Abstract
OBJECTIVE
We previously showed that exenatide (EXE) enhanced insulin secretion after 1 year of treatment, relative to insulin glargine (GLAR), with a similar glucose-lowering action. These effects were not sustained after a 4-week off-drug period. This article reports the results after additional 2 years of exposure.
RESEARCH DESIGN AND METHODS
Sixty-nine metformin-treated patients with type 2 diabetes were randomized to EXE or GLAR. Forty-six patients entered the 2-year extension study in which they continued their allocated therapy. Thirty-six completed (EXE: n = 16; GLAR: n = 20) the 3-year exposure period. Insulin sensitivity (M value) and β-cell function were measured by euglycemic hyperinsulinemic clamp followed by hyperglycemic clamp with arginine stimulation at pretreatment (week 52) and 4 weeks after discontinuation of study medication (week 56 and week 172). First-phase glucose stimulated C-peptide secretion was adjusted for M value and calculated as the disposition index (DI).
RESULTS
At 3 years, EXE and GLAR resulted in similar levels of glycemic control: 6.6 ± 0.2% and 6.9 ± 0.2%, respectively (P = 0.186). EXE compared with GLAR significantly reduced body weight (−7.9 ± 1.8 kg; P < 0.001). After the 4-week off-drug period, EXE increased the M value by 39% (P = 0.006) while GLAR had no effect (P = 0.647). Following the 4-week off-drug period, the DI, compared with pretreatment, increased with EXE, but decreased with GLAR (1.43 ± 0.78 and −0.99 ± 0.65, respectively; P = 0.028).
CONCLUSIONS
EXE and GLAR sustained HbA1c over the 3-year treatment period, while EXE reduced body weight and GLAR increased body weight. Following the 3-year treatment with EXE, the DI was sustained after a 4-week off-drug period. These findings suggest a beneficial effect on β-cell health.
Type 2 diabetes is characterized by progressive β-cell dysfunction against a background of obesity-related peripheral and hepatic insulin resistance (1). Current treatment guidelines promote a stepwise approach starting with lifestyle and metformin and adding a next agent as soon as target HbA1c values cannot be sustained below 7% (2). None of the presently advocated pharmacological interventions, most of which were already used in the UK Prospective Diabetes Study (UKPDS) (3), address the underlying pathophysiological factors of type 2 diabetes, especially β-cell function (4). Because of this progressive decline of β-cell function in the presence of additional glucose toxicity, the majority of patients will require polypharmacy and eventually insulin therapy to maintain acceptable glycemic control (4). Therefore, novel treatment options specifically addressing the β-cell function defect are eagerly awaited.
Exenatide (EXE) is the first-in-class glucagon-like peptide-1 receptor agonist (GLP-1RA) that improves blood glucose in patients with type 2 diabetes by many different mechanisms (5). EXE predominantly lowers postprandial glucose by a glucose-dependent stimulation of insulin secretion, inhibition of an inappropriate glucagon secretion, and by slowing down gastric emptying (6). Additionally, EXE promotes satiety, decreases food intake, and reduces body weight (6).
We previously showed that EXE, as compared with insulin glargine (GLAR), improved pancreatic β-cell secretory function against a background of similar glycemic control (7). However, these findings were not sustained after a 4-week off-drug period, thus it was not possible to demonstrate disease modification (7).
The aim of this extension study was to assess the long-term effects of EXE and GLAR on glycemic control, body weight, and safety, after an additional 2-year treatment period and during a 12-week off-drug period. During the off-drug period, clamp-derived measures of β-cell function and insulin sensitivity were assessed after 4 weeks.
RESEARCH DESIGN AND METHODS
The study was performed between September 2004 and December 2009 at three study sites in Sweden, Finland, and the Netherlands. The 1-year data were previously reported (7). In total, 150 patients were screened of which 69 patients were randomized using a permutated block randomization scheme stratified by site and screenings for HbA1c to receive EXE or GLAR in addition to ongoing metformin treatment. Inclusion criteria were as follows: age 30–75 years, HbA1c 6.5–9.5%, BMI 25–40 kg/m2, and metformin treatment at a stable dose for at least 2 months. No other blood glucose–lowering agents were allowed within 3 months prior to screening. The study protocol was approved by each site’s ethics review committee and was in accordance with the principles described in the Declaration of Helsinki. All participating patients gave their written informed consent prior to screening.
Experimental design
Patients randomized to EXE (n = 36) initiated treatment at a dose of 5 µg b.i.d., injected 15 min before breakfast and dinner for a period of 4 weeks, followed by dose increase to 10 µg b.i.d. EXE was titrated to a maximum dose of 20 µg t.i.d. or the maximum tolerated dose when HbA1c ranged 7.1–7.5% at two consecutive visits or when HbA1c was ≥7.6% at any given visit. Patients randomized to GLAR (n = 33) started at an initial dose of 10 IU once daily (q.d.), which was injected at bedtime. Patients were instructed to increase the daily dose based on their fasting self-monitored blood glucose (SMBG) levels (<5.6 mmol/L) according to a prespecified treat-to-target algorithm (8). When necessary, the importance of proper titration of insulin was emphasized.
After completing the 1-year main treatment period of the study, patients were asked to return to their randomly assigned study medication for an additional 104-week treatment, which was followed by a 12-week off-drug period (Supplementary Fig. 1). Forty-six patients gave their written consent to participate in the extension phase and continued their allocated treatment with EXE (n = 21) or GLAR (n = 25). During the extension phase, patients visited the study centers at 12-week intervals until the end of the treatment period (week 168). At that point the patients stopped the EXE or GLAR treatment and continued their ongoing metformin treatment, which they had been using in an unchanged dose during the total 168-week treatment period. After a 4-week off-drug period, patients returned to the center for their final combined euglycemic hyperinsulinemic and hyperglycemic clamp with arginine stimulation. The final study visit was at week 180 (approximately 3.5 years after randomization).
Combined euglycemic hyperinsulinemic and hyperglycemic clamp with arginine stimulation
Insulin sensitivity and C-peptide secretion measures were measured during a combined euglycemic hyperinsulinemic and hyperglycemic clamp procedure as previously described (Supplementary Fig. 2) (9,10). Clamps were performed prior to randomization, following 52 weeks of treatment, after a 4-week off-drug period (week 56), and finally after a 4-week off-drug period following the total 3 years of treatment (week 172) as previously described (7). During the clamp at week 52, patients randomized to EXE were given the study drug 15 min prior to the onset of the hyperglycemic clamp, and patients randomized to GLAR received their last insulin dose the night before at bedtime. During the clamp at week 56 and week 172, patients did not receive either EXE or GLAR. Arginine was administered at t = 260 min during the hyperglycemic clamp to estimate maximum insulin secretory capacity at a steady-state glucose concentration of 15 mmol/L (11). Whole-body insulin–mediated glucose uptake (M value) was calculated as the mean glucose infusion rate during the last 30 min of the euglycemic hyperinsulinemic clamp between 90–120 min (9). First- and second-phase C-peptide secretion was calculated as area under the curve (AUC) 180–190 min and AUC 190–260 min, respectively. Arginine-stimulated C-peptide secretion (AIRarg) was calculated as the incremental AUC 260–270 min above the C-peptide concentration prior to the start of the hyperglycemic clamp (t = 175 min). The disposition index (DI), a measure of β-cell function and adjusted for insulin sensitivity, was calculated by multiplying the first-phase incremental C-peptide secretion with the M value (AIRgluc*M) (12).
Biochemical analysis
HbA1c (normal range: 4.3–6.1%, Diabetes Control and Complications Trial [DCCT]-standardized Bio-Rad assay), fasting plasma glucose (FPG), and safety parameters were measured by a central laboratory (Quintiles, Livingston, U.K.) prior to randomization and during each follow-up visit until the end of the 12-week off-drug period. Plasma glucose concentrations during the clamp were measured using a YSI 2300 STAT Plus (YSI, Yellow Springs, OH) in Sweden and the Netherlands, and a Beckman-Coulter Glucose Analyzer 2 (Beckman-Coulter, Fullerton, CA) in Finland. C-peptide samples were analyzed at the VU University Medical Center using a single batch immunoradiometric assay (Centaur; Bayer Diagnostics, Mijdrecht, the Netherlands).
Statistical analysis
The extension study’s primary efficacy end point was the treatment effect on the β-cell function measured as the first-phase glucose-stimulated C-peptide secretion adjusted for the M value. Nonnormally distributed data were base-e transformed prior to statistical analysis. Outcome measures were compared between the two treatment groups using an ANCOVA model. The dependent variable used in the model was the change from pretreatment for the β-cell function parameters (AIRarg, first phase, second phase). For all other end points, the dependent value used was the mean at the corresponding visit. The model included factors for treatment group (EXE/GLAR), site (the Netherlands/Sweden/Finland), and baseline HbA1c stratum (≤8.5%/>8.5%), and the pretreatment variable of the corresponding dependent variable as a covariate. If the parameter did not approximate the normal distribution after base-e transformation, a nonparametric test was used (DI statistics using Mann-Whitney test). Statistical analysis was performed using SPSS 16.0 for Mac OS X (SPSS, Chicago, IL). All inferential statistical tests were conducted at a significance level of 0.05 (two-sided). Unless otherwise stated, data are presented as mean ± SEM.
RESULTS
Patient disposition and baseline clinical characteristics
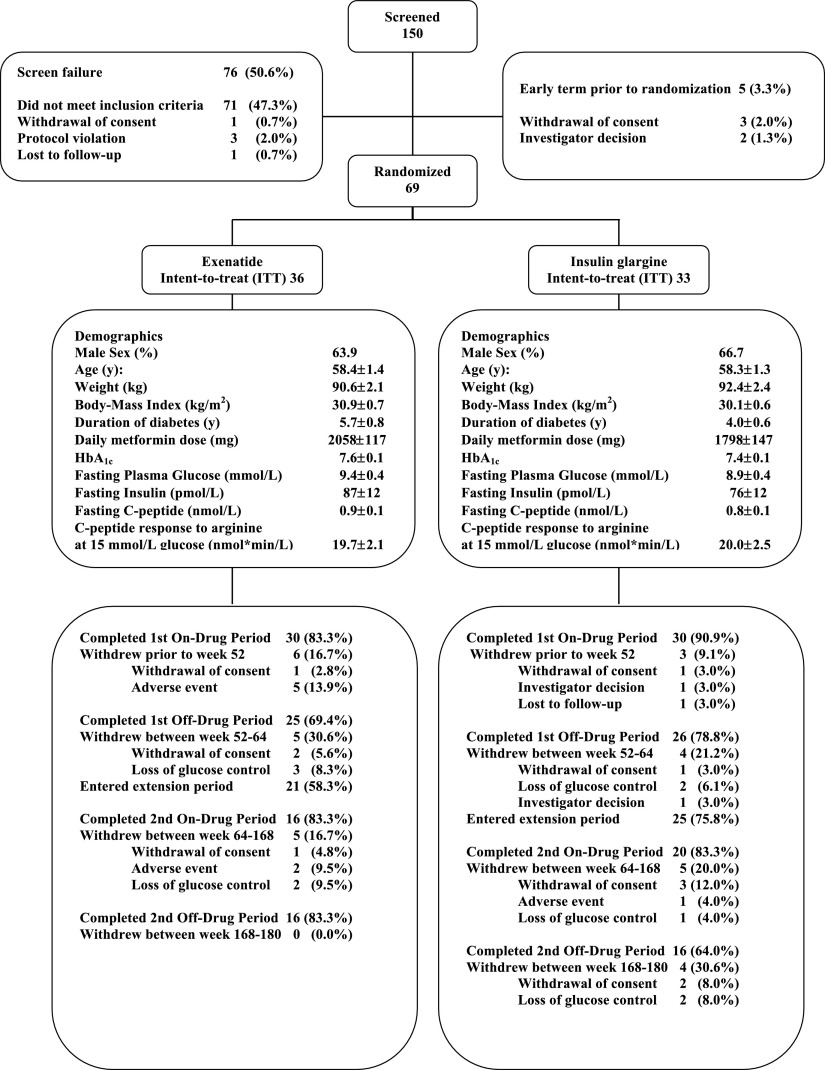
Extension phase patient disposition and baseline clinical characteristics are shown in Fig. 1. No significant between-treatment group differences were present at baseline. At the end of the 1-year main study phase, five patients withdrew their consent and did not participate in the extension phase (one in the GLAR group and four in the EXE group) because of the demanding study procedures. Thirty-six patients completed the 168-week treatment period. Of the patients randomized to EXE, 69% (n = 11) were treated with EXE 10 µg b.i.d. at 168 weeks of treatment. One (6.25%) patient was using 20 µg b.i.d., one 15 µg t.i.d., one a combination of 4 q.d./8 b.i.d., and one a combination of 6 q.d./8 q.d.. The daily EXE dose was reduced to 5 µg b.i.d. in one (6.25%) patient. At 168 weeks, the mean ± SEM daily GLAR dose used was 33.7 ± 4.0 units.
Figure 1.
Protocol flowchart and baseline characteristics of the study population. Data are mean ± SD.
Glycemic control
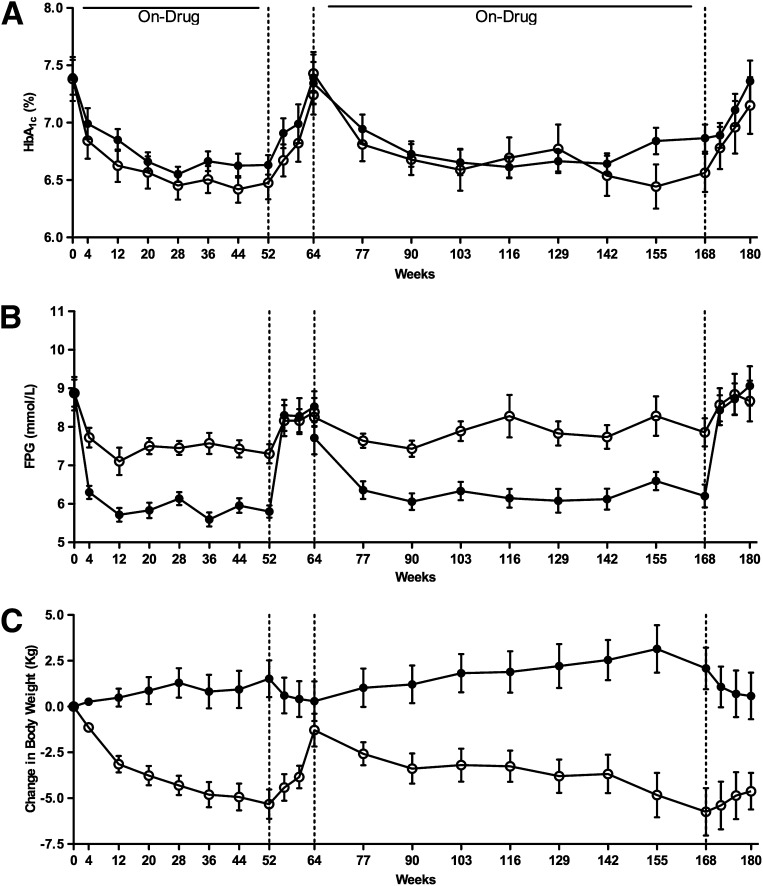
At 3 years, glycemic control was still comparable for EXE and GLAR treatment: the HbA1c values were 6.6 ± 0.2%, and 6.9 ± 0.2% at 168 weeks (between-group difference: P = 0.186) (Fig. 2A). Because of the treat-to-target titration, the GLAR group showed a significantly greater reduction in FPG as compared with EXE (−2.0 ± 0.4 vs. −0.2 ± 0.5 mmol/L; P < 0.0001, respectively) (Fig. 2B). After 12 weeks off-drug, both HbA1c and FPG increased in both groups to pretreatment values (Fig. 2A and B).
Figure 2.
Time course for HbA1c (A), fasting plasma glucose (B), and change in body weight (C). Data are mean (SEM). ○ = EXE; ● = GLAR. Vertical hatched lines at weeks 52, 64, and 168 represent cessation and restart of study medication.
Body weight and insulin sensitivity
After 168 weeks of treatment, EXE reduced body weight by −5.7 ± 1.3 kg, while treatment with GLAR resulted in a body weight increase of 2.1 ± 1.3 kg (between-group least squares mean difference, −7.9 ± 1.8 kg; P < 0.001) (Fig. 2C). During the 12-week off-drug period, body weight slightly trended back toward baseline values in both treatment groups, leaving a statistical significant difference at the end of the study in favor of EXE (between-group difference at week 180: −5.5 ± 1.7 kg; P = 0.004) (Fig. 2C).
Before randomization, whole-body insulin–mediated glucose uptake did not differ between the two treatment groups. Three-year treatment with EXE improved the M value by 39% (P = 0.006) while GLAR had no effect (P = 0.647).
Measures of β-cell function
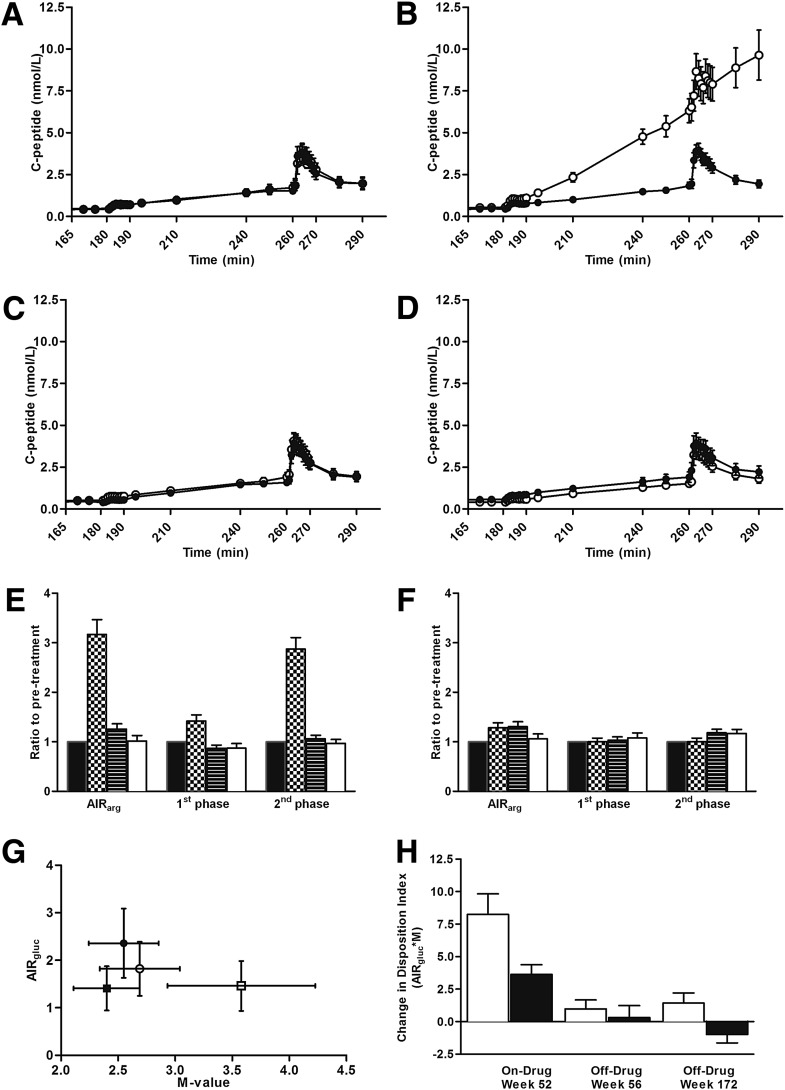
At week 0, both glucose- and arginine-stimulated C-peptide secretion did not significantly differ between the two treatment groups (Fig. 3A). EXE significantly improved β-cell function during 52 weeks of active treatment compared with titrated GLAR (Fig. 3B, E, and F). After 4-week cessation of both EXE and GLAR therapy, β-cell function returned to pretreatment values at week 56 (Fig. 3C, E, and F), as previously reported (7).
Figure 3.
β-Cell function parameters during 3 years of EXE (n = 16) and GLAR (n = 20) treatment. Serum C-peptide concentrations during hyperglycemic clamp are shown at week 0 (A), week 52 (B), week 56 (C), and week 172 (D). ○ = EXE; ● = GLAR. β-Cell secretory capacity ratio-to-pretreatment is shown in the EXE- (E) and GLAR-treated (F) groups. ■ = week 0 (pretreatment); ▦ = week 52 (on-drug); ▤ = week 56 (off-drug); □ = week 172 (off-drug). Mean DI the EXE- and GLAR-treated group (G). ■ = EXE week 0 (pretreatment); □ = EXE week 172 (off-drug); ● = GLAR week 0 (pretreatment); ○ = GLAR week 172 (off-drug). DI change from pretreatment (H). □ = EXE; ■ = GLAR. Data are mean (SEM) in A–D and G–H; geometric mean (SEM) in E–F. AIRarg, C-peptide response to arginine at 15 mmol/L glucose concentration; AIRgluc and 1st phase, first-phase C-peptide response to glucose; 2nd phase, second-phase C-peptide response to glucose. See Research Design and Methods section for calculations of β-cell function measures.
Following 3-year treatment with either EXE or GLAR and a 4-week off-treatment period, the glucose- and arginine-stimulated C-peptide secretion, as compared with baseline, remained similar: 1.02 ± 0.11 and 1.06 ± 0.10 for EXE and GLAR, relative to baseline, respectively (Fig. 3E and F; between-treatment group comparison P = 0.665). Interestingly, first- and second-phase glucose-stimulated C-peptide responses were significantly lower in the EXE-treated patients after 3 years of treatment when compared with GLAR (Fig. 3D): first phase relative to pretreatment EXE 0.88 ± 0.09, GLAR 1.08 ± 0.10, P = 0.038, and second phase relative to pretreatment EXE 0.97 ± 0.08, GLAR 1.17 ± 0.08, P = 0.017. However, the DI change from pretreatment showed a sustained effect on β-cell function 4 weeks after cessation of treatment in the EXE-treated patients, whereas a reduction was observed in the GLAR-treated patients (Fig. 3G and H: 1.43 ± 0.78 and −0.99 ± 0.65, respectively; between-group difference P = 0.028). This is in contrast to the 1-year data, where no sustained effect on the DI was observed after cessation of treatment. No statistical significant between-treatment group difference was observed in the DI calculated over the second-phase C-peptide secretion (36.89 ± 17.51 and 25.02 ± 14.24 for EXE and GLAR, respectively; between-group difference P = 0.763).
Adverse events and tolerability
During the extension phase, most common adverse events with EXE treatment were gastrointestinal in nature (42.9%) and mild to moderate in intensity: nausea 38.1%, vomiting 9.5%, abdominal distention 4.8%, and diarrhea 4.8%. In the EXE-treated group, two patients (9.5%) withdrew their consent as a result of nausea or vomiting. Nineteen percent of EXE-treated subjects experienced treatment-emergent minor hypoglycemia, defined as a self-measured blood glucose concentration <3.0 mmol/L. At week 168, 31% of EXE-treated subjects (5/16) had detectable anti-EXE antibody titers with the majority (4/5) of titers in the low range (<1/25 titer). The anti-EXE antibodies had no predictive effect on the magnitude of an individual’s glycemic response or the incidence of adverse events. Most reported adverse events with GLAR treatment were treatment-related minor hypoglycemia, 28%; gastrointestinal disorders, 16%; and vomiting, 8.0%. No major hypoglycemia and no treatment-related withdrawal because of hypoglycemia were observed in both the EXE and GLAR groups. In the GLAR-treated group, one patient (4.0%) withdrew his consent as a result of a cerebrovascular incident.
CONCLUSIONS
The main result of this 3-year follow-up study in patients treated with EXE is the sustained improvement in first-phase glucose-stimulated C-peptide secretion, adjusted for prevailing insulin sensitivity (the DI) 4 weeks after discontinuation of treatment. No significant effect was seen in the GLAR-treated patients despite achievement of similar glycemic control. Additionally, EXE treatment was associated with continued weight loss and improvement in whole-body insulin sensitivity. Both EXE and GLAR were generally well tolerated with nausea and minor hypoglycemia being the most frequently reported adverse event in the EXE and GLAR groups, respectively.
Although human data are not available, exposure to GLP-1 and GLP-1RA in the preclinical setting results in β-cell proliferation, islet neogenesis, and inhibition of β-cell apoptosis in (human) cell lines, primary rodent islets, and in vivo in different rodent species (13). It has therefore been hypothesized, although human islet-cell biology differs widely from that in rodents, that long-term GLP-1RA treatment may enhance β-cell mass or health in humans, thereby potentially modifying the progressive course of type 2 diabetes (14). The current study, particularly the 3-year data presented herein, reports that EXE treatment may lend some support to this idea, whereas following 1-year EXE exposure, the treatment-related improvement of β-cell function was lost after 4-week drug cessation (7).
The current 3-year treatment data show a small but statistically significant effect on the DI following a 4-week off-therapy period. Our results therefore suggest that a 3-year treatment with a GLP-1RA (such as EXE) is necessary to delineate an effect on β-cell function. This beneficial effect was not enough to sustain glycemic control. Additional factors, such as duration of type 2 diabetes and achieved glycemic control and body weight reduction, may play a role in the ultimate efficacy of the GLP-1RA. An even longer treatment or intervention at an earlier stage of the disease may be necessary given the chronic nature of the disease. Additionally, our results confirm findings in diabetic fatty Zucker rats that prolonged EXE treatment in humans does not result in tachyphylaxis (15).
Prolonged exposure to elevated glucose and lipid concentrations is detrimental to β-cell function (16). These combined glucolipotoxic effects result in impaired insulin secretion and β-cell apoptosis, and may contribute to the loss of β-cell function in the pathogenesis of type 2 diabetes (17). Our results show a similar reduction in hyperglycemia, i.e., glucose toxicity, in the EXE and GLAR groups after 3-year treatment. Although we did find a 0.2% greater HbA1c reduction in the EXE-treated group, this finding did not reach statistical significance because of the decreased number of participants left in the study. Since glycemic control was similar for both treatments, this improvement cannot be solely attributed to an improvement of glycemic control; therefore, a GLP-1RA–related factor should be considered.
In as much as β-cell function integrity is determined by the combined effects of variables related to β-cell stress versus β-cell health, it is important to note that 3-year EXE versus GLAR treatment resulted in body weight reduction of approximately 8 kg with concomitant improvement of insulin sensitivity. Body weight reduction per se has been shown to improve β-cell function in subjects with and without type 2 diabetes (18,19). Interestingly, the improvement in β-cell function reported in our article cannot be explained fully by the reduction in body weight alone. Most patients treated with EXE experienced a reduction in body weight. However, in about half of the patients treated with EXE, a combined improvement in body weight and DI was observed. Additionally, there appeared to be no statistical correlation between the body weight reduction and the DI improvement in both EXE- and GLAR-treated patients. Unfortunately, additional post hoc analysis is not possible because of the small sample size.
We recently demonstrated that EXE predominantly reduces trunkal fat mass, whereas lean body mass was not affected (20). The observed 39% improvement in M value may at least partly be because of a lowering of the (trunkal) body fat mass. Obesity-related insulin resistance is a key feature of type 2 diabetes and is associated with metabolic and cardiovascular complications (16). The landmark study by Zander et al. (21) was the first to report a beneficial effect of continuous GLP-1 infusion on clamp-measured β-cell function as well as insulin sensitivity in the presence of concomitant weight loss of 1.9 kg in spite of the mere 6-week duration. Subsequent clinical studies using the euglycemic hyperinsulinemic clamp, insulin-modified frequently sampled intravenous glucose tolerance test, or homeostasis model assessment of insulin resistance do not provide a clear view of the effects of GLP-1RA on insulin sensitivity and suggest that such an effect may be secondary to the weight reduction (22,23).
Because insulin sensitivity and β-cell secretory function are closely interrelated, it is essential to measure both when studying (long-term) therapeutic interventions that may affect insulin secretion and body weight (1). The DI adjusts for the interaction between changes in insulin sensitivity and insulin secretion as differences in insulin sensitivity must be balanced by reciprocal changes in insulin release in order to maintain glucose tolerance (and prevent hypoglycemia). In the case of EXE, given the observed weight loss–related improvement in insulin sensitivity (+39%), one may have expected that if β-cell secretory function had remained unaltered, the C-peptide response would rather decline. However, inasmuch as the C-peptide remained substantially unaltered (compared with pretreatment), one may conclude that β-cell function, following 3 years of EXE exposure, was improved (1). Additionally, no correlation was observed between treatment-related changes in DI and body weight (Supplementary Fig. A2). Recently, DeFronzo et al. (22) demonstrated a similar phenomenon following 20-week EXE monotherapy as compared with the combination of EXE and rosiglitazone (ROSI). The DI was similar in the EXE alone and EXE/ROSI groups, although the amount of insulin secreted in response to glucose alone or with arginine during the hyperglycemic clamp was markedly reduced in the group receiving EXE/ROSI combination therapy. These findings are in agreement with our results. Interestingly, DeFronzo et al. also showed an improvement in the DI calculated from the second-phase C-peptide secretion, a finding we did not observe in our study. In contrast to the former study, we did not administer EXE prior to the hyperglycemic clamp. This differential study design may account for the observed difference. Additionally, a DI that is not derived from the rapid C-peptide response to intravenous glucose may not present correct physiology (1,12).
The strength of our study is the long-term follow-up and the use of state-of-the-art gold standard methodology to quantify of insulin sensitivity and β-cell function in the study population. Additionally, as patients did not receive study medication prior to the hyperglycemic clamp at the end of the extension phase, the effects of a 3-year treatment period on β-cell health were measured rather than the acute effects of EXE administration. One limitation of our study is the relatively modest proportion of randomized patients who completed the entire study. Fifty-two percent (36/69) of all randomized patients completed the entire 3-year treatment period. Of the original 51 patients who completed the 64-week main study, 46 agreed to participate in the extension phase. From these, 78% completed the additional 2-year treatment period, with similar numbers remaining in both treatment arms. Most patients withdrew their consent and did not participate in the 2-year extension phase of the study because of the demanding nature of the study protocol including long-term follow-up, which included a total of 30 visits (during 3.5 years) to the study center. The proportion of patients who did enter the additional 2-year treatment period show characteristics comparable to those participating in large intervention studies in patients with type 2 diabetes (24,25). Only three patients discontinued the 2-year extension phase because of loss of glycemic control (EXE: n = 2; GLAR: n = 1). Finally, three patients dropped out as a result of an adverse event: one patient randomized to GLAR experienced an ischemic stroke, and two patients randomized to EXE dropped out as a result of nausea following reinitiating of EXE treatment during the first weeks of the extension period. Generally, both EXE and GLAR were well tolerated during the 3-year study period, and reported adverse events were mainly mild to moderate in intensity, confirming previous long-term study tolerability results (6). No renal function deterioration and no pancreatitis were observed. Interestingly, no patients randomized to EXE withdrew their consent during the 3-month off-drug period, whereas four patients in the GLAR group did. After cessation of GLAR, patients continued their SMBG measurements. Of the patients randomized to GLAR, 30.6% did not want to continue with the off-drug period of the study as they observed an increase in SMBG, confirming the important role SMBG plays in the treatment of patients with type 2 diabetes.
In conclusion, 3-year EXE treatment in metformin-treated patients with type 2 diabetes resulted in sustained improvement in β-cell function and progressive weight reduction. Long-term follow-up in a wide variety of patients at earlier stages of type 2 diabetes is needed to study possible disease modifying effects of GLP-1RA.
Supplementary Material
Acknowledgments
This study was sponsored by Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. The study was collectively initiated and designed by the investigators from the three study sites. The investigators had full access to the trial data and had control over the statistical analysis and interpretation of the study results.
B.E. has served on the advisory board and is a speaker for Eli Lilly Sweden AB. R.J.H. is an employee and stockholder of Eli Lilly and Company. During the study, R.J.H. was still employed at the VU University Medical Center. R.M.S. is an employee of Eli Lilly and Company. M.-R.T. is a speaker for Eli Lilly and Company. U.S. served on the advisory board and is a speaker for Amylin Pharmaceuticals, Inc. Through U.S., the Sahlgrenska University Hospital has received research grants from Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. H.Y.-J. serves as a consultant for Amylin Pharmaceuticals, Inc. Through M.-R.T. and H.Y.-J., the Helsinki University Central Hospital has received research grants from Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. M.D. is a consultant and speaker for Eli Lilly and Company. Through M.D., the VU University Medical Center in Amsterdam has received research grants from Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
M.C.B. collected and researched data, contributed to discussion, and wrote the manuscript. A.C. collected data and reviewed and edited the manuscript. B.E. collected data, contributed to discussion, and reviewed and edited the manuscript. R.J.H. researched data, contributed to discussion, and reviewed and edited the manuscript. R.M.S., M.-R.T., and H.Y.-J. contributed to discussion and reviewed and edited the manuscript. U.S. and M.D. researched data, contributed to discussion, and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank the patients for participating in the study.
Footnotes
Clinical trial reg. no. NCT00097500, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0291/-/DC1.
See accompanying editorial, p. 2133.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. UKPDS 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 4.Heine RJ, Diamant M, Mbanya JC, Nathan DM. Management of hyperglycaemia in type 2 diabetes: the end of recurrent failure? BMJ 2006;333:1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SL, Lee N, Thakurta S, Chan BKS. Exenatide efficacy and safety: a systematic review. Diabet Med 2009;26:837–846 [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 7.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yki-Järvinen H, Juurinen L, Alvarsson M, et al. Initiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care 2007;30:1364–1369 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 11.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D, Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 13.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 14.Wajchenberg BL. beta-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007;28:187–218 [DOI] [PubMed] [Google Scholar]
- 15.Gedulin BR, Smith P, Prickett KS, et al. Dose-response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia 2005;48:1380–1385 [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of type 2 diabetes. Biochem Soc Trans 2008;36:348–352 [DOI] [PubMed] [Google Scholar]
- 18.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in β-cell function in older men. J Clin Endocrinol Metab 2004;89:2704–2710 [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Banks MR, Patterson BW, Polonsky KS, Klein S. Weight loss therapy improves pancreatic endocrine function in obese older adults. Obesity (Silver Spring) 2008;16:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunck MC, Diamant M, Eliasson B, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Triplitt C, Qu Y, Lewis MS, Maggs DG, Glass LC. Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care 2010;33:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilsbøll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med 2008;25:152–156 [DOI] [PubMed] [Google Scholar]
- 24.Holman RR, Haffner SM, McMurray JJ, et al. ; NAVIGATOR Study Group. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–1476 [DOI] [PubMed] [Google Scholar]
- 25.Bosch J, Yusuf S, Gerstein HC, et al. ; DREAM Trial Investigators. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–1562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.