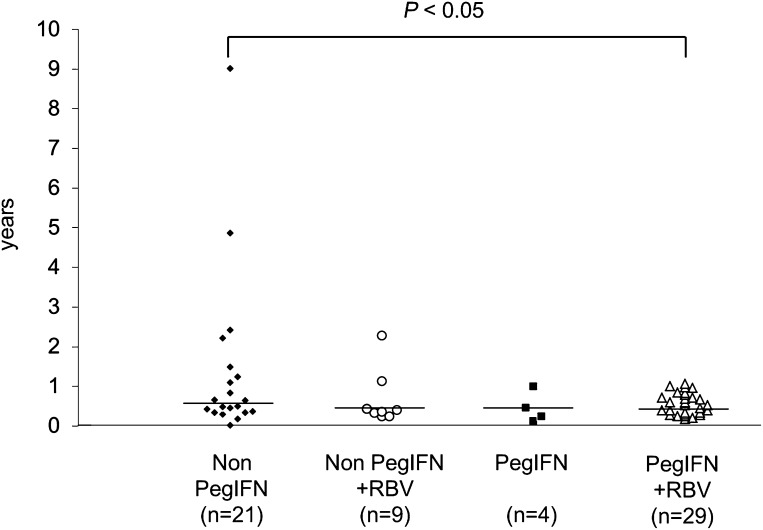
Figure 1 summarizes the mean period from initiation of interferon treatment to the development of type 1 diabetes. Sixty-four patients developed type 1 diabetes during interferon treatment and were divided into four groups based on the type of interferon (nonpegylated interferon [non-PegIFN] or pegylated interferon [PegIFN]) and the use of ribavirin. Compared with the non-PegIFN single-therapy group (median 0.64 years [interquartile range 0.32–1.23; range 0.02–9.01]), the median period was significantly shortened in the PegIFN plus ribavirin group (0.45 [0.29–0.71; 0.17–1.06]; P < 0.05). Furthermore, 92.9% (39 of 42) of patients treated with PegIFN or ribavirin developed type 1 diabetes within 1 year, which was significantly higher than the incidence in the non-PegIFN single-therapy group (66.7% [14 of 21]; P < 0.05). Kaplan-Meier analysis also revealed a faster development of type 1 diabetes in the PegIFN or ribavirin group compared with that in the non-PegIFN single-therapy group (P < 0.05 by log-rank test) (Supplementary Fig. 1).
Figure 1.
Period from initiation of interferon treatment to type 1 diabetes onset. RBV, ribavirin. Data were unavailable for 1 of the 64 patients who developed type 1 diabetes during interferon therapy. The horizontal bar indicates the median year of interferon treatment in each group.
Twenty-six patients (28.6%) developed type 1 diabetes after interferon treatment was terminated, and the median period from the termination of interferon treatment to the onset of type 1 diabetes was 0.75 (range 0.19–1.62) years. The data on the relation between type 1 diabetes development and the initiation or termination time of the interferon treatment were unavailable in the remaining one patient.
Laboratory findings at type 1 diabetes onset
Table 2 summarizes the characteristics of patients before interferon therapy and at type 1 diabetes onset. Although the positivity of HCV RNA was 98.5% at the initiation of interferon treatment, it was 40.6% at type 1 diabetes onset. Therefore, ~60% of patients turned HCV RNA negative before the diagnosis of type 1 diabetes. It is of interest that the incidence of those who were HCV RNA negative was significantly lower in female patients (39.3%) compared with that in male patients (75.0%; P < 0.005). Furthermore, among female patients, the period of interferon treatment before the development of type 1 diabetes was significantly longer in patients with HCV RNA (114.8 ± 123.0 weeks) than in HCV RNA–negative patients (36.4 ± 17.5 weeks; P < 0.05). No associations were observed between the period of interferon treatment before the development of type 1 diabetes and HCV RNA positivity among male patients (data not shown).
Table 2.
Characteristics of patients before interferon therapy and at type 1 diabetes onset
| Before interferon therapy | At type 1 diabetes onset | P | |
|---|---|---|---|
| n | 91 | 91 | |
| BMI (kg/m2) | 24.1 ± 3.5 | 20.8 ± 2.7 | <0.0001 |
| Fasting plasma glucose (mg/dL) | 104.3 ± 19.7 | 360.7 ± 130.1 | <0.0001 |
| Serum HCV RNA (positive/negative) | 65/1 | 26/38 | <0.0001 |
| Aspartate transaminase (IU/L) | 59.1 ± 32.2 | 36.2 ± 24.4 | <0.0001 |
| Alanine transaminase (IU/L) | 71.7 ± 43.7 | 40.6 ± 35.6 | <0.0001 |
| Hemoglobin (g/dL) | 14.4 ± 1.3 | 12.9 ± 1.8 | <0.0001 |
| Platelets (×104/μL) | 17.2 ± 7.3 | 12.8 ± 4.7 | <0.0001 |
| HbA1c (%)* | 6.5 ± 1.5 | 10.5 ± 2.5 | <0.0001 |
| Glycoalbumin (%) | N.D. | 38.4 ± 13.1 | |
| Urine ketone body (positive/negative) | 0/21 | 43/22 | <0.0001 |
| Plasma ketone body (μmol/L) | N.D. | 3,166.9 ± 2,751.5 | |
| Urinary C-peptide (μg/day) | N.D. | 28.8 ± 28.8 | |
| Fasting serum C-peptide (ng/mL) | N.D. | 1.0 ± 1.7 | |
| Stimulated serum C-peptide (ng/mL) | N.D. | 1.4 ± 1.8 | |
| Anti-islet autoantibodies (positive/negative) | N.D. | 86/5 | |
| GADAb (positive/negative) | N.D. | 79/6 | |
| GADAb (units/mL) | N.D. | 14,103.9 ± 32,213.5 | |
| IA-2Ab (positive/negative) | N.D. | 7/24 | |
| ICA (positive/negative) | N.D. | 9/7 | |
| IAA (positive/negative) | N.D. | 2/4 | |
| Dose of daily insulin (units/kg/day) | N.D. | 0.54 ± 0.30 |
Data are means ± SD or n positive/n negative. Data for anti-islet autoantibodies are positive if patients have at least one of GADAb, ICA, IA-2Ab, or IAA. HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = HbA1c (Japan Diabetes Society [JDS]) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and HbA1c (NGSP). N.D., not determined.
*Fourteen patients with type 2 diabetes who had been diagnosed before the initiation of interferon therapy are included.
Mean plasma glucose, HbA1c levels, and the positivity of urinary ketone body were 360.7 ± 130.1 mg/dL, 10.5 ± 2.5%, and 66.2%, respectively. The mean values of daily urinary C-peptide excretion, fasting serum C-peptide concentrations, and daily insulin dose were 28.8 ± 28.8 µg/day, 1.0 ± 1.7 ng/mL, and 0.54 ± 0.30 units/kg/day, respectively. The frequencies of GADAb and IA-2Ab were 92.9 and 22.6%, respectively, and 94.5% of patients were positive for at least one of GADAb, ICA, IA-2Ab, or IAA. In comparison with the previously reported frequency of GADAb in patients with idiopathic fulminant type 1 diabetes (15), the frequency of GADAb was higher (3 of 5; 60%) in interferon treatment–related patients with fulminant type 1 diabetes. Furthermore, GADAb emerged in 2 of 85 patients after the onset of type 1 diabetes. The mean GADAb levels were 14,103.9 ± 32,213.5 units/mL (range 0 to 235,000) and were not associated with the presence or absence of AITD, although extremely high levels of GADAb have been reported in patients with type 1 diabetes and AITD (16) (Supplementary Fig. 2).
HLA-DR allele frequency
Table 3 summarizes the frequencies of the HLA-DR allele in patients with interferon treatment–related type 1 diabetes, classical type 1 diabetes, and healthy control subjects. The frequencies of HLA-DR4, -DR9, and -DR13 were significantly higher in interferon treatment–related type 1 diabetes, whereas those of HLA-DR8 and -DR15 were significantly lower than those in control subjects. The HLA-DR13 allele was observed in 15.4% of interferon treatment–related type 1 diabetic patients but in 7.8% of classical type 1 diabetic patients, with a significant difference in the frequency of HLA-DR13 between these two groups (OR 2.15 [95% CI 1.17–3.93], P < 0.05). The frequency of HLA-DR14 was also significantly higher than that in patients with classical type 1 diabetes (14.2 [3.03–66.7], P < 0.0001).
Table 3.
HLA-DR allele frequency in patients with interferon treatment–related type 1 diabetes, classic type 1 diabetes, and healthy control subjects
| Interferon treatment–related type 1 diabetic patients | Classical type 1 diabetic patients | Control subjects | Interferon treatment vs. control | Interferon treatment vs. classical type 1 diabetes | |||||
|---|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | ||||
| n | 130 | 384 | 608 | ||||||
| DR1 | 4.6 (6) | 2.1 (8) | 5.6 (34) | ||||||
| DR4 | 34.6 (45) | 38.0 (146) | 22.4 (136) | <0.01 | 1.84 | 1.22–2.77 | |||
| DR8 | 7.7 (10) | 9.9 (38) | 15.3 (93) | <0.05 | 0.46 | 0.23–0.91 | |||
| DR9 | 26.9 (35) | 29.4 (113) | 16.4 (100) | <0.01 | 1.87 | 1.20–2.92 | |||
| DR12 | 1.5 (2) | 3.4 (13) | 5.1 (31) | ||||||
| DR13 | 15.4 (20) | 7.8 (30) | 4.3 (26) | <0.0001 | 3.80 | 2.20–7.55 | <0.05 | 2.15 | 1.17–3.93 |
| DR14 | 6.9 (9) | 0.5 (2) | 8.6 (52) | <0.0001 | 14.2 | 3.03–66.7 | |||
| DR15 | 2.3 (3) | 4.7 (18) | 17.8 (108) | <0.0001 | 0.08 | 0.03–0.35 | |||
| Others | 0 (0) | 4.2 (16) | 4.6 (28) | ||||||
Data are % (n) unless otherwise indicated.
CONCLUSIONS
In the current study, we investigated the clinical, immunologic, and genetic characteristics of Japanese patients with type 1 diabetes that developed during or shortly after interferon therapy. This was the first study to investigate the mode of type 1 diabetes onset in this type of diabetes. The median period from the initiation of interferon treatment to type 1 diabetes was found to be short (∼0.7 years), and the majority of patients showed the abrupt-onset form, suggesting rapid β-cell destruction. Among the patients who developed type 1 diabetes after 2002, ribavirin was used in 72.7% of cases and in 75.4% of the patients who developed type 1 diabetes after 2004, when PegIFN began to be covered by health insurance in Japan, PegIFN was used. Moreover, when the period to the development of type 1 diabetes based on the type of interferon (non-PegIFN or PegIFN) and the use of ribavirin were compared, the length of time was significantly shorter in the PegIFN plus ribavirin group (Fig. 1), suggesting that deviation to the Th1-type immune response by ribavirin (17) and extension of the half-life of interferon by pegylation (18) may be among the causes of the increased number of type 1 diabetic patients.
Anti-islet autoantibodies, especially GADAb, were highly detected at the onset of type 1 diabetes. Furthermore, the seroconversion of GADAb during interferon treatment has been reported in ~40% of patients with chronic viral hepatitis who developed type 1 diabetes (19). These findings suggest that measurement of anti-islet autoantibodies before and during interferon treatment is useful for identifying subjects at high risk of developing type 1 diabetes. The sequential study of anti-islet autoantibodies is required to determine the interval from the emergence of anti-islet autoantibodies to the development of type 1 diabetes and possibly to ascertain an effective and economical measurement frequency as early predictive markers.
A genetic predisposition is necessary but not sufficient for the development of type 1 diabetes. To date, there has been no reported investigation of the genetic factors in interferon treatment–related type 1 diabetes. It has been reported that the genetic background of Japanese type 1 diabetes differs from that of Caucasians. The major susceptible class II HLA antigens in Japanese classical type 1 diabetes are DR4 and DR9 (11). In the current study, we have demonstrated the higher frequency of HLA-DR13 in interferon treatment–related type 1 diabetes compared with that in classical type 1 diabetic and healthy control subjects (Table 3). Furthermore, no differences in HLA-DR13 frequency have been reported between healthy control subjects and patients with chronic HCV in the Japanese population (20). These results highlight the differences in the contribution of HLA-DR subtypes to susceptibility to interferon treatment–related and classical type 1 diabetes and the importance of HLA-DR13 in influencing the progression of type 1 diabetes during or after interferon therapy.
The pathogenesis of type 1 diabetes in response to interferon treatment remains unclear. However, evidence is accumulating regarding the association between type 1 interferon and type 1 diabetes. The overexpression of interferon-α in the pancreases of patients with type 1 diabetes (21) and a preventative effect of type 1 diabetes by interferon-α–neutralizing antibody in transgenic mice in which the β-cells express interferon-α have been reported (22). Furthermore, interferon-α is known to increase major histocompatibility complex class I antigen expression on cell membranes and to activate T cells and natural killer cells (23). These findings are in agreement with the hypothesis that interferon-α is involved in the development of type 1 diabetes in humans.
Our study has limitations. We did not assess whether anti-islet autoantibodies were induced in interferon-treated patients who did not develop type 1 diabetes. We also did not examine the frequencies of HLA-DR alleles. Fabris et al. (24) have reported that the prevalence of markers of anti-islet autoimmunity in HCV-positive patients increased during interferon-α therapy from 3 to 7%. Therefore, sequential measurement of blood glucose is important for the early diagnosis of type 1 diabetes. It has been reported that the glucotoxicity effect on β-cells may result in ketoacidosis even in patients with type 2 diabetes. However, the possibility that type 2 diabetes existed in our subjects was considerably low for the following reasons: 1) patients with ketosis-prone type 2 diabetes have a significantly greater rate of insulin discontinuation at 6 months after onset (25), 2) all autoantibody-negative patients with the acute-onset form have absent β-cell function at 12 months after onset, and 3) 14 patients who had been diagnosed as having type 2 diabetes many years before the diagnosis of type 1 diabetes were positive for anti-islet autoantibodies.
In conclusion, interferon treatment–related type 1 diabetes develops rapidly in the majority of patients, and stronger antiviral treatment with PegIFN and ribavirin may induce earlier development of type 1 diabetes. To avoid life-threatening events such as diabetic ketoacidosis, early detection of the development of type 1 diabetes by sequential monitoring of anti-islet autoantibodies and blood glucose before and during interferon therapy is important. Furthermore, patients who develop interferon treatment–related type 1 diabetes are genetically susceptible.
Supplementary Material
Acknowledgments
This study was supported by a grant-in-aid from the Japan Diabetes Society.
No potential conflicts of interest relevant to this article were reported.
K. Nakamura and E.K. wrote the manuscript and researched data. A.I. and T.A. contributed to discussion and reviewed and edited the manuscript. H.I., Y.U., and T.K. reviewed and edited the manuscript. A.S. and K. Nakanishi contributed to discussion and reviewed and edited the manuscript. H.M. and T.M. reviewed and edited the manuscript. T.H. contributed to discussion and reviewed and edited the manuscript.
The authors thank T. Ichikawa, Division of Gastroenterology and Hepatology, Nagasaki University Hospital, Japan, and H. Yatsuhashi, Department of Hepatology, NHO Nagasaki Medical Center, Japan, for their helpful discussions. The authors acknowledge the editorial assistance of Gordon Murphy, KN International, Inc., Hoffman Estates, Illinois.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2274/-/DC1.
A complete list of the members of the Research Committee on Type 1 Diabetes of the Japan Diabetes Society can be found in the Supplementary Data online.
References
- 1.Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by interferon-alpha/beta. Eur J Immunol 2001;31:3138–3146 [DOI] [PubMed] [Google Scholar]
- 2.Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid 2003;13:547–551 [DOI] [PubMed] [Google Scholar]
- 3.Fabris P, Betterle C, Floreani A, et al. Development of type 1 diabetes mellitus during interferon alpha therapy for chronic HCV hepatitis. Lancet 1992;340:548. [DOI] [PubMed] [Google Scholar]
- 4.Waguri M, Hanafusa T, Itoh N, et al. Occurrence of IDDM during interferon therapy for chronic viral hepatitis. Diabetes Res Clin Pract 1994;23:33–36 [DOI] [PubMed] [Google Scholar]
- 5.Guerci AP, Guerci B, Levy-Marchal C, et al. Onset of insulin-dependent diabetes mellitus after interferon-alpha therapy for hairy cell leukemia. Lancet 1994;343:1167–1168 [DOI] [PubMed] [Google Scholar]
- 6.Cozzolongo R, Betterle C, Fabris P, Paola Albergoni M, Lanzilotta E, Manghisi OG. Onset of type 1 diabetes mellitus during peginterferon alpha-2b plus ribavirin treatment for chronic hepatitis C. Eur J Gastroenterol Hepatol 2006;18:689–692 [DOI] [PubMed] [Google Scholar]
- 7.Schreuder TC, Gelderblom HC, Weegink CJ, et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int 2008;28:39–46 [DOI] [PubMed] [Google Scholar]
- 8.Bosi E, Minelli R, Bazzigaluppi E, Salvi M. Fulminant autoimmune type 1 diabetes during interferon-alpha therapy: a case of Th1-mediated disease? Diabet Med 2001;18:329–332 [DOI] [PubMed] [Google Scholar]
- 9.Piquer S, Hernandez C, Enriquez J, et al. Islet cell and thyroid antibody prevalence in patients with hepatitis C virus infection: effect of treatment with interferon. J Lab Clin Med 2001;137:38–42 [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki M, Sato A, Takeda T, Komatsu M. Distinct clinical courses in type 1 diabetes mellitus induced by peg-interferon-alpha treatment for chronic hepatitis C. Intern Med 2010;49:403–407 [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia 2006;49:828–836 [DOI] [PubMed] [Google Scholar]
- 12.Sera Y, Kawasaki E, Abiru N, et al. Autoantibodies to multiple islet autoantigens in patients with abrupt onset type 1 diabetes and diabetes diagnosed with urinary glucose screening. J Autoimmun 1999;13:257–265 [DOI] [PubMed] [Google Scholar]
- 13.Terasaki PI, Bernoco D, Park MS, Ozturk G, Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol 1978;69:103–120 [DOI] [PubMed] [Google Scholar]
- 14.Kawabata Y, Ikegami H, Awata T, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia 2009;52:2513–2521 [DOI] [PubMed] [Google Scholar]
- 15.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003;26:2345–2352 [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki E, Takino H, Yano M, et al. Autoantibodies to glutamic acid decarboxylase in patients with IDDM and autoimmune thyroid disease. Diabetes 1994;43:80–86 [DOI] [PubMed] [Google Scholar]
- 17.Ning Q, Brown D, Parodo J, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 1998;160:3487–3493 [PubMed] [Google Scholar]
- 18.Wedemeyer H, Wiegand J, Cornberg M, Manns MP. Polyethylene glycol-interferon: current status in hepatitis C virus therapy. J Gastroenterol Hepatol 2002;17(Suppl. 3):S344–S350 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Kawasaki E, Abiru N, et al. Trajectories of anti-islet autoantibodies before development of type 1 diabetes in interferon-treated hepatitis C patients. Case reports and a literature review. Endocr J 2010;57:947–951 [DOI] [PubMed] [Google Scholar]
- 20.Kuzushita N, Hayashi N, Moribe T, et al. Influence of HLA haplotypes on the clinical courses of individuals infected with hepatitis C virus. Hepatology 1998;27:240–244 [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Yuang J, Goddard A, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes 1995;44:658–664 [DOI] [PubMed] [Google Scholar]
- 22.Stewart TA, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan NJ. Induction of type I diabetes by interferon-alpha in transgenic mice. Science 1993;260:1942–1946 [DOI] [PubMed] [Google Scholar]
- 23.Taki S. Type I interferons and autoimmunity: lessons from the clinic and from IRF-2-deficient mice. Cytokine Growth Factor Rev 2002;13:379–391 [DOI] [PubMed] [Google Scholar]
- 24.Fabris P, Floreani A, Tositti G, Vergani D, De Lalla F, Betterle C. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment Pharmacol Ther 2003;18:549–558 [DOI] [PubMed] [Google Scholar]
- 25.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008;29:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.