Abstract
OBJECTIVE
The safety of dendritic cells to selectively suppress autoimmunity, especially in type 1 diabetes, has never been ascertained. We investigated the safety of autologous dendritic cells, stabilized into an immunosuppressive state, in established adult type 1 diabetic patients.
RESEARCH DESIGN AND METHODS
A randomized, double-blind, phase I study was conducted. A total of 10, otherwise generally healthy, insulin-requiring type 1 diabetic patients between 18 and 60 years of age, without any other known or suspected health conditions, received autologous dendritic cells, unmanipulated or engineered ex vivo toward an immunosuppressive state. Ten million cells were administered intradermally in the abdomen once every 2 weeks for a total of four administrations. The primary end point determined the proportion of patients with adverse events on the basis of the physician’s global assessment, hematology, biochemistry, and immune monitoring for a period of 12 months.
RESULTS
The dendritic cells were safely tolerated. There were no discernible adverse events in any patient throughout the study. Other than a significant increase in the frequency of peripheral B220+ CD11c− B cells, mainly seen in the recipients of engineered dendritic cells during the dendritic cell administration period, there were no statistically relevant differences in other immune populations or biochemical, hematological, and immune biomarkers compared with baseline.
CONCLUSIONS
Treatment with autologous dendritic cells, in a native state or directed ex vivo toward a tolerogenic immunosuppressive state, is safe and well tolerated. Dendritic cells upregulated the frequency of a potentially beneficial B220+ CD11c− B-cell population, at least in type 1 diabetes autoimmunity.
Type 1 diabetes autoimmunity selectively impairs and destroys pancreatic β-cells. Thymic and peripheral tolerance failure (1,2) involves dendritic cells, which are as important in diabetes onset and progression as pathogenic T cells (3). In general, dendritic cells coordinate immune responses to microenvironmental anomalies (i.e., infection and tissue damage) and orchestrate tolerance to self (4). Many animal studies confirm that exogenous dendritic cell administration prevents autoimmunity and facilitates allograft survival (5). Such dendritic cells often are phenotypically and functionally immature and are largely defined by impaired T-cell costimulation ability. Without costimulation, T cells, including autoreactive cells, either enter into a state of functional impairment (anergy) or undergo apoptosis. Immature dendritic cells also modulate networks of suppressive immune cells, such as T cells expressing the Foxp3 transcription factor.
Our preclinical data in the NOD mouse strain demonstrating prevention and reversal of type 1 diabetes with costimulation-impaired, immunosuppressive dendritic cells (bone marrow–derived dendritic cells treated ex vivo with a mixture of antisense oligonucleotides targeting the primary transcripts of CD40, CD80, and CD86) (6) compelled us to determine the safety of, and possible immune reactions against, such dendritic cells in humans. We therefore generated human dendritic cells analogous to the ones successfully used in those NOD studies (6), concurrently targeting the expression of the same costimulatory molecules ex vivo, envisaging type 1 diabetes cell therapy. We hypothesized that immunosuppressive dendritic cells would primarily be safe and well tolerated and, secondarily, could alter the frequency of immune cell populations potentially beneficial in type 1 diabetes.
RESEARCH DESIGN AND METHODS
This phase I study (ClinicalTrials.gov identifier NCT00445913) was conducted at the University of Pittsburgh Medical Center Clinical Translational Research Center after review and approval by the Food and Drug Administration, the University of Pittsburgh Institutional Review Board, and the Data Safety Monitoring Board and after written informed consent was obtained from each patient. The data herein were reviewed by the Data Safety Monitoring Board and the Food and Drug Administration.
Patients (Table 1) were eligible for enrollment if they were between 18 and 60 years of age, had insulin-requiring diabetes for at least 5 years between the time of clinical diagnosis and the first dendritic cell injection, and met all the inclusion and exclusion criteria (Supplementary Methods Table T1). The patient-selection criteria were recommended by the Food and Drug Administration with institutional review board concurrence.
Table 1.
Study group characteristics
| Control dendritic cells | Immunosuppressive dendritic cells | |
|---|---|---|
| n | 3 | 7 |
| Age (years) | ||
| Mean | 30.3 ± 4.5 | 31.6 ± 13.3 |
| Median | 30 | 27 |
| Range | 26–35 | 19–57 |
| Male sex [n (%)] | 2 (67) | 4 (57) |
| Race or ethnicity (self-reported) [n (%)] | ||
| White | 3 (100) | 7 (100) |
| Non-Hispanic | 3 (100) | 7 (100) |
| Type 1 diabetes autoantibodies [n (%)] | ||
| 0 | 0 (0) | 2 (29) |
| 1 | 2 (67) | 3 (43) |
| 2 | 1 (33) | 1 (14) |
| Years since diagnosis | 18.0 ± 3.0 | 15.0 ± 7.5 |
| Years from diagnosis to first injection of dendritic cells | ||
| Median | 18 | 14 |
| Range | 14–18 | 5–26 |
| Weight (kg) | 84.6 ± 12.5 | 87.0 ± 25.4 |
| BMI | 28.2 ± 3.4 | 27.3 ± 6.4 |
| Total white blood cells (per mm3) (×106/mL) | 8.3 ± 1.9 | 5.9 ± 1.6 |
| Glycated HbA1c at baseline (%) | 8.73 ± 1.76 | 8.26 ± 2.22 |
| C-peptide at baseline | Undetectable | Undetectable |
| Total insulin dose (units/kg) | 0.49 ± 0.20 | 0.32 ± 0.05 |
| Received all four rounds of dendritic cell injections [n (%)] | 3 (100) | 7 (100) |
Data are means ± SD, unless otherwise indicated. BMI is weight in kilograms divided by the square of height in meters.
A power analysis was conducted using simulations of continuously monitored, trial-stopping boundaries to determine the accrual buffer needed to suspend a trial after an adverse event (7). This analysis concluded that in a total sample size of 10 patients, the occurrence of an adverse event in 2 patients would give a 75% probability, and the occurrence of an adverse event in 3 patients would give a 90% probability of hitting the boundary where the boundary is defined as trial suspension (7). Thus, 10 patients who met all inclusion and exclusion criteria (Supplementary Methods Table T1) were enrolled. Peripheral blood was obtained to measure baseline levels of immune cell populations, immune-reactivity indices, serum immune biomarkers, and autoantibodies, as well as for biochemistry and hematology evaluation. Urine was collected to determine kidney function. Enrolled patients were randomly assigned in a 2-to-1 ratio of immunosuppressive dendritic cells to control dendritic cells. Control dendritic cells and immunosuppressive dendritic cells were generated ex vivo from leukapheresis products. The methods for generating control and immunosuppressive dendritic cells are available in the Supplementary Methods. Once generated, the cells were frozen in aliquots of 1 × 107 cells until the day of administration. Participants and research staff were blinded to the type of dendritic cell product administered.
Each patient received 1 × 107 dendritic cells once every 2 weeks for a total of four administrations. For each administration, four aliquots of cells were intradermally injected; each aliquot into one of the vertices of a rectangular quadrant of 3–4 square inches overlying the anterior abdominal wall perpendicularly above the physical location of the stomach and pancreas. After thawing, the cells were slowly delivered by a tuberculin syringe attached to a 27-g 1/2 needle underneath a raised “bleb” of skin at each of the four individual injection sites. Patients were then monitored by the physician for at least 2 h for evidence of local or systemic allergic reaction; inflammation at the injection sites; of any grade ≥2 toxicity or hypersensitivity, including chills, malaise, fever, shortness of breath, palor, or light headedness; or any subjective report of discomfort. Patients maintained their insulin-administration regimen throughout the study, with the objective of maintaining glycated hemoglobin levels within the age-specified range according to the American Diabetes Association guidelines (8,9). In the intervening weeks between administrations, patients continued to be evaluated (physical, biochemical, hematologic, and immune monitoring) to identify possible adverse events. This evaluation was then conducted twice monthly for the first 6 months after the first dendritic cell administration and then once monthly for the remainder of the study period (6 months).
Safety evaluations
Patients were observed for treatment-related toxicity or adverse reactions during and after each course of dendritic cell administration. The Common Toxicity Criteria (ctep.info.nih.gov/reporting/ctc.html) defined toxicity type and grade. The definition of adverse events conformed to 21 CFR 312.32 (a) (serious) and 21 CFR 312.32 (a) (unexpected). Safety evaluations also probed for autoimmunity other than type 1 diabetes in serum, indices of systemic immunosuppression measured as an in vitro cellular response to alloantigens and vaccination antigens, white blood cell counts, and flow cytometry–based measurement of frequencies of specific immune cell populations (Supplementary Methods Tables T2 and T3).
Laboratory measurements
Standard hematology and biochemistry were used to screen blood, serum, and urine samples and also to detect specific pathogens, antinuclear, antithyroglobulin, antithyroperoxidase, and type 1 diabetes–relevant autoantibodies. Immune monitoring included serum cytokine detection, multiparameter flow cytometry for immune cell subsets, and cellular proliferation assays to vaccination and alloantigens in vitro (see also Supplementary Methods Tables T3 and T4).
Statistical analysis
The trial aimed at assuring that the toxicity rate was acceptably low to warrant additional study of the dendritic cell products. The following stopping rule was imposed: If at any time during the trial the observed proportion of the sum of all grade ≥2 toxicities or adverse events and grade 2 autoimmune toxicities equaled or exceeded 33% of treated patients, additional treatment would be held pending review by the Data Safety Monitoring Board. An upper bound on the rate of serious toxicity was chosen to claim, with at least 90% probability, that the true serious toxicity rate was no greater than 20% (7,10). To determine whether the differences in specific cell-population frequencies between baseline and in-trial time points were statistically relevant, the Wilcoxon signed-rank test for paired observations was used. Elsewhere, and as indicated, standard two-tailed and repeated-measures ANOVA and two-tailed t tests were used to ascertain statistical significance to apparent differences in trends and values.
RESULTS
There was no apparent effect of sex or ethnicity on any of the outcomes or results of the trial.
Dendritic cell administration is well tolerated without any adverse events
Ten insulin-requiring type 1 diabetic patients were enrolled in the study (Table 1). There were no detectable adverse events in any patient throughout the study duration. There were no difficulties experienced during the series of intradermal injections in any patient. During follow-up, no patient reported experiences outside routine activities of daily living. None of the patients exhibited any acute changes in diabetes control. No patient demonstrated acute illnesses that met the standard of an adverse event. The physical examination outcomes of all patients during the course of the study were unchanged from pretreatment/baseline and were considered normal and unremarkable. The average insulin dose remained unchanged for each patient throughout the duration of the study when compared with pretreatment/baseline even though the specific insulin formulation varied (Lantus long-acting insulin with multiple daily injections of Humalog, Humulin injectable or pump, or Novolog injectable). The only notable physical observation was a predictable “wheal and flare” reaction at the abdominal rectangular quadrant defined by the injection-site vertices, which resolved within the first 60 min, on average. We did not observe or detect any subjective or objective evidence of fever, systemic hypersensitivity, malaise, chills, pains, or cardiac or ventilation abnormalities. None of the enrolled patients reported any adverse subjective sensation by 72 h after each of the dendritic cell administrations suggestive of a treatment-related anomaly.
Laboratory outcomes
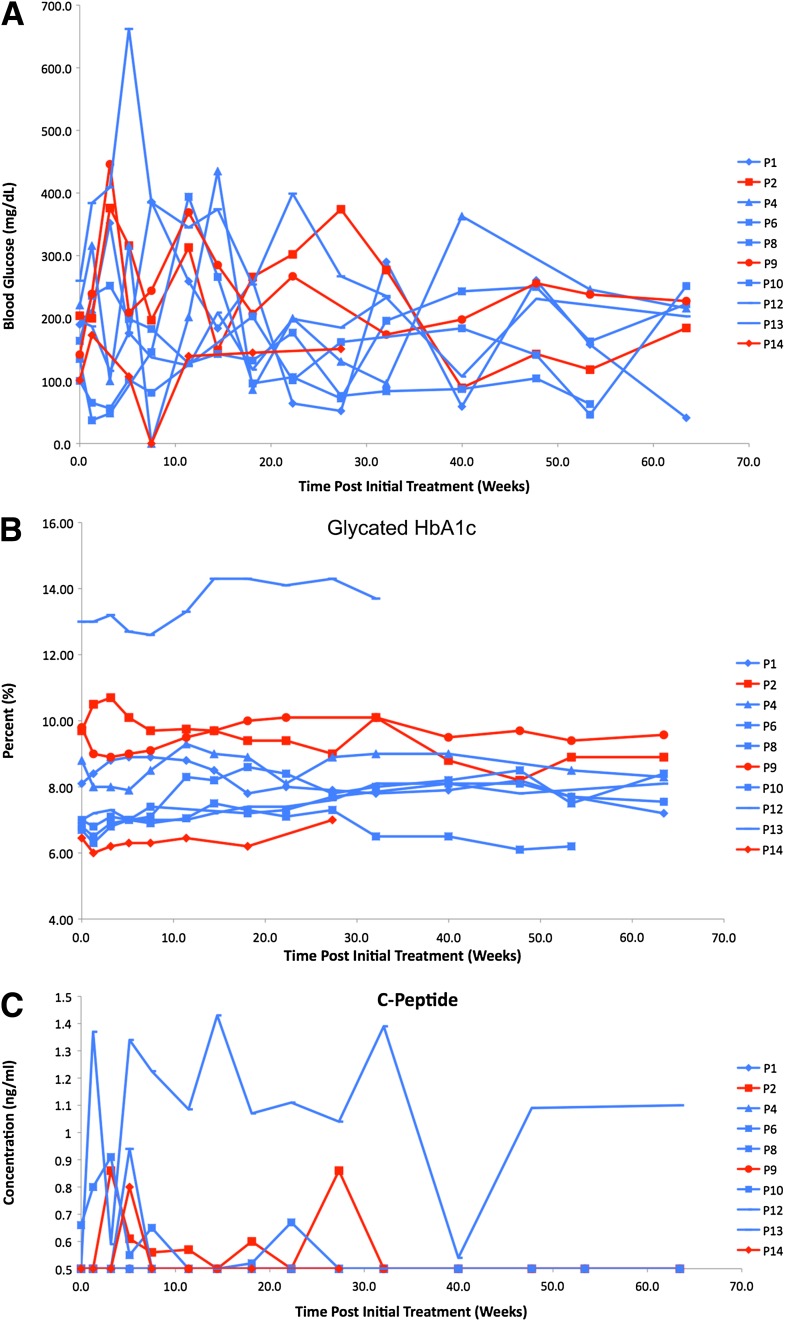
Nonfasting blood glucose levels fluctuated compared with baseline in all the patients treated with control or immunosuppressive dendritic cells (Fig. 1A). Compared with pretreatment/baseline, glycated hemoglobin levels did not change in any significant manner in control or immunosuppressive dendritic cell recipients (Fig. 1B) with the exception of one immunosuppressive dendritic cell recipient (patient 13, Fig. 1B), who had historically exhibited poor glycemic control. However, more intriguing was the observation that C-peptide levels in some of the subjects became detectable, whereas previously and at the time of enrollment they were undetectable (Fig. 1C).
Figure 1.
Effects of control and immunosuppressive dendritic cells on nonfasting blood glucose levels (A), nonfasting glycated HbA1c levels (B), and nonfasting, nonstimulated C-peptide levels in serum (C). Week 0 represents pretreatment/baseline levels. The symbols and lines in blue represent the immunosuppressive dendritic cell recipients and those in red represent the control dendritic cell recipients. The legend to the right of each graph shows the symbols that correspond to each individual patient (P). The values were measured in freshly obtained blood and serum at each of the weeks of the trial, shown on the x-axis in the graph. For C-peptide levels, only values >0.5 ng/mL are shown based on standard clinical reference.
Hematology assessment at all times during and after the dendritic cell administrations did not reveal any differences in total leukocyte frequency or specific general leukocyte population compared with the expected standard reference range (Supplementary Fig. 1).
The frequency of relevant peripheral blood immune cell populations (total and activated CD4+ and CD8+ T cells, total B cells, and peripheral blood CD11c+ dendritic cells) was measured by fluorescence-activated cell sorter at key time points of the trial (Table 2). There were no statistically distinguishable differences in the frequencies of any of these populations between control and immunosuppressive dendritic cell recipients as well as within each group compared with baseline at any time during the trial, with one exception. Compared with baseline, control and immunosuppressive dendritic cell recipients exhibited a statistically relevant increase in B220+ CD11c− cells during the dendritic cell administration period (Supplementary Fig. 2). However, despite sustained increases compared with baseline, statistical significance was lost by the end of the treatment period (Table 2). The frequencies returned to baseline through month 12, although immunosuppressive dendritic cell recipients exhibited lower-than-baseline levels at this time (Table 2). Additional characterization of this population uncovered immunosuppressive activity in vitro (Supplementary Fig. 3A and B).
Table 2.
Immune cell populations
| Cell population | Baseline | Week 12 (DC4) | 6 Months | 12 Months |
|---|---|---|---|---|
| CD3+ CD4+ | ||||
| Control dendritic cells | 37.7 ± 0.5 | 33.9 ± 9.9 | 47.1 ± 1.9 | 48.2 ± 2.7 |
| Immunosuppressive dendritic cells | 46.3 ± 2.7 | 45.5 ± 3.7 | 40.8 ± 4.6 | 42.9 ± 3.7 |
| CD3+ CD8+ | ||||
| Control dendritic cells | 20.9 ± 3.4 | 21.8 ± 3.2 | 28.6 ± 3.0 | 29.7 ± 2.7 |
| Immunosuppressive dendritic cells | 23.6 ± 1.9 | 18.6 ± 2.9 | 20.4 ± 2.2 | 22.4 ± 1.8 |
| CD4+ CD69+ | ||||
| Control dendritic cells | 0.6 ± 0.4 | 2.4 ± 1.2 | 0.6 ± 0.3 | 0.8 ± 0.5 |
| Immunosuppressive dendritic cells | 1.7 ± 0.8 | 2.0 ±1.0 | 0.7 ±0.2 | 1.4 ± 1.0 |
| CD8+ CD69+ | ||||
| Control dendritic cells | 1.7 ± 1.2 | 4.2 ± 2.4 | 0.8 ± 0.2 | 1.1 ± 0.5 |
| Immunosuppressive dendritic cells | 3.4 ± 0.7 | 3.8 ± 1.6 | 0.6 ± 0.1 | 1.7 ± 0.4 |
| CD4+ CD45RA+ | ||||
| Control dendritic cells | 25.2 ± 2.2 | 23.9 ± 6.7 | 24.4 ± 1.6 | 23.4 ±12.3 |
| Immunosuppressive dendritic cells | 29.5 ± 3.9 | 27.2 ± 6.0 | 29.7 ± 4.6 | 29.5 ± 2.1 |
| CD8+ CD45RA+ | ||||
| Control dendritic cells | 12.3 ± 2.0 | 15.8 ± 0.5 | 16.7 ±2.6 | 16.9 ±1.5 |
| Immunosuppressive dendritic cells | 13.9 ± 1.7 | 15.0 ±2.8 | 13.8 ± 2.4 | 15.7 ±1.8 |
| CD4+ CD25HIGH FOXP3+ | ||||
| Control dendritic cells | 2.1 ± 1.2 | 2.9 ± 1.9 | 1.3 ± 0.5 | 1.8 ± 0.7 |
| Immunosuppressive dendritic cells | 1.1 ± 0.3 | 1.7 ±0.5 | 1.8 ± 0.7 | 1.9 ± 0.7 |
| B220+ CD11c− | ||||
| Control dendritic cells | 3.5 ± 2.9 | 9.6 ± 5.1 | 3.2 ± 2.6 | 5.0 ± 2.1 |
| Immunosuppressive dendritic cells | 5.0 ± 2.9 | 13.9 ± 8.8 | 5.3 ± 2.7 | 1.6 ± 0.96 |
| CD11c+ CD83+ HLA-DR+ | ||||
| Control dendritic cells | 73.9 ± 6.7 | 62.6 ± 13.1 | 65.6 ± 10.1 | 63.6 ± 4.7 |
| Immunosuppressive dendritic cells | 71.9 ± 6.9 | 71.0 ± 5.4 | 70.2 ± 4.9 | 57.8 ± 8.0 |
Data are means ± SEM and indicate the percentage of gated cells by fluorescence-activated cell sorter. “Immunosuppressive dendritic cells” refers to antisense oligonucleotide-treated dendritic cell recipients. DC4 at week 12 indicates the measurement of the cell populations at 1 week after the last dendritic cell administration.
Two of three control dendritic cell recipients and five of seven immunosuppressive dendritic cell recipients exhibited GAD autoantibodies at baseline. There were no apparent differences in GAD autoantibody concentrations at all times the serum was collected before and after dendritic cell administration. One of three control dendritic cell recipients and one of seven immunosuppressive dendritic cell recipients exhibited insulinoma-associated protein-2 antibodies, whose concentrations did not demonstrate any apparent change before and after dendritic cell administration (Supplementary Fig. 4). Also, the levels of antinuclear and antithyroglobulin antibodies were below the threshold considered to be clinically relevant (Supplementary Fig. 5).
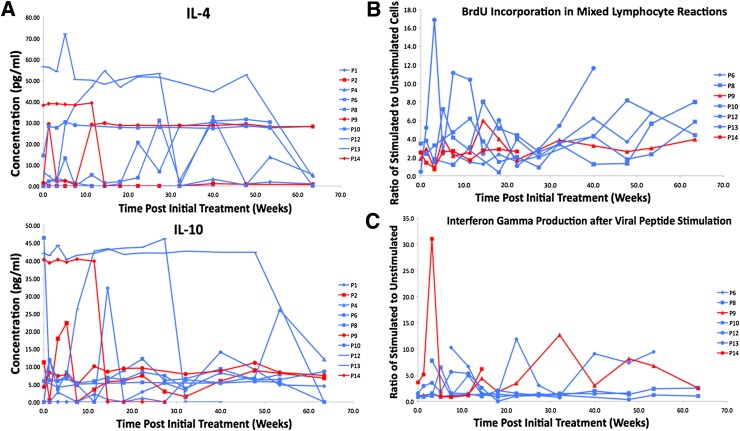
For what concerns serum cytokine profiles and concentration, the only two cytokines whose levels increased in control and immunosuppressive dendritic cell recipients compared with baseline were interleukin (IL)-4 and IL-10, even if inconsistently (Fig. 2A). The source of these cytokines currently is unknown, but their established role in immune tolerance suggests that control or immunosuppressive dendritic cell administration may provide some benefit by upregulating their production in one or more immune cell populations. We note that control and immunosuppressive dendritic cells do not themselves produce IL-4 or IL-10 in vitro, although whether they do so in vivo is unknown.
Figure 2.
A: Serum levels of IL-4 and IL-10 in dendritic cell recipients. The presence and the concentration of serum cytokines was measured by Luminex multianalyte assay systems (LincoPLEX and Beadlyte). Serum IL-4 and IL-10 were reproducibly detectable. The points and lines in red indicate cytokine concentration in the serum of control dendritic cell recipients and the blue points and lines show the concentration in immunosuppressive dendritic cell recipients. The graph at the top shows the levels of IL-4 and that on the bottom shows IL-10. The legend to the right shows the symbols that correspond to each individual patient (P). B: Dendritic cell administration does not confer systemic immunosuppression. Proliferation of dendritic cell-recipient T cells in allogeneic MLR in vitro. 2 × 105 PBMCs from the freshly obtained blood of the dendritic cell recipients were cultured alone or mixed with an equal number of allogeneic irradiated PBMCs in standard one-way MLR. The cells were cultured for 4 days before BrdU addition. BrdU incorporation into proliferating cells was measured on day 5 by flow cytometry. The graph shows the ratio of BrdU+ cells in the presence of allogeneic stimulators to BrdU+ cells in the absence of stimulators at each of the indicated weeks of the trial in seven individual patients compared with baseline (visit 2). Patients 6, 8, 10, 12, and 13 received immunosuppressive dendritic cells; patients 9 and 14 received control dendritic cells. The volumes of blood (and hence cell number) obtained from the remainder of the patients were not permissive to properly conduct biologically meaningful MLR. The legend to the right shows the symbols that correspond to each individual patient (P). C: The ELISPOT response of dendritic cell-recipient T cells to vaccination antigens in vitro. PBMCs from freshly isolated blood were frozen and, for this assay, thawed at a later time. The thawed cells were plated on interferon-γ ELISPOT strips (Human Interferon ELISPOT Pro; Mabtech) at densities of 1 × 105 or 3 × 105 cells. PBMCs were plated alone, with a viral peptide pool (CEF Peptide Pool; Mabtech) at 2 µg/mL, or with a kit-supplied CD3 agonistic antibody. An ELISPOT assay was conducted as recommended by the manufacturer, and the spots were counted electronically (KS ELISPOT; Zeiss) in an Axioplan 2 microscope with ELISPOT adapter/module. The data in the graph are shown for each patient where frozen PBMCs from each of the indicated weeks of the trial were available. The data are represented as the ratio of interferon-γ spots in viral peptide–stimulated PBMCs to the spot number in unstimulated cells. Viability of frozen PBMCs after thawing from the remainder of the patients precluded the conduct of a biologically meaningful MLR assay. The legend to the right shows the symbols that correspond to each individual patient (P).
To determine their potential to induce systemic immunosuppression, we conducted a standard allogeneic mixed-leukocyte reaction (MLR) in vitro, measuring the proliferation of dendritic cell–recipient T cells in culture with allogeneic peripheral blood mononuclear cells (PBMCs). The stimulation indices remained at the baseline level and even increased in some dendritic cell recipients over the study period (Fig. 2B). We also measured the reactivity of dendritic cell–treated patient PBMCs to a panel of viral peptide antigens by interferon γ enzyme-linked immunosorbent spot (ELISPOT). In all screened individuals, there was no loss of response compared with baseline (Fig. 2C). Altogether, these data indicate that control and immunosuppressive dendritic cells do not induce nonspecific systemic immunosuppression.
CONCLUSIONS
To date, all known dendritic cell clinical trials aimed at boosting immunity. Despite the convincing evidence supporting dendritic cell–based prevention or reversal of autoimmunity and in facilitating allograft survival (5,11,12), no human trials have ever been conducted to determine immunosuppressive dendritic cell safety and potential benefits. Herein, we report, for the first time, that multiple intradermal injections of autologous dendritic cells, untreated or stabilized ex vivo toward an immunoregulatory tolerogenic state into an abdominal region overlying the anatomical location of the pancreas, are well tolerated and safe in adult type 1 diabetic patients with established disease longer than 5 years. There were no observable adverse events or toxicities.
Dendritic cells are strong T-cell modulators, and control and/or immunosuppressive dendritic cell administration could potentially cause general T-cell activation (with or without proliferation). By measuring the frequency of CD69+ T cells at key trial time points, we concluded that neither control nor immunosuppressive dendritic cells induced any changes in CD69+ T-cell frequency compared with baseline (Table 2). This observation is in line with the tolerogenic nature of immature dendritic cells, which, on their own, do not activate T cells. Nevertheless, we cannot rule out the possibility that T cells underwent unmeasurable differentiation activity not involving activation, per se.
Next, we probed the possibility that control or immunosuppressive dendritic cells could affect the frequency of naive antigen-inexperienced T cells (CD45RA+ T cells), which, if activated to proliferate, could induce diabetes-unrelated autoimmunities. The frequency of CD4+ CD45RA+ and CD8+ CD45RA+ T cells was statistically indistinguishable at all trial time points compared with baseline, between and within control and immunosuppressive dendritic cell recipients (Table 2), indicating that this possibility was unlikely.
Administration of immature dendritic cells in vivo increases the prevalence of Foxp3+ T-regulatory cells (Tregs), and these cells partially mediate the beneficial effects of immunoregulatory dendritic cells (13). We observed a slight increase in CD4+ CD25HIGH Foxp3+ T cells in immunosuppressive dendritic cell recipients compared with control dendritic cell recipients, mainly during the dendritic cell administration period (Supplementary Fig. 6). The differences, however, between immunosuppressive and control dendritic cell recipients as well as the difference between before and after dendritic cell injections, with the exception of three patients (Supplementary Fig. 6), were not statistically relevant (Table 2).
We noted an increased frequency of PBMC B220+ CD11c− lymphocytes of all dendritic cell recipients as early as 1 week after dendritic cell administration. On average, this cell population comprises between 1.0 and 3.3% of the total PBMCs in normal individuals (data not shown). In four of seven immunosuppressive dendritic cell–treated patients, the increases were substantial during the dendritic cell administration period compared with baseline. These differences were statistically relevant only during the first 6 weeks after the first round of immunosuppressive dendritic cell administration (P < 0.05; one-tailed Wilcoxon signed-rank test) (Supplementary Fig. 2). Although there were no statistically distinguishable differences in B220+ CD11c− cell frequency at any time during the trial between immunosuppressive dendritic cell and control dendritic cell recipients (P > 0.05, two-tailed Mann-Whitney U test), further characterization and in vitro functional assays suggest that this population contains at least one novel suppressive subpopulation (Supplementary Fig. 3A and B). A number of studies confirm the existence of immunosuppressive B cells referred to as B-regulatory cells (Bregs) (14–16). In murine models of inflammation and autoimmunity, including collagen-induced arthritis, colitis, lupus, and type 1 diabetes, exogenously administered Bregs suppress disease (14–16). Possible roles of Bregs in human autoimmunity recently have been identified (17,18). Our putative Bregs do not seem to be like the recently described B10 and CD24HIGH CD38HIGH Bregs (Supplementary Fig. 7), even though CD24 and CD27, characteristic of the recently described human B10 Bregs, were expressed strongly on the surface of the parental B220+ CD11c− cells (17,18). This does not exclude the possibility that our Bregs could in fact be a heterogeneous population consisting of memory and transitional B cells in different states of activation. That even untreated dendritic cells upregulate potentially beneficial B cells in humans is not surprising in light of analogous observations of B-cell upregulation in pioneering diabetes prevention studies with pancreatic immature dendritic cells in NOD mice (19). It would be of significant interest to determine whether type 1 diabetic patients and high-risk relatives exhibit perturbations in one or more Breg populations. We currently are exploring this possibility.
Autoantibodies often reflect an underlying autoimmunity and are an appropriate biomarker of de novo autoimmunity. One concern of our trial was the potential to induce de novo autoimmunity, especially thyroid-specific autoimmunity, which has been shown in some populations to cosegregate with type 1 diabetes. We did not observe any antinuclear antibodies or any de novo thyroid-specific autoantibodies arguing against the possibility that control or immunosuppressive dendritic cell administration could provoke a generalized autoimmunity or a potential type 1 diabetes cosegregating secondary autoimmunity.
All dendritic cell recipients exhibited normal immune responses to vaccination antigens and alloantigen stimulation in vitro, indicating that control and immunosuppressive dendritic cells do not induce any measurable level of systemic immunosuppression. In light of the outcome of this study, we believe, like others, that the widely reported adverse events, including elevated proinflammatory cytokines, fever, chills, and general malaise, associated with immunostimulatory dendritic cell immunotherapy are attributed to the normally adopted priming of the patients with cytokines such as IL-2 and granulocyte macrophage–colony-stimulating factor (20–22).
Our findings demonstrate, for the first time, that nonmanipulated autologous dendritic cells, or autologous dendritic cells directed ex vivo toward a costimulation-impaired immunologically suppressive state, are well tolerated and safe. Our observations herein also portend beneficial outcomes when considering tolerogenic dendritic cell intervention in autoimmune diseases such as type 1 diabetes at a time closer to the clinical onset of the disease.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Award R33-DK-63499-03 (to M.T.).
No potential conflicts of interest relevant to this article were reported.
N.G. wrote and edited all draft versions and the final version of the manuscript, performed the immunological and mechanistic assays, analyzed the immunological and mechanistic data, and had full access to all data in the study and had the final responsibility for the decision to submit the manuscript for publication. B.P. performed the immunological and mechanistic assays and analyzed the immunological and mechanistic data. D.F. analyzed the safety and physical patient data. J.H. performed the immunological and mechanistic assays. M.T. wrote and edited all draft versions and the final version of the manuscript, analyzed the immunological and mechanistic data, analyzed the safety and physical patient data, and had full access to all data in the study and had the final responsibility for the decision to submit the manuscript for publication.
The authors thank Carl Engman, Robert Lakomy, and Alexis Styche for excellent technical support and assistance. Carl Engman, J.H., B.P., Robert Lakomy, and Alexis Styche are fully compensated employees of the Children's Hospital of Pittsburgh. N.G., M.T., and D.F. are fully compensated employees of the University of Pittsburgh School of Medicine.
Footnotes
Clinical trial reg. no. NCT00445913, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0472/-/DC1.
References
- 1.Eisenbarth GS. Insulin autoimmunity: immunogenetics/immunopathogenesis of type 1A diabetes. Ann N Y Acad Sci 2003;1005:109–118 [DOI] [PubMed] [Google Scholar]
- 2.Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J 2009;28:2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo J, Clare-Salzler MJ. Dendritic cell subsets and type I diabetes: focus upon DC-based therapy. Autoimmun Rev 2006;5:419–423 [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA 2002;99:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transpl Int 2006;19:525–538 [DOI] [PubMed] [Google Scholar]
- 6.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol 2004;173:4331–4341 [DOI] [PubMed] [Google Scholar]
- 7.Lazaridis E, Gonin R. A new program to compute and evaluate continuously monitored stopping boundaries for clinical trials. Comput Methods Programs Biomed 2000;61:187–194 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon R, Thall PF, Ellenberg SS. New designs for the selection of treatments to be tested in randomized clinical trials. Stat Med 1994;13:417–429 [DOI] [PubMed] [Google Scholar]
- 11.Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis 2008;67(Suppl. 3):iii90–iii96 [DOI] [PubMed] [Google Scholar]
- 12.Phillips BE, Giannoukakis N, Trucco M. Dendritic cell mediated therapy for immunoregulation of type 1 diabetes mellitus. Pediatr Endocrinol Rev 2008;5:873–879 [PubMed] [Google Scholar]
- 13.Yamazaki S, Dudziak D, Heidkamp GF, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol 2008;181:6923–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 2008;224:201–214 [DOI] [PubMed] [Google Scholar]
- 15.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol 2008;29:34–40 [DOI] [PubMed] [Google Scholar]
- 16.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol 2008;8:391–397 [DOI] [PubMed] [Google Scholar]
- 17.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008;28:639–650 [DOI] [PubMed] [Google Scholar]
- 18.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117:530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest 1992;90:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 2001;193:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redman BG, Chang AE, Whitfield J, et al. Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. J Immunother 2008;31:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correale P, Campoccia G, Tsang KY, et al. Recruitment of dendritic cells and enhanced antigen-specific immune reactivity in cancer patients treated with hr-GM-CSF (Molgramostim) and hr-IL-2. Results from a phase Ib clinical trial. Eur J Cancer 2001;37:892–902 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.