Abstract
OBJECTIVE
Long-term implications of childhood obesity and BMI change over the life course for risk of type 2 diabetes remain uncertain. The objective was to establish whether there are effects on adult glucose metabolism of 1) sensitive periods of BMI gain or 2) long duration of overweight and obesity.
RESEARCH DESIGN AND METHODS
Participants in the 1958 British birth cohort with child to adult BMI and glycosylated hemoglobin (HbA1c) at 45 years (n = 7,855).
RESULTS
Prevalence of type 2 diabetes or HbA1c ≥7 was 2%. BMI gains in child- and adulthood were associated with higher HbA1c: for every SD of 5-year BMI increase from 0 to 7 years, there was a 75% (95% CI 1.42–2.16) increased risk of HbA1c ≥7, increasing to a 4.7-fold (3.12–7.00) risk for the interval 23–33 years. Associations for BMI gain in adulthood were related to attained BMI but were independent for the longer period birth (or 7 years) to 45 years. Duration of obesity was also associated with HbA1c; compared with the never obese, those with childhood onset had a 23.9-fold risk (13.5–42.1) of HbA1c ≥7%; odds ratios were 16.0 (10.6–24.2) and 2.99 (1.77–5.03), respectively, for young and midadulthood onset. Similar trends by onset age were found in mean HbA1c levels and for onset of overweight. Those with the earliest age of onset had higher BMI and waist circumference at 45 years, which markedly explained the associations for onset age and HbA1c.
CONCLUSIONS
Excessive BMI gain across the life span and earlier onset of overweight/obesity are associated with impaired glucose metabolism, in part through attained adult BMI.
The global and societal implications of the type 2 diabetes epidemic are recognized increasingly, with reductions in life expectancy and increased ill-health burden. Thinness and accelerated weight gain in childhood (1–4) and weight gain in adulthood (5,6) have been found to affect glucose tolerance or risk of type 2 diabetes. Thus, weight gain over different life stages from early childhood is implicated in the development of type 2 diabetes, but uncertainty remains about whether some life stages are more influential than others. There is also some evidence that longer duration of adiposity may increase the risk of type 2 diabetes (6–9), from studies that are mostly limited to small samples (9) or to retrospective recall of weight from midadulthood (6,8). Evidence on duration of adiposity therefore lacks a firm foundation, although it is urgently needed given that a growing number of adults will be overweight or obese for long periods of their lives as a result of increased prevalence of childhood overweight in many populations (10). Large population-based studies with repeated adiposity measures are required to confirm or refute claims about the timing and duration of adiposity gain and to determine effect sizes. We therefore examined the influence of adiposity gain over the life course on glucose metabolism in midadulthood in a British population. Our aims were to establish whether there are 1) sensitive or critical periods of adiposity gain for glucose metabolism in midadulthood and 2) long-lasting effects of childhood overweight (or obesity), either in the absence of or with persistence (duration) of overweight (or obesity) into midadulthood.
RESEARCH DESIGN AND METHODS
Study sample
The 1958 cohort consists of 17,638 individuals born one week in March 1958 in England, Scotland, and Wales and enrolled at birth with 920 immigrants recruited during childhood; this is largely a white European population (98%). Survivors were seen at ages 7, 11, 16, 23, 33, 42, and 45 years. The 45-year survey included physical assessments and nonfasted venous blood collection, to which all participants gave written informed consent; ethical approval was given by the South-East Multi-Centre Research Ethics Committee. Details of the 45-year study are provided elsewhere (11). In brief, 11,971 cohort members who were still living in the U.K. and in contact with the study were invited to a clinical assessment at 45 years; 9,377 participated. These participants were broadly similar to the original birth population but with slight underrepresentation of some groups (e.g., those with poor cognitive ability) (12). Individuals with type 1 diabetes (59 self-reported at 42 years and 8 prescribed insulin at 45 years, British National Formulary code 060101) were excluded, leaving 9,310 participants, of whom 7,855 had glycosylated hemoglobin (HbA1c) and diabetes medication data at 45 years.
Measures
HbA1c was measured using high performance liquid chromatography and the results standardized to the Diabetes Control and Complications Trial as described previously (13). HbA1c was analyzed as a continuous variable and as two binary variables using cutoffs 7% (3 SDs above the mean) and 6% (2 SDs) (14). Individuals with type 2 diabetes were grouped with HbA1c ≥7. A total of 155 cases were identified from 1) self-reports at 42 years that a doctor had told them that they had “non–insulin-dependent diabetes that is controlled by diet or tablets” (n = 67), 2) oral antidiabetic drugs at 45 years (n = 108, British National Formulary code 060102), and 3) HbA1c ≥7 (n = 116). Thus, there was overlap of the three sources of identification, with only 13 individuals classified from self-report alone. Other studies found self-reports to agree well with information from other sources (15,16).
BMI was calculated at each age (kg/m2). Thinness, overweight, and obesity in childhood were defined using international BMI cutoffs (17); for adulthood, thinness (moderate) was defined as ≤17, overweight as ≥25, and obesity as ≥30 kg/m2. Age of onset of overweight was identified as the first age when BMI was defined as overweight (including obese); in a similar manner, categories were derived for obesity onset (details are given in table footnotes). Waist circumference at 45 years was measured midway between the costal margin and iliac crest to the nearest 1 mm. Birth weight for gestational age was calculated as birth weight standardized within gestational week. Family history of diabetes, ethnicity, social class in childhood, plus social class, education level, smoking and alcohol consumption (42 years), and total and HDL cholesterol (45 years), were considered as potential confounding factors.
Analysis
Main analyses examined associations between BMI and HbA1c using linear and logistic regression for HbA1c as a continuous or dichotomous (≥7, including type 2 diabetes) outcome, respectively. Models of HbA1c (continuous) were fitted using robust variance estimation and were adjusted for type 2 diabetes treatment. We tested whether associations between BMI and HbA1c differed for men and women using an interaction term for sex.
Sensitive or critical periods of BMI gain
Sex-specific SD scores (z scores) were calculated for BMI at each age because the distribution of BMI changes with age (Table 1). Birth weight for gestational age was also converted to a z score. To examine BMI gain over different age intervals, HbA1c outcomes were regressed on zBMI at each age, conditioned on zBMI at the previous age. For example, the model for the interval 7–11 years is specified as: HbA1c = a + b zBMI7 + c zBMI11. To allow for varying age intervals, we reparameterized this model so that increase of zBMI between each age is expressed as a rate of change per 5 years:
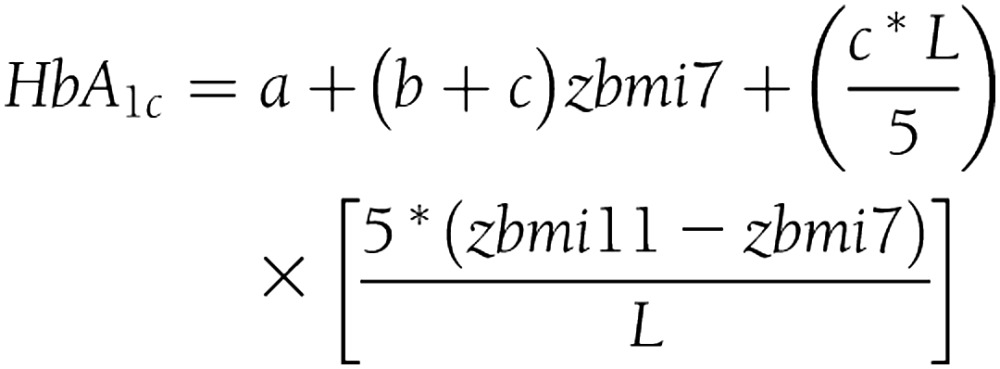 |
where L is the number of years (e.g., 7–11 years, L = 4). Thus, the coefficient (c*L/5) can be interpreted as the estimated change in HbA1c per SD increase in BMI per 5 years for the interval 7–11 years, given BMI at 7 years, and accordingly, representing the change in relative position in the distribution between ages. Odds ratios (ORs) estimated for dichotomous outcomes were similarly adjusted to allow for varying age intervals (i.e., ORL/5). To assess the effect of change in BMI over longer periods from the earliest ages to 45 years, we examined models of BMI at each age adjusted for BMI at 45 years: the model HbA1c = a + c zBMI7 + d zBMI45 was used to estimate change in HbA1c associated with an SD increase in BMI at 7 years. When rewritten as HbA1c = a − c (zBMI45 − zBMI7) + (c + d) zBMI45, we interpret − c as the estimated effect of an SD increase in BMI, 7–45 years, allowing for BMI at 45 years. If c is negative, this indicates that HbA1c will be higher in association with BMI gain (e.g., 7–45 years); if c is positive, HbA1c will be lower in association with BMI gain. All models were adjusted for sex, family history of diabetes, ethnicity, and social class in childhood and adulthood. To determine whether early BMI modifies the association between concurrent BMI and HbA1c, we examined associations between BMI at 45 years and HbA1c, stratified by BMI categories (i.e., lowest 10%, 10–50%, 50–90%, and highest 10%) in childhood with a test of the interaction between early and concurrent BMI.
Table 1.
Elevated HbA1c (%) and mean HbA1c at age 45 years by BMI at several ages, child- to adulthood (n = 7,855)
| n (%) | Mean (SD) | BMI at 45 years mean (SD) | HbA1c geometric mean (SD) | HbA1c ≥7‡ |
||
|---|---|---|---|---|---|---|
| n (%) | OR (95% CI) | |||||
| Men | 3,937 (50.1) | 27.7 (4.2) | 5.26 (0.64) | 95 (2.4) | — | |
| Women | 3,918 (49.9) | 26.9 (5.5) | 5.14 (0.64) | 60 (1.5) | — | |
| BMI at 7 years# | 15.8 (1.7) | |||||
| Thin | 140 (2.2) | 26.1 (4.6) | 5.23 (0.50) | 2 (1.4) | 0.9 (0.2–3.5) | |
| Normal | 5,631 (88.6) | 27 (4.6) | 5.19 (0.48) | 94 (1.7) | 1.0 | |
| Overweight | 480 (7.6) | 29.8 (5.9) | 5.27 (0.68) | 23 (4.8) | 3.0 (1.9–4.7) | |
| Obese | 104 (1.6) | 34.3 (7.7) | 5.31 (0.66) | 6 (5.8) | 3.6 (1.5–8.4) | |
| BMI at 11 years# | 17.3 (2.4) | |||||
| Thin | 92 (1.5) | 23.8 (3.7) | 5.21 (0.35) | 0 (0) | — | |
| Normal | 5,579 (89.6) | 26.9 (4.5) | 5.19 (0.46) | 83 (1.5) | 1.0 | |
| Overweight | 475 (7.6) | 31.5 (6.3) | 5.30 (0.68) | 26 (5.5) | 3.8 (2.4–6.0) | |
| Obese | 82 (1.3) | 33.7 (6.9) | 5.57 (1.11) | 10 (12.2) | 9.2 (4.6–18.4) | |
| BMI at 16 years# | 20.6 (2.7) | |||||
| Thin | 105 (1.8) | 23 (3.1) | 5.18 (0.35) | 1 (1) | 0.8 (0.1–5.5) | |
| Normal | 5,117 (89.4) | 26.8 (4.4) | 5.18 (0.43) | 64 (1.3) | 1.0 | |
| Overweight | 433 (7.6) | 32.3 (5.9) | 5.36 (0.77) | 29 (6.7) | 5.7 (3.6–8.9) | |
| Obese | 71 (1.2) | 37.3 (7.9) | 5.89 (1.49) | 18 (25.4) | 26.8 (14.9–48.3) | |
| BMI at 23 years§ | 22.5 (2.9) | |||||
| Thin | 52 (0.8) | 21 (3.1) | 5.11 (0.29) | 0 (0) | — | |
| Normal | 5,638 (84.2) | 26.3 (3.9) | 5.16 (0.41) | 53 (0.9) | 1.0 | |
| Overweight | 856 (12.8) | 32.2 (4.7) | 5.35 (0.71) | 42 (4.9) | 5.4 (3.6–8.2) | |
| Obese | 147 (2.2) | 39.4 (6.1) | 5.85 (1.27) | 31 (21.1) | 28.2 (17.4–45.5) | |
| BMI at 33 years§ | 24.9 (4.1) | |||||
| Thin | 28 (0.4) | 22.7 (3.5) | 5.16 (0.35) | 0 (0) | — | |
| Normal | 4,019 (58.4) | 24.8 (3) | 5.12 (0.35) | 16 (0.4) | 1.0 | |
| Overweight | 2,191 (31.9) | 29.5 (3.4) | 5.23 (0.50) | 37 (1.7) | 4.3 (2.4–7.7) | |
| Obese | 640 (9.3) | 35.8 (5.6) | 5.54 (0.95) | 68 (10.6) | 29.7 (17.1–51.6) | |
| BMI at 45 years§ | 27.3 (4.9) | |||||
| Thin | 8 (0.1) | 16.4 (0.8) | 5.13 (0.28) | 0 (0) | — | |
| Normal | 2,733 (34.8) | 22.7 (1.7) | 5.11 (0.35) | 11 (0.4) | 1.0 | |
| Overweight | 3,236 (41.2) | 27.3 (1.4) | 5.17 (0.42) | 37 (1.1) | 2.9 (1.5–5.6) | |
| Obese | 1,868 (23.8) | 34.1 (4) | 5.40 (0.74) | 105 (5.6) | 14.7 (7.9–27.5) | |
All trends are statistically significant (P < 0.001) across categories of BMI.
‡Includes known type 2 diabetes.
#BMI in childhood classified using international age and sex-specific cutoffs (17,25): thinness (moderate, i.e., grade 2), respectively, for ages 7, 11, and 16 years as 13.08, 13.72, and 16.08 kg/m2 for boys and 12.91, 13.79, and 16.44 kg/m2 for girls; overweight, respectively, as ≥17.92, ≥20.55, and ≥23.90 kg/m2 for boys and ≥17.75, ≥20.74, and ≥24.37 kg/m2 for girls; and obesity, respectively, as ≥20.63, ≥25.10, and ≥28.88 kg/m2 for boys and ≥20.51, ≥25.42, and ≥29.43 kg/m2 for girls.
§BMI in adulthood, cutoff for thinness (grade 2) is ≤17 kg/m2; for overweight, ≥25 kg/m2; and for obesity, ≥30 kg/m2.
Age of onset (overweight or obesity)
We examined associations between age of obesity onset and HbA1c using “never obese” as the reference group. Trends across onset groups representing increasing duration of obesity were tested. Analyses were repeated for onset of overweight, including obesity. Models were adjusted for family history of diabetes, social class in childhood and adulthood, education, smoking, alcohol consumption, menopausal status, total and HDL cholesterol, BMI, and waist circumference.
Missing data
To minimize any bias associated with missing data and to maximize precision of results, we used the multiple imputation by chained equations (MICE) procedure in STATA version 10.1. A total of 10 imputed datasets were created using 20 cycles per imputation. Variables in imputation models included all model variables plus key predictors of missing data previously identified: social class at birth and in adulthood and cognitive ability and behavior problems at 7 years (12). BMI z scores and onset variables were derived from imputed BMI within each dataset; distributions were similar for observed and imputed values. We compared results from multiple imputations to those from complete case analyses and found similar results. In general, associations for complete case analyses were stronger with wider CIs, although adjustment for concurrent adiposity reduced associations to a similar degree. Imputed results are presented.
All analyses for elevated HbA1c were repeated using a lower threshold (≥6, including type 2 diabetes) to confirm the pattern of results in a larger group, as presented in the Supplementary Tables.
RESULTS
Of 45-year-olds, 2% had HbA1c ≥7% or had been diagnosed with type 2 diabetes (Table 1). A further 2.0% had HbA1c 6–6.9%. Men had higher HbA1c than women (median = 5.2 and 5.1, respectively) and more had HbA1c ≥7%. For all ages, those who were overweight or obese had more than double the risk of elevated HbA1c, and there were trends in HbA1c levels across categories of BMI. Associations were found to be similar for men and women and, thus, combined analyses are presented (adjusted for sex).
Change in BMI and adult HbA1c
For each separate age interval, an increase in BMI was associated with higher HbA1c at 45 years, after allowing for BMI at the start of the interval, which strengthened with age (Table 2). For example, from 0 to 7 years, for every SD of 5-year BMI gain, there was a 78% increased risk of HbA1c ≥7, which strengthened to a fivefold risk for 23–33 years. An exception was for 33–45 years, where no association was found for HbA1c ≥7, although BMI gain was associated with higher mean HbA1c and a fourfold increased risk for the lower threshold of HbA1c ≥6 (Supplementary Table 1). This exception may be the result of weight loss related to diabetes onset for some individuals; individuals with type 2 diabetes increased on average from 31.6 to 32.9 kg/m2 at age 33–45 years, compared with 24.9 to 27.3 kg/m2 among others. When associations were examined for BMI change over longer periods, we found that allowing for concurrent (45-year) BMI gain 0–45 years and 7–45 years was associated with higher HbA1c by 0.02 for every SD increase in BMI, but there was no effect on HbA1c ≥7 (Table 2). From 11 years onward, BMI gain was associated with reduced risk of HbA1c ≥7, given concurrent BMI; for example, the OR was 0.39 (95% CI 1–2.56) for a 1 SD increase in BMI, 33–45 years. In analyses of the association between concurrent BMI and HbA1c, stratified by childhood BMI, there was some evidence of a strengthening association from the lowest to the highest BMIs at 11 years but no modifying effect for HbA1c ≥7 (Supplementary Table 2).
Table 2.
ORs for elevated HbA1c ≥7 at 45 years and difference in HbA1c (%) per SD of 5-year BMI change during different life periods adjusted for baseline BMI and per SD of BMI at each age adjusted for BMI at 45 years (n = 7,855)
| HbA1c ≥7† OR (95% CI) | HbA1c§ difference (95% CI) | |
|---|---|---|
| BMI change between ages (years) adjusted for baseline BMI | ||
| Birth to 7 | 1.75 (1.42–2.16) | 0.029 (0.008–0.050) |
| 7–11 | 1.66 (1.45–1.90) | 0.040 (0.020–0.061) |
| 11–16 | 2.06 (1.69–2.51) | 0.042 (0.013–0.070) |
| 16–23 | 2.99 (2.31–3.87) | 0.095 (0.063–0.127) |
| 23–33 | 4.67 (3.12–7.00) | 0.205 (0.142–0.268) |
| 33–45 | 1.24 (0.74–2.07) | 0.155 (0.092–0.218) |
| BMI SD score, adjusted for BMI at age 45 years, for ages (years) | ||
| 0 | 0.90 (0.75–1.07) | −0.024 (−0.037 to −0.011) |
| 7 | 1.02 (0.86–1.21) | −0.015 (−0.030 to 0.000) |
| 11 | 1.21 (1.03–1.43) | −0.005 (−0.023 to 0.013) |
| 16 | 1.44 (1.23–1.68) | −0.002 (−0.025 to 0.021) |
| 23 | 1.83 (1.52–2.21) | 0.004 (−0.019 to 0.027) |
| 33 | 2.56 (2.08–3.14) | 0.048 (0.012–0.085) |
All models adjusted for sex, social class in childhood and at 42 years, family history of diabetes, and ethnicity. Results estimated using multiple imputation.
†Includes diagnosed type 2 diabetes.
§Models control for type 2 diabetes treatment.
Age of (overweight or obesity) onset
Of the population, ∼25% were obese by 45 years, most (59%) with onset in midadulthood, whereas 7.6% were obese from childhood (Table 3). There was an increasing trend in mean BMI at 45 years with longer obesity duration; waist circumference also differed, by almost 10 cm on average, between child- and midadulthood onset groups. Duration of obesity was associated also with all HbA1c outcomes (trend P < 0.05); for example, those with childhood onset had a 24-fold increased risk of HbA1c ≥7 versus a 3-fold risk for midadulthood onset (Table 3). Adjustment for adiposity and other factors substantially reduced, but did not entirely eliminate, the associations and trend for HbA1c ≥7. For those obese in childhood, only a fivefold increased risk of HbA1c ≥7 persisted after adjustment. Similar trends were found for HbA1c ≥6; however, the association for onset of obesity in childhood was almost abolished after adjustment (Supplementary Table 3). Mean HbA1c levels were also higher with longer duration of obesity, with substantial reductions after adjustment, but individuals with childhood obesity only did not have higher levels. An estimated 68% of the population was overweight by 45 years, with onset commonly in early adulthood or in childhood (Table 3). Patterns of results were similar, albeit weaker, when duration of overweight was examined; hence, associations were largely abolished after adjustment.
Table 3.
Association between age of onset of overweight or obesity and adult HbA1c: ORs for elevated HbA1c ≥7 and regression coefficients for change in HbA1c (%)
| Duration/age of onset (N = 7,855)† | n (%)‡ | BMI at 45 years (kg/m2) mean (95% CI)‡ | Waist circumference (cm) mean (95% CI)‡ | HbA1c ≥7* OR (95% CI) |
HbA1c coefficient (95% CI)§ |
||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted$ | Unadjusted | Adjusted$ | ||||
| Obesity | |||||||
| Never | 5,819 (74.1) | 25.1 (25.0–25.2) | 86.9 (86.6–87.2) | — | — | — | — |
| Childhood only | 62 (0.8) | 26.3 (25.5–27.0) | 90.6 (87.9–93.4) | 7.01 (1.89–25.89) | 4.95 (1.30–18.93) | 0.034 (−0.147 to 0.214) | 0.012 (−0.158 to 0.181) |
| Onset in midadulthood | 1,171 (14.9) | 32.5 (32.3–32.7) | 103.5 (102.8–104.1) | 2.99 (1.77–5.03) | 1.13 (0.61–2.08) | 0.151 (0.112–0.189) | 0.026 (−0.026 to 0.078) |
| Onset in young adulthood | 652 (8.3) | 35.2 (35.0–35.5) | 109.2 (108.3–110.0) | 16.04 (10.63–24.17) | 3.96 (2.10–7.43) | 0.280 (0.211–0.349) | 0.115 (0.032–0.199) |
| Onset in childhood | 151 (1.9) | 37.8 (37.2–38.4) | 113.2 (111.4–115.1) | 23.86 (13.52–42.14)# | 4.38 (1.86–10.31)# | 0.363 (0.173–0.554)# | 0.170 (−0.009 to 0.348)# |
| Overweight (including obesity) | |||||||
| Never | 2,366 (30.1) | 22.6 (22.5–22.7) | 80.0 (79.5–80.4) | — | — | — | — |
| Childhood only | 163 (2.1) | 22.6 (22.1–23.2) | 78.1 (76.4–79.7) | — | — | −0.040 (−0.100 to 0.021) | −0.035 (−0.094 to 0.023) |
| Onset in midadulthood | 1,729 (22.0) | 27.2 (27.1–27.4) | 92.2 (91.7–92.6) | 1.88 (0.66–5.33) | 0.77 (0.27–2.19) | 0.044 (0.022–0.066) | −0.083 (−0.117 to −0.050) |
| Onset in young adulthood | 2,396 (30.5) | 29.9 (29.8–30.1) | 99.2 (98.7–99.6) | 8.63 (3.85–19.32) | 1.87 (0.79–4.45) | 0.112 (0.083–0.141) | −0.088 (−0.133 to −0.044) |
| Onset in childhood | 1,201 (15.3) | 32.1 (31.9–32.3) | 101.4 (100.8–102.1) | 21.63 (9.77–47.85)# | 3.25 (1.30–8.14)# | 0.183 (0.134–0.231)# | −0.059 (−0.116 to −0.001)# |
*Type 2 diabetes or HbA1c ≥7.
§Models control for type 2 diabetes treatment.
†Age at onset of obesity/overweight defined as: never (BMI less than World Health Organization cutoff at all ages); childhood only (BMI greater than or equal to cutoff at 7, 11, or 16 years but not at 23, 33, and 45 years); onset in childhood or adolescence (BMI greater than or equal to cutoff at 7, 11, or 16 years plus 23, 33, or 45 years); onset in young adulthood (BMI greater than or equal to cutoff at 23 or 33 years but not in childhood); or onset in midadulthood (BMI greater than or equal to cutoff at 45 years only). BMI in childhood classified using international age and sex-specific cutoffs (17, 25): thinness (moderate, i.e., grade 2), respectively, for ages 7, 11, and 16 years as 13.08, 13.72, and 16.08 kg/m2 for boys and 12.91, 13.79, and 16.44 kg/m2 for girls; overweight, respectively, as ≥17.92, ≥20.55, and ≥23.90 kg/m2 for boys and ≥17.75, ≥20.74, and ≥24.37 kg/m2 for girls; and obesity, respectively, as ≥20.63, ≥25.10, and ≥28.88 kg/m2 for boys and ≥20.51, ≥25.42, and ≥29.43 kg/m2 for girls.
‡Averaged across imputed datasets.
$Sex, total cholesterol, HDL cholesterol, family history of diabetes, menopausal status, smoking at 42 years, alcohol consumption at 42 years, social class in childhood, social class at 45 years, and qualifications by 42 years.
#Linear trend from never to onset in midadulthood P < 0.05 (tested including and excluding category of childhood only).
CONCLUSIONS
Our population-based study of BMI from early life and adult glucose metabolism provides three main findings. First, excessive BMI gain at all life stages was associated with elevated HbA1c levels, cumulatively across the life span. When concurrent (45-year) BMI is taken into account, associations for adult BMI gain appeared to be largely due to their effect on attained BMI, whereas BMI gain from the first decade of life remained associated with mean HbA1c levels and HbA1c ≥6%, although not HbA1c ≥7%. Second, adults with overweight or obesity onset in childhood or young adulthood had the highest mean BMIs and waist circumference at 45 years and greatest risk of elevated HbA1c. Childhood obesity onset was associated with an almost 24-fold risk of HbA1c ≥7% compared with the never obese, and there was a 22-fold risk for the more prevalent group with childhood overweight onset. Associations between earlier onset of overweight or obesity and adult HbA1c levels were largely due to the greater adiposity at 45 years of those with earlier onset. These findings are important given recent increasing trends in overweight and obesity in many countries (10). Young children today are overweight earlier in their lives than previous generations, including our cohort. Third, for a minority of overweight children who were not overweight in adulthood, mean levels and risk of HbA1c ≥6% were not elevated, suggesting that detrimental effects of childhood overweight can be averted if BMI gain with increasing age can be controlled. However, those who were obese in childhood and not thereafter had a fivefold risk of HbA1c ≥7% or type 2 diabetes.
Glucose metabolism is indicated by HbA1c (13,16) because it was impractical to obtain fasting samples in this working-age population, resident throughout the U.K. Reassuringly, prevalence of type 2 diabetes is comparable to other U.K. studies using different data ascertainment methods (13), although at 45 years this is an uncommon outcome. A threshold of 7% is associated with a diagnosis of type 2 diabetes based on the oral glucose tolerance test (18); although a continuum of risk has been demonstrated for type 2 diabetes (16), and for cardiovascular disease and mortality (19), justifying analysis of HbA1c as a continuous variable. Regarding clinical significance, others have shown that among women with baseline HbA1c ≥7 and 6.0–6.9%, respectively, 81.1 and 60% developed diabetes within 10 years’ follow-up (16). Our results for HbA1c ≥7% were confirmed for a larger group with HbA1c ≥6%. BMI change was assessed using repeated prospective measures rather than the retrospective reports of some previous studies (5,6,8,20). A further advantage is prospectively measured birth weight, to assess body size changes from birth to 7 years, although our lack of early life measures is a limitation. Some bias in the participating adult sample has arisen in association with loss to follow-up, although less so for factors examined here than for others (12). A further source of missing data is item nonresponse; hence, we conducted analyses using imputation.
Changes in BMI, child- to adulthood
Studies of type 2 diabetes have mainly focused on weight or BMI change during child- or adulthood, and such studies cannot elucidate whether some ages are more important than others. Our study of multiple intervals over an individual’s lifetime shows that excessive BMI gain at every life stage was associated with HbA1c levels at 45 years. Because we allowed for BMI at the beginning of each age interval, our analyses separate associations for BMI gain during each interval from prior BMI. Strong effects of adult adiposity gain are suggested by the literature. For example, those 40- to 59-year-old men gaining >10% of their weight had a two- to threefold increased risk of type 2 diabetes compared with men whose weight remained stable (6). With risk of HbA1c ≥7% of more than fourfold for an SD gain in BMI per 5 years over the interval 23–33 years, our study confirms the importance of adult adiposity gain for risk of diabetes. As reported elsewhere (21), our findings suggest that adult BMI gains are associated with glucose metabolism via BMI attained in midadulthood. Indeed, lower HbA1c was observed for some life stages, after we had taken account of concurrent BMI, which may be partly the result of disease-related weight loss. (In the absence of HbA1c measurement at earlier life stages, we are unable to assess this possibility.) But higher HbA1c associated with BMI gains over longer periods, from the first decade of life to 45 years, was not because of concurrent BMI. Despite the lack of an association for HbA1c ≥7%, our study suggests that BMI gain in the first decade may be important outside any influence of gain on adult adiposity. Thus, our results are compatible with others’ (2,4) suggesting that accelerated weight gain in childhood influences diabetes risk, even in the absence of obesity (2), although we found no support for associations with thinness in childhood (1). We were unable to examine narrow intervals to test the argument that rapid weight gain in the first 2 weeks of life is a sensitive period for risk of diabetes (22). Yet we previously demonstrated an inverse association of birth weight with diabetes risk and HbA1c levels in this population, showing the greater risk among those with small size at birth to be dependent on subsequent weight gain (23,24). Our findings for BMI gain during different life stages may correspond to different changes in body composition, with changes in lean mass possibly more involved in BMI change in childhood than in adulthood.
Duration of adiposity
A dose-response relationship was found for overweight and obesity duration with elevated HbA1c in midadulthood; associations strengthened from those with the shortest to the longest duration, the latter identified as onset from childhood. Our study therefore adds to previous studies suggesting an association between overweight duration and diabetes or impaired glucose tolerance (9). Our findings are also consistent with the possibility that those with longer overweight duration have greater abdominal obesity (8). Because elevated levels of HbA1c associated with longer duration of overweight or obesity were largely attenuated after adjustment for current BMI and waist circumference, our study suggests that alterations in glucose metabolism for this group are primarily the result of their greater adiposity. Positive associations were markedly reduced but persisted for HbA1c ≥7 after adjustment for several factors, including current adiposity, suggesting that there may be some residual confounding or alternatively, some effect of adiposity duration. Our findings from stratified analysis, suggesting a strengthening association between concurrent BMI and HbA1c, from the lowest to the highest childhood BMIs, may be relevant here. These results (although not seen for HbA1c ≥7) provide some support for an effect of longer duration on glucose metabolism. It has been argued that duration of obesity decreases glucose tolerance by way of progressively increased insulin resistance and with long duration, with decreased insulin secretion (20), but underlying biological mechanisms remain unclear.
It is important that for a minority of overweight children who were not overweight later in life, HbA1c was not elevated relative to those who had never been overweight. A similar finding has been reported for metabolic syndrome, although using lower cutoffs for overweight (7). Our study suggests that outcome depends on BMI threshold, with a fivefold risk of elevated HbA1c among those with obesity in childhood only, suggesting a long-lasting risk for impaired glucose metabolism even in the absence of adult obesity. This finding could be due to overweight in adulthood (72% at 45 years); although adjustment for adiposity at 45 years did not explain the association, and other factors in childhood may be important. If confirmed in other studies, this finding argues for primary prevention in childhood.
Associations observed in this study suggest that there are benefits of delaying onset of overweight and obesity, in that risks of elevated HbA1c were lower for 45-year-olds becoming overweight or obese in the previous 12 years than for earlier onset. Arguably, therefore, interventions to prevent obesity are best targeted in childhood. Moreover, normal weight groups in our study population who gained BMI excessively were at risk for elevated HbA1c, albeit via their attained BMI, suggesting that weight gain across the full distribution should be a focus for preventive strategies for type 2 diabetes.
Supplementary Material
Acknowledgments
This survey was supported by a grant from the Medical Research Council (G0000934). The study was undertaken by the University College London (UCL) Institute of Child Health and was supported initially by the Secretary of State for Health, Department of Health, England (National Health Service Research & Development Programme) and latterly by the Department of Health Policy Research Programme through the Public Health Research Consortium (PHRC). The views expressed herein are those of the authors and not necessarily those of the Department of Health. Information about the wider program of the PHRC is available from www.york.ac.uk/phrc. The Great Ormond Street Hospital/UCL Institute of Child Health was supported in part by the Department of Health’s National Institute for Health Research Biomedical Research Centre. The Centre for Paediatric Epidemiology and Biostatistics was supported in part by the Medical Research Council in its capacity as the MRC Centre of Epidemiology for Child Health.
No potential conflicts of interest relevant to this article were reported.
C.P. designed the study, interpreted results, undertook analysis, wrote the manuscript, and reviewed and edited the manuscript. C.T. designed the study, interpreted results, undertook analysis, and reviewed and edited the manuscript.
Leah Li and Catherine Law at the UCL Institute of Child Health gave useful comments on an earlier draft. The authors are grateful to participants in the 2002–2004 clinical follow-up of the 1958 birth cohort.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1482/-/DC1.
References
- 1.de Lauzon-Guillain B, Balkau B, Charles M-A, Romieu I, Boutron-Ruault M-C, Clavel-Chapelon F. Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale (E3N) Cohort. Diabetes Care 2010;33:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava SK, Sachdev HS, Fall CHD, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev HP, Osmond C, Fall CH, et al. Predicting adult metabolic syndrome from childhood body mass index: follow-up of the New Delhi birth cohort. Arch Dis Child 2009;94:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 2000;133:176–182 [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care 1999;22:1266–1272 [DOI] [PubMed] [Google Scholar]
- 7.Vanhala M, Vanhala P, Kumpusalo E, Halonen P, Takala J. Relation between obesity from childhood to adulthood and the metabolic syndrome: population based study. BMJ 1998;317:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol 2004;14:585–591 [DOI] [PubMed] [Google Scholar]
- 9.Sakurai Y. Duration of obesity and risk of non-insulin-dependent diabetes mellitus. Biomed Pharmacother 2000;54:80–84 [DOI] [PubMed] [Google Scholar]
- 10.Lobstein T, Baur L, Uauy R; IASO International Obesity Task Force. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(Suppl. 1):4–104 [DOI] [PubMed] [Google Scholar]
- 11.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol 2006;35:34–41 [DOI] [PubMed] [Google Scholar]
- 12.Atherton K, Fuller E, Shepherd P, Strachan DP, Power C. Loss and representativeness in a biomedical survey at age 45 years: 1958 British birth cohort. J Epidemiol Community Health 2008;62:216–223 [DOI] [PubMed] [Google Scholar]
- 13.Thomas C, Hyppönen E, Power C. Diabetes risk in British adults in mid life: a national prevalence study of glycated haemoglobin. Diabet Med 2007;24:317–321 [DOI] [PubMed] [Google Scholar]
- 14.Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemia for the diagnosis of type 2 diabetes mellitus. Ann Intern Med 2002;137:263–272 [DOI] [PubMed] [Google Scholar]
- 15.Harlow SD, Linet MS. Agreement between questionnaire data and medical records. The evidence for accuracy of recall. Am J Epidemiol 1989;129:233–248 [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 2007;120:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters AL, Davidson MB, Schriger DL, Hasselblad V; Meta-analysis Research Group on the Diagnosis of Diabetes Using Glycated Hemoglobin Levels. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. JAMA 1996;276:1246–1252 [PubMed] [Google Scholar]
- 19.Khaw K-T, Wareham NJ, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson S, Persson PG, Alvarsson M, et al. Weight history, glucose intolerance, and insulin levels in middle-aged Swedish men. Am J Epidemiol 1998;148:539–545 [DOI] [PubMed] [Google Scholar]
- 21.Jacobs-van der Bruggen MA, Spijkerman A, van Baal PH, et al. Weight change and incident diabetes: addressing an unresolved issue. Am J Epidemiol 2010;172:263–270 [DOI] [PubMed] [Google Scholar]
- 22.Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 2003;361:1089–1097 [DOI] [PubMed] [Google Scholar]
- 23.Hyppönen E, Power C, Smith GD. Prenatal growth, BMI, and risk of type 2 diabetes by early midlife. Diabetes Care 2003;26:2512–2517 [DOI] [PubMed] [Google Scholar]
- 24.Thomas C, Hyppönen E, Power C. Prenatal exposures and glucose metabolism in adulthood: are effects mediated through birth weight and adiposity? Diabetes Care 2007;30:918–924 [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007;335:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


