Abstract
OBJECTIVE
To evaluate the efficacy and safety of α-lipoic acid (ALA) over 4 years in mild-to-moderate diabetic distal symmetric sensorimotor polyneuropathy (DSPN).
RESEARCH DESIGN AND METHODS
In a multicenter randomized double-blind parallel-group trial, 460 diabetic patients with mild-to-moderate DSPN were randomly assigned to oral treatment with 600 mg ALA once daily (n = 233) or placebo (n = 227) for 4 years. Primary end point was a composite score (Neuropathy Impairment Score [NIS]–Lower Limbs [NIS-LL] and seven neurophysiologic tests). Secondary outcome measures included NIS, NIS-LL, nerve conduction, and quantitative sensory tests (QSTs).
RESULTS
Change in primary end point from baseline to 4 years showed no significant difference between treatment groups (P = 0.105). Change from baseline was significantly better with ALA than placebo for NIS (P = 0.028), NIS-LL (P = 0.05), and NIS-LL muscular weakness subscore (P = 0.045). More patients showed a clinically meaningful improvement and fewer showed progression of NIS (P = 0.013) and NIS-LL (P = 0.025) with ALA than with placebo. Nerve conduction and QST results did not significantly worsen with placebo. Global assessment of treatment tolerability and discontinuations due to lack of tolerability did not differ between the groups. The rates of serious adverse events were higher on ALA (38.1%) than on placebo (28.0%).
CONCLUSIONS
Four-year treatment with ALA in mild-to-moderate DSPN did not influence the primary composite end point but resulted in a clinically meaningful improvement and prevention of progression of neuropathic impairments and was well tolerated. Because the primary composite end point did not deteriorate significantly in placebo-treated subjects, secondary prevention of its progression by ALA according to the trial design was not feasible.
Diabetic distal symmetric sensorimotor polyneuropathy (DSPN) is a chronic progressive disease affecting around one-third of the diabetic population and accounts for considerable morbidity, increased mortality, and reduced quality of life (1,2). Recent long-term studies in type 2 diabetic patients indicate that the current strategies of intensive diabetes therapy or multifactorial cardiovascular risk intervention are not sufficient to slow the progression of DSPN (3–5). Thus, effective treatment of DSPN remains challenging for the physician (1,6).
Based on the pathogenetic mechanisms of DSPN, potential disease-modifying therapeutic approaches have been developed including antioxidants such as α-lipoic acid (ALA) (7–9) to diminish increased oxidative stress (10). Other potential modalities include the aldose reductase inhibitors (11), growth factors (12), and the protein kinase C-β inhibitor ruboxistaurin (13). These drugs have been designed to favorably influence the underlying pathophysiology of the disorder rather than for symptomatic pain relief. However, several problems have been encountered previously in designing appropriate clinical trials in DSPN. Among these, the most important are as follows: 1) the lack of homogeneity of patients studied with respect to both the form of neuropathy and the degree of glycemic control; 2) different pathogenetic pathways, the relative importance of which may vary intraindividually; 3) stages of neuropathy that are too advanced; 4) the use of end points with rather large variability between individuals and between centers; 5) the unknown relevance of end points used; and 6) study durations too short to allow for a favorable functional or structural effect (6,14,15).
Treatment with ALA administered intravenously or orally for several weeks or months improves neuropathic symptoms and deficits (7–9). However, based on the assumption that a therapeutic agent may prevent worsening of DSPN but not cause improvement, clinical trials should be conducted for a minimum period of 3 years to achieve a clinically meaningful change of two Neuropathy Impairment Score (NIS) points (16,17). Therefore, we assessed the efficacy and safety of oral treatment with 600 mg ALA once daily in mild-to-moderate DSPN.
RESEARCH DESIGN AND METHODS
The Neurological Assessment of Thioctic Acid in Diabetic Neuropathy (NATHAN) 1 trial was a multicenter (36 centers in the U.S., Canada, and Europe [Supplementary Materials]), randomized, double-blind, placebo-controlled, two-arm, 1:1 allocation ratio, parallel-group clinical trial using film-coated tablets containing 600 mg ALA (Thioctacid HR; MEDA Pharma, Bad Homburg, Germany) that were administered once daily or matching placebo tablets with increased amounts of cellulose and lactose that were identical in appearance in diabetic patients with mild-to-moderate DSPN (18). The trial consisted of a 2-week screening phase, 6-week placebo run-in phase, 4-year double-blind phase, and 4-week washout phase. Approvals were obtained from the local ethics committees of all participating centers.
Inclusion criteria at the screening visit were age 18–64 years; type 1 or type 2 diabetes defined by the American Diabetes Association criteria (1997); diabetes duration ≥1 year; presence of stage 1 or 2a DSPN attributable to diabetes (18); stable insulin regimen, weight, diet, and physical activity level as judged by the investigator; NIS–Lower Limbs and seven nerve conduction tests (NIS-LL+7) score ≥97.5th percentile (corresponding to 4.43 transformed score points); NIS-LL ≥2 points; one of two abnormalities [either 1) abnormal nerve conduction attributes in two separate nerves ≥99th percentile for distal latency or ≤1st percentile for nerve conduction velocity or amplitude or 2) abnormal heart rate during deep breathing (HRDB) ≤1st percentile or total symptom score (TSS) in the feet <5 points]; and being of the female sex and surgically sterilized, ≥1 year postmenopausal, or practicing an acceptable method of contraception.
Exclusion criteria were neuropathies other than DSPN; myopathy and other neurologic diseases that may might interfere with the assessment of the severity of DSPN; previous bilateral sural nerve biopsies; peripheral vascular disease with intermittent claudication; foot ulcers; high risk for visual loss; psychiatric, psychological, or behavioral symptoms that would interfere with the patient’s ability to participate in the trial; active neoplastic disease except basal cell carcinoma; uncontrolled atrial fibrillation; clinically significant cardiac, pulmonary, gastrointestinal, hematologic, or other endocrine disease; organ transplants; aspartate aminotransferase or alanine aminotransferase >2 times normal; serum creatinine >1.8 and >1.6 mg/dL for men and women, respectively; drug or alcohol abuse within the last year; use of investigational drug within the last 6 months; severe or anaphylactic reaction to multiple drugs, sulfur products, or biologic products; ketoacidosis or hypoglycemia within the last 3 months resulting in hospital admission; antioxidant therapy (>400 IU vitamin E, >200 mg vitamin C, or >30 mg/day β carotene) or pentoxyphylline within the last month; γ-linolenic acid and ALA >50 mg/day within the last 3 months; history of use of medications or vitamins known to cause peripheral neuropathy including but not limited to use of phenytoin or carbamazepine ≥15 years or use of >100 mg/day pyridoxine within the last 12 months; and use of pain medications except for standard doses of salicylates, ibuprofen, indoles, fenamates, oxicams, or pyrazoles.
Outcomes
The primary outcome measure was a composite score including the NIS-LL+7 suggested by Dyck et al. (16) including 1) vibration detection threshold, 2) peroneal motor nerve conduction velocity (MNCV), 3) peroneal motor nerve distal latency, 4) peroneal compound muscle action potential (CMAP), 5) tibial motor nerve distal latency, 6) sural sensory nerve action potential amplitude, and 7) change in HRDB. The primary criterion of efficacy in the confirmatory analysis was the absolute change in the NIS-LL+7 score expressed as normal deviates (nds from percentiles correcting for age and other applicable variables) between baseline (mean of visit during weeks 1 and 2 or last available value before randomization) and end point (mean of weeks 191 and 192 or last available value after randomization).
Secondary outcome measures included the NIS, NIS-LL, Neuropathy Symptoms and Change (NSC) score, TSS, cooling detection threshold, heat pain response slope (0.5–5.0), tibial nerve CMAP and MNCV, sural sensory nerve action potential latency, and sensory nerve conduction velocity (SNCV). In a response/progression analysis after 2 and 4 years, a clinically meaningful response was defined as a decrease in NIS or NIS-LL by ≥2 points, respectively, while clinically meaningful progression was defined as an increase in NIS or NIS-LL by ≥2 points, respectively (16,17).
Duplicate measurements of these measures within 1 week were performed at baseline, after 2 years, and after 4 years, whereas single measurements were carried out at screening and after 6 months, 1 year, and 3 years except for the NIS and NSC, which were assessed as single assessments at screening and thereafter at 6-month intervals, and the TSS and foot inspection, assessed at 3-month intervals.
The NIS is the sum score of examinations of muscle weakness, reflex loss, touch pressure, vibration, joint position and motion, and pinprick of index finger and great toe and is scored for both sides of the body (19). The NSC scores (number, severity, and change) are derived from answers to 38 questions (muscle weakness, questions 1–19; sensation, questions 20–29; and autonomic symptoms, questions 30–38) (7). Experienced, trained, and certified (by P.J.D. and colleagues) physicians evaluated the NIS and NSC. Study physicians had participated in training sessions and actual examination of patients under observation using a formal certification process. The nerve conduction, quantitative sensory tests (QSTs), and autonomic tests were performed by trained and certified personnel (by P.A.L., W.J.L., P.J.D., and colleagues). All results were interactively evaluated by the Reading and Quality Assurance Centers (at Mayo Clinic and Health Partners). Eligibility, baseline conditions, wave forms, stimulus response patterns, and test values were also assessed.
Safety measures included monitoring of adverse events, vital signs, weight, 12-lead resting electrocardiogram, chest X-ray at baseline, concomitant medication, global assessment of tolerability, and physical examination. Laboratory tests including blood chemistry, hematology, blood glucose, and HbA1c were performed at screening, baseline, and 2-month (1st year), 3-month (2nd year), and 6-month (3rd year) intervals.
Randomization
Screened patients were assigned a unique five-digit number. Randomized patients were additionally assigned a four-digit randomization number at baseline. Patients were assigned to the two treatment groups according to a randomization list generated by the Biostatistics Department of MEDA Pharma. The random allocation was balanced using an undisclosed block size of six. The investigators and the monitor received sealed envelopes to enable decoding the individual blinded treatment in case of emergency.
Statistical analysis
Confirmatory analysis.
The following hypothesis was tested: H0:μT = μP vs. H1:μT≠μP, where μT and μP denote the mean change in NIS-LL+7 tests from baseline to end point in the ALA and placebo groups, respectively. A two-way ANOVA including the factors treatment and center was performed. Variances were allowed to differ between treatments. Degrees of freedom were derived according to Kenward-Rogers. A 95% CI for the treatment difference based on least squares (LS) estimates from the model without interactions was calculated. A second model including treatment and center interaction was fitted explanatorily.
Interim analysis.
An interim analysis was performed as soon as the 2-year data of most subjects were available. The complete table part of the study had been provided to and assessed by an independent supervisory committee. The decision on continuation of the study was based on the confirmatory test of the primary variable. At P < 0.005, the study would have been stopped. To ensure a global type 1 error of 5%, the error level for the final analysis was set to α2 = 0.0452 according to the Šidák (20) inequality.
Depending on the structure of data, either contingency tables [n (%)] or descriptive statistics (n, mean, SD, median, range, quartiles) were presented for time courses and changes from baseline. Secondary end points were analyzed by applying a Wilcoxon Mann-Whitney test.
Adverse events were coded according to the World Health Organization-Adverse Reactions Terminology (ART). Global incidences on preferred term and body system class level were calculated based on different causality filters. Vital and laboratory variables were screened for notable individual and trend-like changes.
Sample size calculation.
We used a conservative approach to suggest that treatment with ALA may prevent worsening of DSPN but not necessarily cause improvement (16). A clinically meaningful treatment difference of 2.0 nds for the changes from baseline for NIS-LL+7 tests and statistical error probabilities α = 0.05 and β = 0.1 were assumed. Based on a two-sided t test, the following scenarios for SDs and sample size per group (n) were considered: SD 3.57, n = 68; SD 4, n = 86; SD 5, n = 133; SD 6, n = 191; SD 7, n = 211. To account for a relatively high dropout rate expected in this long-term study, randomization of 250 patients per arm was proposed. Analysis of the intention-to-treat population was primary for all efficacy variables. Homogeneity of baseline characteristics was investigated by exploratory statistical tests based on the intention-to-treat population on selected baseline variables.
RESULTS
Patient disposition, clinical characteristics, HbA1c, and vital signs
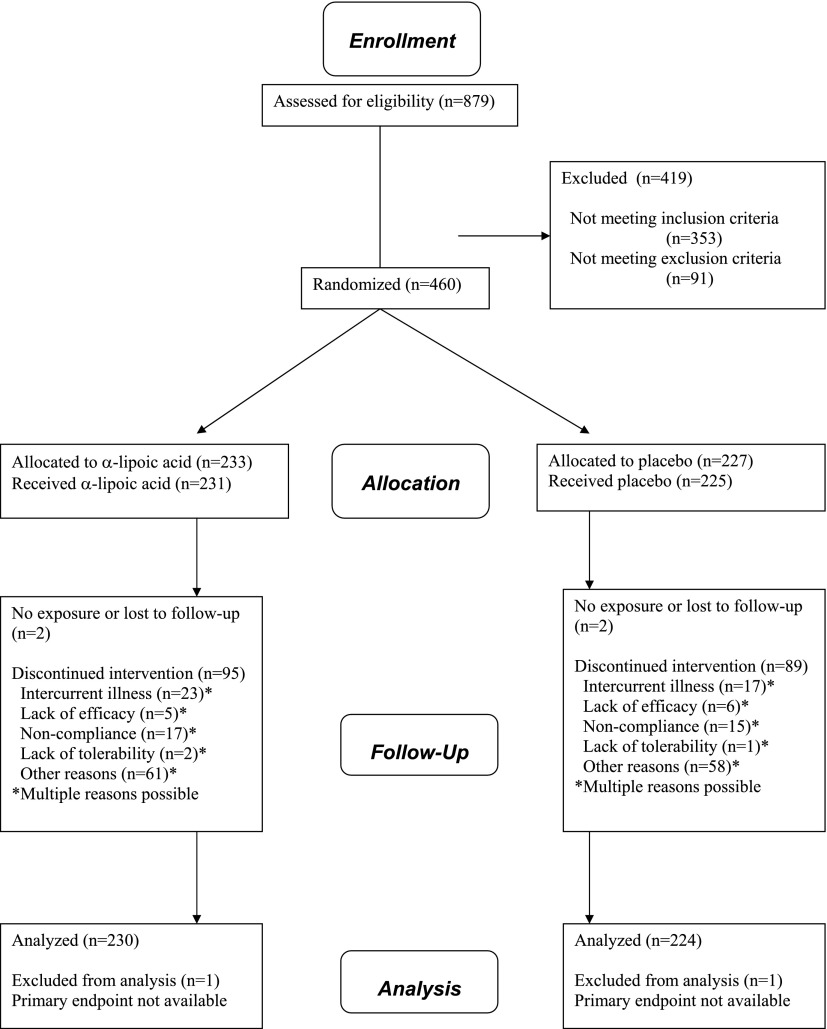
The patient disposition throughout the trial according to the CONSORT Statement 2010 flow diagram (21) is shown in Fig. 1. The demographic variables and outcome measures at baseline in both groups are shown in Table 1. As a sign of homogeneity, no significant differences among the groups were noted for any of the parameters listed except for HRDB (P = 0.0193).
Figure 1.
Patient disposition.
Table 1.
Clinical characteristics of the intention-to-treat population at baseline
| ALA | Placebo | P | |
|---|---|---|---|
| n | 230 | 224 | |
| Age (years) | 53.3 ± 8.3 | 53.9 ± 7.6 | 0.3607 |
| Sex (% male) | 66.1 | 67.0 | 0.8430 |
| BMI (kg/m2) | 29.7 ± 6.1 | 29.8 ± 6.1 | 0.9226 |
| Heart rate (bpm) | 76.3 ± 12.3 | 74.6 ± 12.6 | 0.1603 |
| Type 1/type 2 diabetes | 27.4 / 72.6 | 21.0 / 79.0 | 0.1111 |
| Diabetes duration (years) | 13.3 (0.8–56.1) | 13.5 (0.9–46.7) | 0.4190 |
| Neuropathy duration (years) | 3.0 (0.0–25.4) | 3.2 (0.0–21.1) | 0.2588 |
| Insulin treatment | 58.9 | 55.1 | 0.4170 |
| HbA1c | 8.9 ± 1.8 | 8.8 ± 1.9 | 0.6354 |
| Fasting blood glucose (mmol/L) | 11.1 ± 4.68 | 10.9 ± 4.26 | 0.6529 |
| Nephropathy | 11.7 | 12.0 | 0.5935 |
| Retinopathy | 45.5 | 43.6 | 0.6833 |
| Neuropathy stage 1/stage 2a | 11.3 / 88.7 | 9.8 / 90.2 | 0.6074 |
| NIS-LL+7 (nds) | 17.1 ± 8.4 | 16.8 ± 8.0 | 0.6740 |
| NIS (points) | 12.7 ± 8.6 | 12.2 ± 7.8 | 0.5062 |
| NIS-LL (points) | 9.8 ± 5.6 | 9.5 ± 5.3 | 0.6087 |
| Peroneal MNCV (m/s) | 38.5 ± 5.03 | 38.1 ± 6.48 | 0.4957 |
| Sural SNAP (µV) | 2.49 ± 3.38 | 2.43 ± 3.21 | 0.8387 |
| Vibration perception threshold (JND) | 21.27 ± 3.18 | 21.21 ± 3.52 | 0.8393 |
| Cold detection threshold (JND) | 17.86 ± 5.14 | 17.58 ± 5.33 | 0.5765 |
| Heart rate deep breathing (bpm) | 7.26 ± 5.44 | 8.59 ± 6.59 | 0.0193 |
| NSC weakness (number) | 0.06 ± 0.30 | 0.03 ± 0.23 | 0.2353 |
| NSC weakness (severity) | 0.10 ± 0.56 | 0.04 ± 0.31 | 0.1613 |
| TSS (points) | 2.4 ± 1.9 | 2.6 ± 1.8 | 0.2752 |
Data are means ± SD, median (range), or %. P values derived from χ2 test for binary data and from t tests otherwise. JND, just noticeable difference; SNAP, sensory nerve action potential.
Mean HbA1c decreased from baseline by 0.67 ± 1.41% in the ALA group and by 0.48 ± 1.46% on placebo after 2 years. After 4 years, HbA1c declined compared with baseline by 0.62 ± 1.59% with ALA and by 0.60 ± 1.78% during placebo without significant differences between the groups (P = 0.9313 after 4 years). After 4 years, systolic blood pressure decreased by 0.1 ± 16.1 mmHg in the ALA group and by 0.2 ± 16.5 mmHg in the group given placebo (P = 0.9770). The corresponding reductions in diastolic blood pressure were 3.5 ± 11.1 mmHg for ALA compared with 2.7 ± 9.8 for placebo (P = 0.5121). Heart rate declined by 2.6 ± 10.9 bpm with ALA treatment and by 2.1 ± 10.4 bpm in the placebo group after 4 years compared with baseline (P = 0.7115). Weight increased slightly by 1.3 ± 5.4 kg in the ALA-treated group compared with 0.9 ± 8.6 in the placebo group (P = 0.6157).
The changes in the clinical neuropathy scores and nerve function tests from baseline to 2 and 4 years are summarized in Table 2. The NIS-LL+7 composite score decreased after 4 years compared with baseline on ALA (LS mean −0.45) and increased on placebo (LS mean +0.34) without reaching statistical significance between the groups (ALA-placebo difference in LS means 0.78 [95% CI −0.16 to 1.73]; P = 0.105). After 4 years, NIS improved significantly on ALA and worsened on placebo (P = 0.028) while the differences from baseline between the groups reached borderline significance for the pinprick test of NIS (P = 0.074) and NIS-LL (P = 0.0505). The muscular weakness subscore of NIS-LL improved significantly on ALA and deteriorated on placebo (P = 0.045). No significant differences between the groups after 4 years were noted for the NIS-LL subscores sensory function and reflexes. Response analysis after 4 years revealed that the percentages of clinical responders (decrease in NIS or NIS-LL by ≥2 points) were higher while at the same time those of progressors (increase in NIS or NIS-LL by ≥2 points) were lower on ALA compared with placebo. This difference was statistically significant for both NIS (P = 0.013) and NIS-LL (P = 0.025). The NSC scores for weakness number and severity, respectively, improved on ALA and worsened on placebo (P = 0.005 and P = 0.008). No significant differences between both groups after 4 years were noted for the nerve conduction parameters, QST, HRDB, or TSS.
Table 2.
Changes in clinical neuropathy scores and nerve function tests from baseline to 2 and 4 years
| 2 Years |
4 Years |
|||
|---|---|---|---|---|
| ALA |
Placebo |
ALA |
Placebo |
|
| n | 214 | 207 | 215 | 207 |
| Composite score | ||||
| NIS-LL+7 (nds) | −0.40 ± 4.92 | 0.19 ± 4.74 | −0.37 ± 5.59* | 0.29 ± 5.37 |
| NIS and subscores | ||||
| NIS | −0.54 ± 6.62 | 0.12 ± 6.13 | −0.68 ± 6.44† | 0.61 ± 6.61 |
| NIS pinprick | −0.06 ± 1.48 | −0.05 ± 1.44 | −0.07 ± 1.60‡ | 0.05 ± 1.43 |
| NIS-LL | −0.38 ± 4.52 | 0.03 ± 4.22 | −0.34 ± 4.48§ | 0.43 ± 4.49 |
| NIS-LL sensory function | −0.34 ± 3.02 | −0.09 ± 2.92 | −0.12 ± 3.01 | 0.10 ± 2.89 |
| NIS-LL muscular weakness | −0.15 ± 1.66 | 0.05 ± 1.85 | −0.21 ± 1.57† | 0.17 ± 2.12 |
| NIS-LL reflexes | 0.10 ± 1.63 | 0.07 ± 1.57 | 0.03 ± 1.75 | 0.16 ± 1.80 |
| NIS responders | 37.9 | 35.2 | 41.1† | 30.0 |
| NIS unchanged | 35.6 | 32.4 | 29.7† | 31.9 |
| NIS progressors | 26.5 | 32.4 | 29.2† | 38.1 |
| NIS-LL responders | 34.7 | 34.8 | 35.6† | 29.0 |
| NIS-LL unchanged | 42.0 | 35.2 | 40.2† | 36.2 |
| NIS-LL progressors | 23.3 | 30.0 | 24.2† | 34.8 |
| Nerve function tests | ||||
| Peroneal MNCV (m/s) | 0.04 ± 3.89 | 0.18 ± 3.99 | −0.35 ± 4.23 | −0.06 ± 4.07 |
| Sural SNAP (µV) | −0.00 ± 2.17 | −0.07 ± 1.96 | −0.20 ± 2.34 | −0.15 ± 2.43 |
| Foot VPT (JND) | 0.47 ± 2.12 | 0.58 ± 2.11 | 0.87 ± 2.35 | 0.76 ± 2.38 |
| Cold detection threshold (JND) | 0.65 ± 3.56 | 0.87 ± 3.33 | 1.12 ± 3.96 | 1.28 ± 3.43 |
| Heart rate deep breathing (bpm) | −0.68 ± 3.39 | −1.06 ± 3.23 | −0.67 ± 4.44¶ | −1.35 ± 3.72 |
| Neuropathic symptoms | ||||
| NSC weakness (number) | −0.02 ± 0.30 | 0.04 ± 0.42 | −0.04 ± 0.26† | 0.04 ± 0.42 |
| NSC weakness (severity) | −0.03 ± 0.40 | 0.03 ± 0.48 | −0.05 ± 0.39† | 0.04 ± 0.50 |
| TSS | −0.27 ± 2.46 | −0.04 ± 2.16 | −0.22 ± 2.42 | −0.21 ± 2.45 |
Data are means ± SD or %. All P values calculated vs. placebo, with two-way ANOVA for NIS-LL+7 and Wilcoxon Mann-Whitney tests otherwise. JND, just noticeable difference; SNAP, sensory nerve action potential; VPT, vibration perception threshold.
*P = 0.105.
†P < 0.05.
‡P = 0.074.
§P = 0.0505.
¶P = 0.087.
Safety analysis
The incidences of treatment-emergent adverse events (TEAEs) were 214 (92.6%) on ALA and 203 (90.2%) on placebo, respectively. Two (0.9%) patients on ALA and one (0.7%) patient on placebo discontinued study treatment as a result of lack of tolerability (i.e., because of an adverse event with “likely” causal relationship to study medication as judged by the investigator). Analysis of TEAEs by the body system class showed that heart rate, rhythm disorders, and myocardial, endocardial, pericardial, valvular, and urinary system disorders tended to be more frequent on ALA than on placebo (Supplementary Table 1). No significant differences in the incidences of the individual cardiovascular disorders were observed between the groups (Supplementary Table 2). Corrected QT interval (QTc) prolongation >60 ms was significantly more frequent on placebo than that on ALA (10 [5.0%] vs. 3 [1.4%]; P = 0.0497). Global assessment of treatment tolerability by investigators and patients showed no differences between the groups (Supplementary Table 3).
Serious adverse events (SAEs) occurred in 88 (38.1%) patients in the ALA group and in 63 (28.0%) of those receiving placebo. The incidence of SAEs in the ALA group versus that in the placebo group was 31 (13.4%) vs. 20 (8.9%), respectively, (P = 0.125) for cardiovascular disorders; 5 (2.2%) vs. 7 (3.1%) (P = 0.528) for cerebrovascular disorders; 20 (8.7%) vs. 13 (5.7%) (P = 0.235) for infections; 23 (10.0%) vs. 14 (6.2%) for inflicted injuries (including surgical interventions, coronary angiography or angioplasty, and fractures; P = 0.144); and 3 (1.3%) vs. 6 (2.7%) (P = 0.294) for deaths.
CONCLUSIONS
The results of this longest randomized clinical trial ever conducted in DSPN demonstrate that 4-year treatment with the antioxidant ALA did not influence a composite score consisting of the NIS-LL+7 but improved neuropathic impairments scored by the NIS and NIS-LL, particularly small fiber and muscular function. The lack of improvement in the composite score was predominantly due to the fact that nerve conduction deficits in the placebo-treated group did not progress. Thus, secondary prevention of progression of the composite end point by treatment with ALA was not readily achievable. In contrast, a response analysis of clinically meaningful improvement and progression in these scores by at least two points (16,17) revealed that the percentages of NIS and NIS-LL responders with mild-to-moderate DSPN were significantly higher on ALA, whereas the progressors were more frequent with placebo. To achieve these favorable effects, 4 years of treatment using 600 mg ALA once daily were necessary. Safety analysis showed that SAEs were slightly more frequent in patients receiving ALA, but more deaths occurred in the placebo group.
Similar to recent multicenter trials in DSPN (22), only a minor progression of the various measures of DSPN was observed in the placebo group over 4 years in the present trial. Therefore, improvement rather than slowing of progression was required for most outcomes to demonstrate efficacy. Because nerve conduction attributes did not deteriorate in the placebo group and did not improve with ALA, the composite primary end point could not be expected to ameliorate despite the improvement in neuropathic deficits. One reason for the lack of deterioration of nerve conduction attributes during placebo treatment could be the reduction in mean HbA1c by 0.6% in this group. However, the calculation of the sample size and trial duration was based on the assumption that a treatment effect slows the progression of DSPN without improving it (16). This concept has been challenged by recent findings, suggesting that DSPN progresses very slowly or may even improve in placebo-treated patients participating in randomized clinical trials (11,13,22). A progressive improvement in neuropathic symptoms over 1 year was observed in placebo-administered patients with mild symptomatic DSPN (22). In a recent multicenter study, patients with mild-to-moderate DSPN randomized to placebo showed an improvement of 2.0 ± 8.0 m/s in summed median and sural SNCV after 1 year, which was associated with improvements in glycemic control and serum triglyceride levels (23). Dyck et al. (15) assessed clinimetric performance of DSPN end points in single and multicenter trials. They concluded that the main reasons why it is difficult to demonstrate monotonic worsening of neuropathic end points appears to be a very slow worsening of DSPN, improvement of symptoms and signs in the placebo group, and measurement noise. Demonstrating disease progression in DSPN trials may be more likely when 1) patients with developing rather than established DSPN are selected, 2) type 1 diabetic patients are preferentially recruited, 3) patients are selected who cannot achieve ideal glycemic control, 4) end points chosen are known to show monotonic worsening, and 5) a restricted number of centers and expert examiners are used.
The reasons for the disparity between the effects of ALA on neuropathic deficits and nerve conduction or QST are not understood, but the same pattern was observed in the short-term Symptomatic Diabetic Neuropathy (SYDNEY) trial over 3 weeks (7). The mechanisms of the improvement in neuropathic impairments may be related to an improvement in nerve blood flow mediated by the antioxidant action of the drug. In the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study, oral administration of 300 mg ALA per day to patients with the metabolic syndrome resulted in a significant increase in endothelium-dependent brachial artery flow–mediated vasodilation and reductions in plasma levels of interleukin-6 and plasminogen activator-1 compared with placebo after 4 weeks, suggesting that the drug may improve endothelial dysfunction via anti-inflammatory and antithrombotic mechanisms (23). Interestingly, prevention of endothelial dysfunction via activation of AMP-activated protein kinase by ALA was postulated as a possible mechanism to explain the weight loss achieved after 20 weeks of ALA treatment in obese Korean subjects (24).
There is no firm explanation for the higher incidence of SAEs on ALA than placebo, given that only two patients on ALA and one patient on placebo discontinued study treatment due to adverse events with “likely” causal relationship to study medication as judged by the investigator. Moreover, when SAEs due to cardiovascular/cerebrovascular disorders/procedures and deaths were summarized, 34 (14.7%) patients on ALA and 27 (12.0%) patients on placebo were affected (P = 0.394). On the other hand, QTc prolongation >60 ms was significantly more frequent on placebo than on ALA (5.0 vs. 1.4%; P = 0.0497) and more deaths were observed with placebo than with ALA (3 [1.3%] vs. 6 [2.7%]). Thioctacid is an over-the-counter drug that has been widely prescribed in Germany for decades, and postmarketing surveillance data sources from Germany showed low rates of adverse drug reactions comparable with those reported in previous clinical trials (25).
In conclusion, 4-year treatment with ALA in mild-to-moderate DSPN was well tolerated and was associated with improvement of neuropathic impairments but not nerve conduction attributes. Therefore, the primary composite end point did not change significantly. The clinical progression of DSPN in the placebo group within the setting of this randomized clinical trial with good retention of patients throughout a 4-year period was relatively slow. Hence, prediction of a treatment effect aimed at halting the progression of DSPN based on epidemiologic data indicating significant progression may not be appropriate. The designs of future trials in similar diabetic populations should anticipate a long-term stable neuropathic condition. For demonstration of efficacy, the drug or combinations of drugs that impact the different nerve fibers affected in DSPN that are tested in the future must achieve a clinically relevant neurologic improvement.
Supplementary Material
Acknowledgments
D.Z. received honoraria for consulting, review, administrative, and speaking activities and support for travel from MEDA. P.A.L. received honoraria for consulting activities from MEDA. W.J.L. received honoraria for consulting and review activities from MEDA. A.J.M.B. received grants to his institution from MEDA. A.I.V. received grants, fees for patents, and royalties to his institution from MEDA. R.S. was an employee of MEDA from 1993 to 2006. H.T., U.M., and J.M. are employees of MEDA. No other potential conflicts of interest relevant to this article were reported.
D.Z. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. P.A.L. and W.J.L. researched data, contributed to discussion, and reviewed and edited the manuscript. A.J.M.B. and A.I.V. contributed to discussion and reviewed and edited the manuscript. R.F. researched data, contributed to discussion, and reviewed and edited the manuscript. R.S. researched data and reviewed and edited the manuscript. H.T. researched data, contributed to discussion, and reviewed and edited the manuscript. U.M. researched data and reviewed and edited the manuscript. J.M. reviewed and edited the manuscript. K.S. and P.J.D. researched data, contributed to discussion, and reviewed and edited the manuscript.
Footnotes
Clinical trial reg. no. NCT00977483, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0503/-/DC1.
A complete list of the NATHAN 1 trial participating centers can be found in the Supplementary Data.
References
- 1.Boulton AJM, Vinik AI, Arezzo JC, et al. ; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 3.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler D, Luft D. Clinical trials for drugs against diabetic neuropathy: can we combine scientific needs with clinical practicalities? Int Rev Neurobiol 2002;50:431–463 [DOI] [PubMed] [Google Scholar]
- 7.Ametov AS, Barinov A, Dyck PJ, et al. ; SYDNEY Trial Study Group. The sensory symptoms of diabetic polyneuropathy are improved with α-lipoic acid: the SYDNEY trial. Diabetes Care 2003;26:770–776 [DOI] [PubMed] [Google Scholar]
- 8.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 2006;29:2365–2370 [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a meta-analysis. Diabet Med 2004;21:114–121 [DOI] [PubMed] [Google Scholar]
- 10.Ziegler D, Sohr CGH, Nourooz-Zadeh J. Oxidative stress and antioxidant defense in relation to the severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care 2004;27:2178–2183 [DOI] [PubMed] [Google Scholar]
- 11.Bril V, Hirose T, Tomioka S, Buchanan R; Ranirestat Study Group. Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care 2009;32:1256–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ropper AH, Gorson KC, Gooch CL, et al. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: a randomized, double-blinded trial. Ann Neurol 2009;65:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinik AI, Bril V, Kempler P, et al. ; MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther 2005;27:1164–1180 [DOI] [PubMed] [Google Scholar]
- 14.Boulton AJM. Whither clinical research in diabetic sensorimotor peripheral neuropathy? Problems of end point selection for clinical trials. Diabetes Care 2007;30:2752–2753 [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJ, Norell JE, Tritschler H, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care 2007;30:2619–2625 [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997;49:229–239 [DOI] [PubMed] [Google Scholar]
- 17.Peripheral Nerve Society. Diabetic polyneuropathy in controlled clinical trials: Consensus Report of the Peripheral Nerve Society. Ann Neurol 1995;38:478–482 [DOI] [PubMed] [Google Scholar]
- 18.Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve 1988;11:21–32 [DOI] [PubMed] [Google Scholar]
- 19.Dyck PJ, Karnes JL, O’Brien PC, Litchy WJ, Low PA, Melton LJ, 3rd. The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992;42:1164–1170 [DOI] [PubMed] [Google Scholar]
- 20.Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 1967;62:626–633 [Google Scholar]
- 21.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726–732 [DOI] [PubMed] [Google Scholar]
- 22.Tesfaye S, Tandan R, Bastyr EJ, 3rd, Kles KA, Skljarevski V, Price KL; Ruboxistaurin Study Group. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care 2007;30:2626–2632 [DOI] [PubMed] [Google Scholar]
- 23.Sola S, Mir MQ, Cheema FA, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 2005;111:343–348 [DOI] [PubMed] [Google Scholar]
- 24.Koh EH, Lee WJ, Lee SA, et al. Effects of alpha-lipoic acid on body weight in obese subjects. Am J Med 2011;124:e1–e8 [DOI] [PubMed]
- 25.Rathmann W, Haastert B, Delling B, Gries FA, Giani G. Postmarketing surveillance of adverse drug reactions: a correlational study approach using multiple data sources. Pharmacoepidemiol Drug Saf 1998;7:51–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.