Abstract
OBJECTIVE
The cannabinoid receptor type 2 (CB2) has protective effects in chronic degenerative diseases. Our aim was to assess the potential relevance of the CB2 receptor in both human and experimental diabetic nephropathy (DN).
RESEARCH DESIGN AND METHODS
CB2 expression was studied in kidney biopsies from patients with advanced DN, in early experimental diabetes, and in cultured podocytes. Levels of endocannabinoids and related enzymes were measured in the renal cortex from diabetic mice. To assess the functional role of CB2, streptozotocin-induced diabetic mice were treated for 14 weeks with AM1241, a selective CB2 agonist. In these animals, we studied albuminuria, renal function, expression of podocyte proteins (nephrin and zonula occludens-1), and markers of both fibrosis (fibronectin and transforming growth factor-β1) and inflammation (monocyte chemoattractant protein-1 [MCP-1], CC chemokine receptor 2 [CCR2], and monocyte markers). CB2 signaling was assessed in cultured podocytes.
RESULTS
Podocytes express the CB2 receptor both in vitro and in vivo. CB2 was downregulated in kidney biopsies from patients with advanced DN, and renal levels of the CB2 ligand 2-arachidonoylglycerol were reduced in diabetic mice, suggesting impaired CB2 regulation. In experimental diabetes, AM1241 ameliorated albuminuria, podocyte protein downregulation, and glomerular monocyte infiltration, without affecting early markers of fibrosis. In addition, AM1241 reduced CCR2 expression in both renal cortex and cultured podocytes, suggesting that CB2 activation may interfere with the deleterious effects of MCP-1 signaling.
CONCLUSIONS
The CB2 receptor is expressed by podocytes, and in experimental diabetes, CB2 activation ameliorates both albuminuria and podocyte protein loss, suggesting a protective effect of signaling through CB2 in DN.
Diabetic nephropathy (DN) is characterized by increased glomerular permeability to proteins and excessive extracellular matrix deposition in the mesangium, eventually resulting in glomerulosclerosis and progressive renal impairment (1).
Both hyperglycemia and hypertension are established key determinants in the development of DN (2). In addition, increasing evidence suggests that a low-grade inflammatory response also plays a role. In particular, binding of the chemokine monocyte chemoattractant protein 1 (MCP-1) to the CC chemokine receptor 2 [CCR2] receptor, expressed by monocytes and glomerular resident cells, appears to contribute to both increased glomerular permeability and glomerulosclerosis (3–6).
The endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are synthesized on demand from arachidonic acid–containing phospholipid precursors and bind to G protein–coupled cannabinoid receptors. The cannabinoid receptor type 1 (CB1) is predominantly expressed in the central nervous system (7), but we have recently reported that glomerular podocytes overexpress this receptor in experimental diabetes and that CB1 blockade ameliorates albuminuria by preventing loss of podocyte proteins important in preserving glomerular permselectivity (8).
Endocannabinoids also bind to the cannabinoid receptor type 2 (CB2), which is present primarily in cells of the immune system (9). Recent studies have shown that CB2 receptors are also expressed by other cell types, such as nonparenchymal liver cells, cardiomyocytes, vascular smooth muscle cells, and endothelial cells (10–13). Furthermore, there is in vivo evidence that CB2 activation has beneficial effects in animal models of chronic degenerative diseases, such as atherosclerosis and liver fibrosis (10,14,15), by reducing inflammatory, oxidative, and fibrotic processes. This raises the hypothesis that CB2 receptor activation may have a protective role in DN, counteracting the deleterious effects of signaling through CB1 receptors.
To test this hypothesis we studied CB2 expression in kidney biopsies from patients with advanced DN, in early experimental diabetes, and in cultured podocytes. Furthermore, we assessed the effect of CB2 activation in streptozotocin (STZ)-induced diabetic mice.
RESEARCH DESIGN AND METHODS
Materials.
All materials were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Human kidney biopsies.
The study was performed on nine renal biopsies from diabetic patients with overt nephropathy (persistent proteinuria >0.5 g/24 h) and normal kidney portions of eight control subjects who underwent surgery for hypernephromas and did not have proteinuria or glomerular abnormalities, as detected by light and immunofluorescence microscopy. The study was approved by the ethical committee of Genoa University, the procedures were in accordance with the Helsinki Declaration, and informed consent was obtained from all subjects. Patient biopsies presented classic histological features of DN. One biopsy, showing severe nephroangiosclerotic abnormalities without typical glomerular diabetic damage, was excluded. Control subjects were selected to be comparable for age and sex, and individuals with diabetes and/or hypertension were excluded. Hypertension was defined as a blood pressure ≥140/90 mmHg on at least three different occasions. Diabetic retinopathy was assessed by direct funduscopic examination. Twenty-four–hour urinary protein content was measured using the pyrogallol-red method in three separate urine collections, plasma creatinine by the kinetic Jaffé method, and HbA1c by ion-exchange liquid chromatography. Creatinine clearance was estimated using the Cockcroft-Gault equation (16).
Drugs.
(2-Iodo-5-nitrophenyl)-(1-[1-methylpiperidin-2-ylmethyl]-1H-indol-3-yl) methanone (AM1241), a potent and selective CB2 agonist (17) and 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone (AM630), a selective CB2 antagonist (18), were purchased from Cayman (Ann Arbor, MI) and Tocris Bioscience (Bristol, U.K.), respectively. AM1241 was dissolved in DMSO to a stock concentration of 10 mg/mL and stored at −20°C. Stock solutions were diluted in a 18:1:1 ratio of saline/emulphor-620/DMSO immediately prior to use.
Animals and induction of diabetes.
The study was approved by the ethical committee of Turin University, and both housing and care of laboratory animals were in accordance with Italian law (D.L.116/1992). Male C57BL6/J mice from Jackson Laboratories (Bar Harbor, ME) were maintained on a normal diet under standard animal house conditions. Diabetes was induced in mice, aged 8 weeks, by intraperitoneal injection of STZ in citrate buffer, pH 4.5 (55 mg/kg body wt/day), delivered in five consecutive daily doses. Mice that were sham injected with sodium citrate buffer were used as controls. Diabetes onset was confirmed by blood glucose levels >250 mg/dL 4 weeks after the first dose of STZ.
Experimental protocol.
Animals were divided into the following groups: nondiabetic mice given vehicle (n = 6), nondiabetic mice given AM1241 (n = 6), diabetic mice given vehicle (n = 14), and diabetic mice given AM1241 (n = 7). AM1241 was administered daily via intraperitoneal injection at the dosage of 3 mg/kg on the basis of previous studies that have proven both efficacy and selectivity of this dose in mice (19). Mice that were sham injected with a mixture 18:1:1 of saline/emulphor-620/DMSO were used as controls. After 14 weeks of diabetes, mice were killed by decapitation. The kidneys were dissected and weighed. The right kidney was frozen in N2 and stored at −80°C for both mRNA and protein analysis. Half of the left kidney was fixed in 10% PBS–formalin and then paraffin embedded for light microscopy; the remaining tissue was embedded in optimal cutting temperature compound and snap frozen in N2.
Metabolic and physiological parameters.
Before killing, blood samples were taken via saphenous vein puncture on alert 4-h–fasted animals, and glucose levels were measured using a glucometer (Accu-chek; Roche, Milan, Italy). Systolic blood pressure was assessed by tail-cuff plethysmography. Urine was collected over 18 h, with each mouse individually housed in a metabolic cage and provided with food and water ad libitum. Urinary albumin concentration was measured by a mouse albumin ELISA kit (Bethyl Laboratories, Milan, Italy). Creatinine clearance was estimated from serum and urine creatinine concentrations, as determined by high-performance liquid chromatography (20). Glycated hemoglobin was measured by quantitative immunoturbidimetric latex determination (Sentinel Diagnostic, Milan, Italy). Urinary N-acetylglucosamine (NAG) activity was assessed by colorimetric assay (Roche), and results were expressed as urinary NAG activity-to-creatinine ratio.
Extraction and quantification of endocannabinoids.
Endocannabinoids and cannabinoid-related molecules were measured by isotope-dilution liquid chromatography–mass spectrometric analysis in lipid extracts of frozen kidney tissue samples from diabetic (n = 3) and nondiabetic (n = 5) mice, as detailed in the Supplementary Data.
Glomerular isolation.
Glomeruli were isolated immediately after mice were killed, using a modified Dynabeads method as we have previously described (5,21) (see Supplementary Data).
Glomerular volume.
Glomerular volume was calculated as previously described (22) and reported in the Supplementary Data.
Immunohistochemistry.
Immunohistochemical staining for both human and murine CB2 detection was performed on 4-μm paraffin sections of formalin-fixed tissue. After antigen retrieval, endogenous peroxidase activity quenching, and blocking with avidin–biotin and BSA, sections were incubated overnight at 4°C with an anti-CB2 antibody (Cayman) and the specific staining detected using the LSAB+ system-HRP (Dako, Glostrup, Denmark). Sections exposed to a primary antibody preincubated with a control peptide as well as sections from CB2−/− mice were used as negative controls. Sections were visualized with an Olympus-Bx4I microscope and digitized with a high resolution camera (Carl Zeiss, Oberkochen, Germany). Results were expressed as percent area of positive staining per glomerulus. Evaluations were performed by two investigators in a blinded fashion. Glomerular accumulation of monocytes/macrophages was determined by immunohistochemistry using a rat anti-mouse F4/80 antibody (Serotec, Oxford, U.K.) as previously described (23).
Immunofluorescence.
Staining for nephrin, zonula occludens-1 (ZO-1), synaptopodin, and fibronectin was performed by immunofluorescence on snap-frozen sections as we have previously described (5,8).
Double immunofluorescence.
Sections were incubated with primary anti–WT-1, anti-F4/80, or anti-CD68 antibodies for 18 h at 4°C, followed by a fluorescein isothiocyanate–conjugated donkey anti-rat antibody (Invitrogen, Milan, Italy). After further blocking in avidin–biotin, sections were incubated with a rabbit anti-CB2 antibody for 1 h, followed by incubation with a biotinylated swine anti-rabbit IgG antibody (Dako, Glostrup, Denmark) and Alexa 555–conjugated streptavidin (Invitrogen).
Immunoblotting.
Expression of CB2, CB1, nephrin, ZO-1, diacylglycerol lipase (DAGLα), monoacylglycerol lipase (MAGL), transforming growth factor-β1 (TGF-β1), CCR2, extracellular signal–related kinase (ERK), Akt, and p38 was measured in total protein extracts from either renal cortex or cultured podocytes by immunoblotting (available in the Supplementary Data).
mRNA analysis.
RNA extraction and both real-time and traditional PCR were performed as previously described (5,8) and detailed in the Supplementary Data.
Podocyte cell culture.
Conditionally immortalized mouse podocytes were provided by P. Mundel (University of Miami, Miami, FL) and cultured as previously described (24). Expression of CB1/CB2 receptors was also assessed in immortalized podocytes of human origin, which were established, characterized, and cultured as we have previously reported (5,25). Selected experiments were performed in murine primary podocytes obtained from both wild-type C57BL6 and knockout CB2 mice (B6.129P2-Cnr2tm1Dgen/J mice; Jackson Laboratories). Glomeruli were isolated using the Dynabeads method (21), and primary human podocytes were established as we have previously described (5,25).
CB1 silencing.
To investigate the effect of CB1 on CB2 expression/signaling, CB1 expression was stably knocked down in murine podocytes using HuSH 29mer short hairpin RNA (shRNA) constructs against CB1 in a (pGFP)-V-RS vector (OriGene Technologies, Rockville, MD). A scrambled shRNA and an empty vector served as controls. Transfection was performed in undifferentiated podocytes using Lipofectamine LTX reagent (Invitrogen). Stable transfectants were selected by addition of 10 μg/mL puromycin (Santa Cruz, Heidelberg, Germany). Selected clonal cell lines were placed under nonpermissive conditions and tested for CB1 expression.
In vitro experiments.
After serum deprivation for 24 h, differentiated podocytes were exposed to either AM1241 (10 μmol/L) or AM630 (10 μmol/L) for 24 h. Appropriate dilutions of DMSO were added as vehicle controls in all experiments. In a subset of experiments, cells were exposed to AM1241 either in the presence or in the absence of an Akt (inhibitor VIII, 1 μmol/L) (26) or a p38 (SB202190, 1 μmol/L) inhibitor.
Data presentation and statistical analysis.
Data, presented as means ± SEM, geometric means (25–75% percentile), or fold change over control, were analyzed by Student t test or ANOVA, as appropriate. Fisher's least significant difference test was used for post hoc comparisons. Values for P < 0.05 were considered significant.
RESULTS
Human study
Glomerular CB2 receptor expression in advanced human DN.
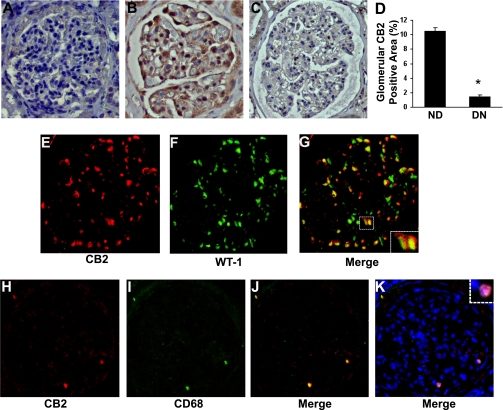
Clinical and laboratory characteristics of both type 2 diabetic patients with overt DN and control subjects are shown in Table 1. CB2 expression was assessed by immunohistochemistry, and specificity of the antibody was confirmed by the disappearance of the signal when the tissue was preabsorbed with a 10-fold excess of blocking peptide (Fig. 1A). In normal renal cortex, several glomerular cells stained positively for CB2 and the pattern of staining was suggestive of podocyte labeling (Fig. 1B). In patients with DN, CB2 protein expression was reduced (Fig. 1C), and semiquantitative analysis showed that the percentage of positive area was sixfold lower than in healthy subjects (Fig. 1D). Double immunofluorescence for CB2 and the podocyte marker WT-1 confirmed that in control subjects, CB2 was predominantly expressed by podocytes (Fig. 1E–G). In patients with DN, only a few glomerular CB2-positive cells were found and colocalized with the macrophage marker CD68 (Fig. 1H–K).
TABLE 1.
Control subjects and patients with DN: clinical parameters
| Diabetic patients | Control subjects | |
|---|---|---|
| n | 8 | 8 |
| Age (years) | 57.3 ± 2.9 | 65.4 ± 6.3 |
| Sex (male/female) | 6/2 | 6/2 |
| Diabetes duration (years) | 14 ± 2.9 | — |
| A1C (%) | 7.2 ± 0.6 | — |
| Serum creatinine (mg/dL) | 2.2 ± 0.5 | 1 ± 0.1 |
| Creatinine clearance (mL/min) | 44.8 ± 8.8 | — |
| Proteinuria (g/24 h) | 4.11 ± 0.75 | — |
| Retinopathy (%) | 90 | — |
| Hypertension (%) | 100 | 0 |
Data are means ± SEM.
FIG. 1.
CB2 receptor expression in human DN. Glomerular CB2 protein expression was assessed by immunohistochemistry as detailed in the Supplementary Data. Specificity of the antibody was confirmed by the disappearance of the signal when the tissue was preabsorbed with a 10-fold excess of blocking peptide (A). B and C: CB2 staining in renal sections from nondiabetic subjects (ND) and diabetic patients with overt nephropathy (DN), respectively (original magnification ×200). The percent area of positive staining, quantified by a computer-aided image analysis system, is shown in the graph (D) (*P < 0.001 DN vs. ND). Double immunofluorescence for CB2 (E) and the podocyte marker WT-1 (F) was performed in control renal sections, whereas renal sections from diabetic patients were used for double staining of CB2 (H) and the macrophage marker CD68 (I). Positive staining colocalized as shown by merging (G and J). K: Nuclei were counterstained with DAPI. The dashed squares in G and K delimit areas shown at higher magnification. (A high-quality digital representation of this figure is available in the online issue.)
Animal study
Metabolic and physiological parameters in experimental animals.
As shown in Table 2, after 14 weeks of diabetes, both blood glucose and glycated hemoglobin levels were significantly higher in diabetic than in nondiabetic mice. Furthermore, compared with sham-injected control animals, diabetic mice showed a significant decrease in body weight and a significant increase in kidney weight-to-body weight ratio. Treatment with AM1241 did not affect glycemic control, body weight, kidney weight-to-body weight ratio, and systolic blood pressure.
TABLE 2.
Metabolic and physiological parameters of diabetic and control mice treated with either the CB2 agonist (AM1241) or vehicle
| ND | ND+AM1241 | DM | DM+AM1241 | |
|---|---|---|---|---|
| Body weight (g) | 28.80 ± 0.61 | 27.23 ± 0.43 | 24.92 ± 0.33* | 24.83 ± 0.93* |
| BG (mg/dL) | 114 ± 5.44 | 111 ± 5.14 | 326 ± 16.41† | 323 ± 41.10† |
| Glycated Hb (%) | 4.18 ± 0.35 | 4.00 ± 0.15 | 12.79 ± 0.35† | 12.18 ± 1.10† |
| sBP (mmHg) | 125 ± 6.83 | 119 ± 2.43 | 133 ± 3.16 | 132 ± 5.14 |
| KW/BW ratio | 5.81 ± 0.03 | 6.12 ± 0.08 | 7.25 ± 0.29‡ | 6.83 ± 0.30‡ |
| AER (μg/18 h) | 78.8 (73.8–86.6) | 71.9 (69.5–79.6) | 315.4 (291.9–356.6)† | 162.9 (139.5–161.7)†§ |
Data are shown as means ± SEM or geometric means (25th–75th percentile). BG, blood glucose; sBP, systolic blood pressure; KW/BW, kidney weight/body weight; AER, albumin excretion rate.
*P < 0.01 DM and DM+AM1241 vs. ND and ND+AM1241.
†P < 0.001 DM and DM+AM1241 vs. ND and ND+AM1241.
‡P < 0.05 DM and DM+AM1241 vs. ND.
§P < 0.001 DM+AM1241 vs. DM.
CB2 receptor expression in early experimental diabetes.
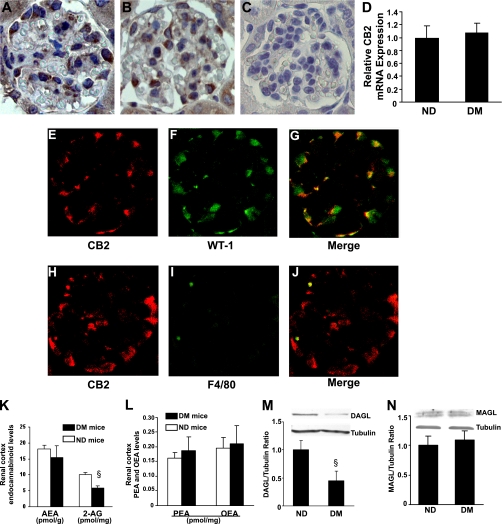
Immunohistochemical analysis showed a positive CB2 staining with a predominant podocyte distribution in both control and diabetic mice (Fig. 2A and B). Specificity of the antibody binding was confirmed by lack of staining in sections from CB2−/− mice (Fig. 2C). Semiquantitative analysis did not show significant differences in the percentage of positive area between groups (nondiabetic, 5.21 ± 0.02 vs. diabetic, 4.78 ± 0.41%; P = not significant [NS]). Consistently, mRNA levels encoding for CB2 were similar in diabetic and nondiabetic mice, as assessed by real-time PCR on isolated glomeruli (Fig. 2D). Double immunofluorescence for the CB2 receptor and the podocyte marker WT-1 confirmed that CB2 was mainly expressed by podocytes (Fig. 2E–G), though sporadic CB2-F4/80–positive macrophages were also observed in the diabetic glomeruli (Fig. 2H–J).
FIG. 2.
Endocannabinoid system in early experimental DN. CB2 protein expression was assessed in renal sections from nondiabetic (A) and diabetic mice (B) by immunohistochemistry. Renal sections from CB2 knockout mice served as negative control (C) (original magnification ×400). D: Glomerular CB2 mRNA levels were measured in both diabetic (DM) and nondiabetic (ND) mice by real-time PCR and corrected for the expression of the housekeeping gene HPRT. Double immunofluorescence for CB2 (E and H) and either the podocyte marker WT-1 (F) or the macrophage marker F4/80 (I) was performed on renal sections from diabetic mice. Colocalization was demonstrated by merging (G and J). Concentrations of endocannabinoids (AEA and 2-AG) (K) and endocannabinoid-related molecules (palmitoylethanolamide [PEA] and oleoylethanolamide [OEA]) (L) were measured in the renal cortex from both ND and DM mice by isotope dilution liquid chromatography mass spectrometry (§P < 0.01 DM vs. ND). Protein expression of DAGLα and MAGL was assessed in total renal cortex protein extracts by immunoblotting. Tubulin was used as internal control. Representative immunoblots and results of densitometry analyses are shown in M and N (§P < 0.05 DM vs. ND). (A high-quality digital representation of this figure is available in the online issue.)
Endocannabinoid levels in early experimental diabetes.
Levels of AEA, 2-AG, and endocannabinoid-related molecules were measured in the renal cortex from both diabetic and nondiabetic mice (Fig. 2K and L). The concentrations of AEA, which binds with higher affinity to, and activates with higher efficacy, the CB1 receptor (27), were similar in the two groups. On the contrary, the levels of 2-AG, which equally binds to both CB1 and CB2, and is more potent than AEA at CB2 receptors (27), were diminished in diabetic mice. Expression of DAGLα, the primary enzyme responsible for 2-AG synthesis (9), was significantly reduced in diabetic mice (Fig. 2M), whereas the expression of the 2-AG–degrading enzyme MGL was unchanged (Fig. 2N).
Effect of CB2 receptor activation on albuminuria, NAG activity, and renal function.
Diabetes caused a fourfold rise in the albumin excretion rate that was significantly reduced by AM1241, whereas this compound did not alter albuminuria in control animals (Table 2). Urinary NAG activity/creatinine ratio was also significantly increased in diabetic mice, but no differences were observed between treated and untreated mice (nondiabetic, 59.67 ± 14.65; nondiabetic+AM1241, 48.45 ± 6.75; diabetic, 140.14 ± 14.88; diabetic+AM1241, 143.68 ± 41.57 units/g; P < 0.05 diabetic and diabetic+AM1241 vs. nondiabetic). Renal function was similar among groups (nondiabetic, 0.42 ± 0.05; diabetic, 0.45 ± 0.06; nondiabetic+AM1241, 0.45 ± 0.04; diabetic+AM1241, 0.48 ± 0.06 mL/min).
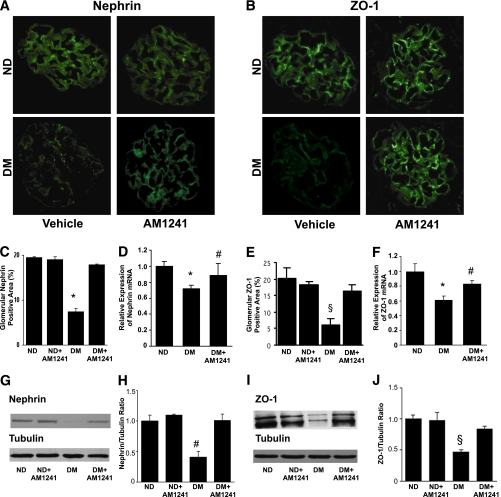
AM1241 prevented diabetes-induced nephrin and ZO-1 loss.
In diabetic mice there was a significant reduction in both nephrin and ZO-1 mRNA and protein expression. This effect was significantly reduced by AM1241, as assessed by immunofluorescence (Fig. 3A and B). Although immunofluorescence is poorly quantitative, the results of immunofluorescence quantitation were also confirmed by real-time PCR (Fig. 3C–F). Moreover, immunoblotting analysis showed that AM1241 treatment almost completely prevented diabetes-induced nephrin and ZO-1 downregulation (Fig. 3G–J). No significant changes in glomerular synaptopodin expression were observed among groups, as assessed by immunofluorescence (nondiabetic, 41.5 ± 1.2; nondiabetic+AM1241, 42.4 ± 1.6; diabetic, 37.0 ± 1.6; diabetic+AM1241, 40.4 ± 1.1%; glomerular positive area; P = NS).
FIG. 3.
Activation of the CB2 receptor by AM1241 prevented diabetes-induced downregulation of both nephrin and ZO-1. Control nondiabetic (ND) and diabetic (DM) mice were treated with either vehicle or the CB2 agonist AM1241 (3 mg/kg) via intraperitoneal injection for 14 weeks. Nephrin and ZO-1 mRNA and protein expression were assessed by immunofluorescence, immunoblotting, and real-time PCR. Representative immunofluorescence images of nephrin (A) and ZO-1 (B) are shown (original magnification ×400) and quantification of glomerular positive staining reported in the graphs in C and E (nephrin, *P < 0.001 DM vs. others; ZO-1, §P < 0.01 DM vs. others). Nephrin and ZO-1 mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex. Results were corrected for the expression of WT-1 as described in research design and methods and shown in the graphs in D and F (*P < 0.001 DM vs. ND; #P < 0.05 DM vs. DM+AM1241). G and I: Representative immunoblots of nephrin and ZO-1 protein expression in total protein extracts from the renal cortex. Tubulin was used as internal control. Results of densitometry analyses are reported in H and J (nephrin, #P < 0.05 DM vs. others; ZO-1, §P < 0.01 DM vs. others). (A high-quality digital representation of this figure is available in the online issue.)
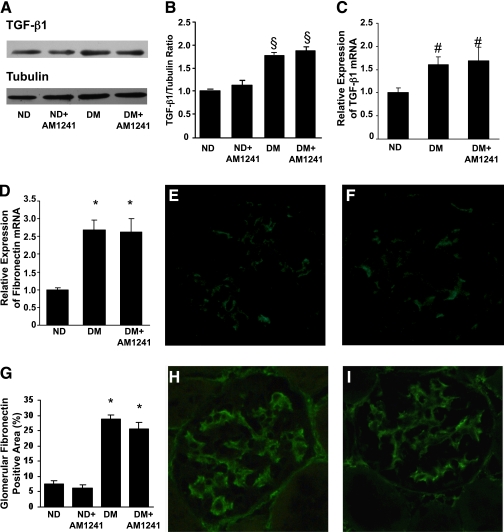
CB2 activation effect on glomerular hypertrophy and early markers of fibrosis.
AM1241 administration did not prevent diabetes-induced glomerular hypertrophy (glomerular volume: nondiabetic, 157 ± 14.66; diabetic, 316 ± 25.73; nondiabetic+AM1241, 171 ± 6.24; diabetic+AM1241, 286 ± 41.51 μm3; P < 0.01 diabetic and diabetic+AM1241 vs. nondiabetic). Furthermore, in diabetic mice the significant increase in both TGF-β1 and fibronectin mRNA and protein levels was left unchanged by AM1241 (Fig. 4). Differences in type I collagen mRNA expression among groups were not significant (nondiabetic, 1.33 ± 0.08; diabetic, 1.79 ± 0.14; diabetic+AM1241, 1.49 ± 0.23; P = NS).
FIG. 4.
CB2 activation did not affect overexpression of early markers of fibrosis in diabetic mice. Control nondiabetic mice (ND) and diabetic mice (DM) were treated with either vehicle or the CB2 agonist AM1241 (3 mg/kg) via intraperitoneal injection for 14 weeks. A: Representative immunoblot of TGF-β1 protein expression in total protein extracts from the renal cortex. Tubulin was used as internal control. Results of densitometry analysis are reported in B (§P < 0.01 DM and DM+AM1241 vs. ND). Fibronectin and TGF-β1 mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex. Results were corrected for the expression of HRPT and shown in the graphs in C and D (*P < 0.001, #P < 0.05 DM and DM+AM1241 vs. ND). Representative immunofluorescence images of fibronectin (E: ND, F: ND+AM1241, H: DM, I: DM+AM1241) are shown (original magnification ×400), and quantification of glomerular staining is reported in the graph in G (*P < 0.001 DM and DM+AM1241 vs. ND). (A high-quality digital representation of this figure is available in the online issue.)
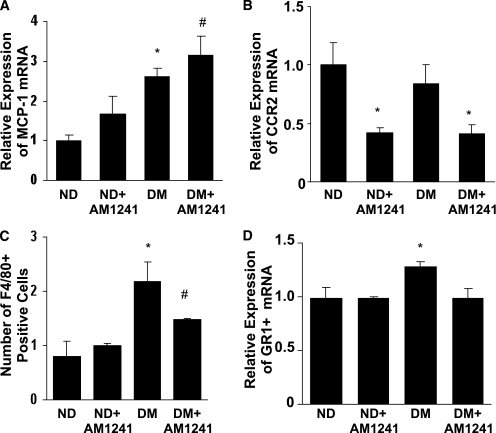
Effect of CB2 activation on inflammatory markers.
CB2 activation did not alter diabetes-induced MCP-1 overexpression (Fig. 5A). However, treatment with AM1241 caused a marked reduction in CCR2 expression in both control and diabetic mice (Fig. 5B). The number of F4/80-positive cells was greatly enhanced in the glomeruli of diabetic mice and significantly reduced by AM1241 (Fig. 5C). Furthermore, diabetes-induced overexpression of GR1, a marker highly expressed by inflammatory monocytes recruited by MCP-1 (28), was completely abolished by AM1241 (Fig. 5D).
FIG. 5.
Effect of CB2 activation on diabetes-induced markers of inflammation. Nondiabetic (ND) and diabetic (DM) mice treated with either vehicle or the CB2 agonist AM1241 (3 mg/kg/die i.p.) were studied 14 weeks after diabetes onset. MCP-1 (A), CCR2 (B), and GR1+ (D) mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex, and results were corrected for the expression of the housekeeping gene HRPT (A: *P < 0.01 DM vs. ND; #P < 0.05 DM+AM1241 vs. ND+AM124; B: *P < 0.05 DM+AM1241 and ND+AM1241 vs. ND and DM; D: *P < 0.01 DM vs. others). C: Glomerular monocyte accrual was assessed by counting the number of F4/80-positive cells within the glomeruli (*P < 0.01 DM vs. ND and ND+AM1241; #P < 0.05 DM+AM1241 vs. DM).
In vitro study
Endocannabinoid receptor expression in cultured podocytes.
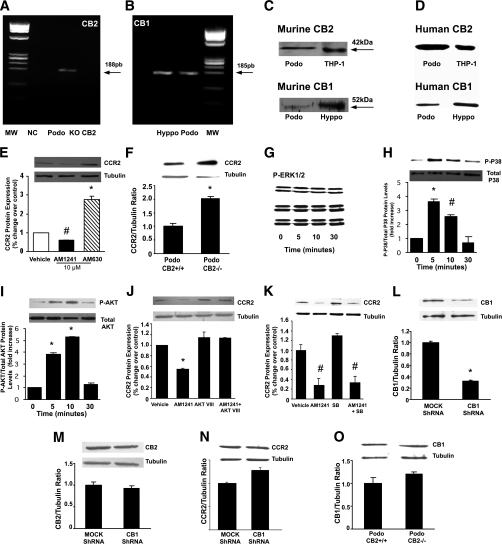
Murine differentiated podocytes constitutively express the mRNA encoding for both the CB2 and the CB1 receptor (Fig. 6A and B). In addition, immunoblotting results showed expression of both CB2 and CB1 receptors in protein extracts from both murine (Fig. 6C) and human podocytes (Fig. 6D).
FIG. 6.
CB2 receptor expression and signaling in cultured podocytes. A and B: mRNA expression of both CB2 (A) and CB1 (B) was studied in murine-cultured podocytes by traditional real-time PCR, and representative 2% agarose gels stained with ethidium bromide are shown. MW, molecular weight marker; NC, negative control; Podo, murine podocytes; KO-CB2, renal tissue from CB2 knockout mice (negative control); Hyppo, hyppocampal tissue (positive control). C and D: CB2 and CB1 protein expression was assessed in protein extracts from murine- (Podo) (C) and human- (D) cultured podocytes by immunoblotting. Total protein extracts from a monocyte cell line (THP-1) and hyppocampal tissue (Hyppo) were used as positive control. E and F: CCR2 expression was assessed by immunoblotting on total protein extracts from murine podocytes (Podo) exposed to vehicle, AM1241 (10 μmol/L), or AM630 (10 μmol/L) for 24 h (E) and from CB2+/+ and CB2−/− murine podocytes (F). Tubulin was used as internal control. Representative immunoblots and results of densitometric analyses are shown (E: *P < 0.001 AM630 vs. vehicle, #P < 0.05 AM1241 vs. vehicle; F: *P < 0.05 podo CB2+/+ vs. podo CB2−/−). G–I: Murine podocytes were exposed to AM1241 for 0, 5, 10, and 30 min, and then expression of both phosphorylated and total forms of ERK (G), p38 (H), and Akt (I) was assessed by immunoblotting (H: *P < 0.001 AM1241 at 5 min vs. control, #P < 0.01 AM1241 at 10 min vs. control; I: *P < 0.001 AM1241 at 5 and 10 min vs. control). J and K: CCR2 expression was studied in murine podocytes exposed to AM1241 for 24 h either in the presence or in the absence of the Akt inhibitor VIII (1 μmol/L) (J) or the p38 inhibitor SB202190 (SB) (K) by Western blotting (J: *P < 0.01 AM1241 vs. others; K: #P < 0.01 AM1241 and AM1241+SB vs. others). L–N: Murine podocytes were stably transfected with either shRNA constructs designed to silence CB1 or mock shRNA. Expression of CB1 (L), CB2 (M), and CCR2 (N) was studied by immunoblotting (*P < 0.01 CB1 shRNA vs. mock shRNA). O: CB1 expression was studied in total protein extracts from wild-type and CB2 knockout podocytes (Podo) by immunoblotting. A representative immunoblot and results of densitometric analysis are shown.
The CB2 receptor modulates CCR2 expression.
Podocyte exposure to AM1241 (10 μmol/L) for 24 h significantly reduced CCR2 protein expression, as assessed by immunoblotting. By contrast, the addition of a specific CB2 antagonist, AM630 (18), markedly enhanced CCR2 expression (Fig. 6E). A strong increase in CCR2 expression was also observed in CB2−/− podocytes (Fig. 6F).
To investigate the intracellular signaling pathway involved in CB2-induced CCR2 downregulation, podocytes were exposed to AM1241 (10 μmol/L) for 5, 10, and 30 min, and then phosphorylated levels of Akt, ERK, and p38 were measured by immunoblotting. No significant changes were observed in phospho-ERK expression in response to AM1241 (Fig. 6G). On the contrary, AM1241 induced a rapid and strong activation of both p38 and Akt (Fig. 6H and I). The addition of Akt inhibitor VIII (26) completely abolished AM1241-induced CCR2 downregulation (Fig. 6J) at 24 h, whereas no changes were observed in the presence of the p38 inhibitor SB202190 (Fig. 6K).
To establish if the CB1 receptor can modulate CB2 receptor expression/signaling, CB1 expression was knocked down by stably transfecting murine podocytes with shRNA constructs specifically designed to silence CB1. CB1 expression was suppressed efficiently (Fig. 6L); however, this did not affect either CB2 expression or CB2-induced CCR2 downregulation (Fig. 6M and N). Similarly, no changes in CB1 protein expression were observed in CB2−/− podocytes (Fig. 6O).
DISCUSSION
Our results suggest a protective role of the CB2 receptor in DN by showing 1) CB2 expression by podocytes both in vitro and in vivo, 2) CB2 downregulation in kidney biopsies from patients with advanced DN, 3) reduced kidney levels of the CB2 ligand 2-AG in early experimental diabetes, and 4) beneficial effects of a selective CB2 agonist on albuminuria, nephrin downregulation, and glomerular monocyte infiltration in diabetic mice.
In both human and murine healthy kidneys the CB2 receptor was expressed within the glomeruli predominantly by podocytes. In addition, detectable levels of both AEA, 2-AG, and related metabolic enzymes were present in the renal cortex from normal mice. This, together with our previous report showing in vivo CB1 expression by podocytes (8), demonstrates that an endocannabinoid system is present within the kidney and that podocytes are a primary target of endocannabinoid action.
In patients with advanced DN, there was a marked decrease in podocyte CB2 expression. The underlying mechanism is unclear; however, the prosclerotic cytokine TGF-β1 diminishes CB2 expression in lymphocytes (29). Therefore, diabetes-induced TGF-β1 overexpression may be involved in this phenomenon. The remaining glomerular CB2-positive cells were macrophages. This is not surprising as the CB2 receptor is highly expressed by monocytes/macrophages (9) and enhanced glomerular monocyte accrual occurs in DN (30). However, at variance with other chronic inflammatory diseases, such as atherosclerosis and liver fibrosis, where CB2 expression is enhanced due to monocyte infiltration (14,15), overall glomerular CB2 expression was reduced in human DN because of the prominent effect of CB2 downregulation in podocytes.
In early STZ-induced experimental diabetes, we found here that podocyte CB2 expression was preserved; however, we observed in this case a significant reduction in 2-AG concentrations without significant changes in the levels of AEA. A diabetes-related defect in renal 2-AG production is likely to be implicated, as DAGLα, the primary enzyme responsible for 2-AG synthesis (9), was significantly downregulated. Insulin deficiency is a potential underlying mechanism, as insulin has been shown to enhance DAGLα expression in cultured adipocytes (31).
It is not unusual that AEA and 2-AG tissue concentrations change in different, or even opposing, ways in pathophysiological conditions (32). A decrease in 2-AG levels with normal/elevated AEA levels has been reported in adipose tissue from obese diabetic patients (33), retinas from patients with diabetic retinopathy (34), and murine ischemic brain regions (35), whereas an increase in AEA without changes in 2-AG concentration was found in the renal cortex from mice with cisplatin-induced nephropathy (36). Differential changes in the levels of the two endocannabinoids may have functional relevance, particularly as AEA is known to activate the CB1 receptor with strong efficacy, while 2-AG is equally efficacious for both the CB1 and the CB2 receptor (27). This raises the hypothesis that, in our study, reduced availability of 2-AG, the predominant CB2 ligand, may lower signaling through the CB2 receptor. In addition, the CB1 receptor is overexpressed by podocytes in this animal model (8) and this may further shift endocannabinoid signaling from the CB2 to the CB1 receptor.
To investigate the functional role of CB2 in DN, we studied the effect of CB2 activation in early experimental diabetes. Treatment with the selective CB2 agonist AM1241 (17) induced a significant 50% reduction in diabetes-induced albuminuria without significant changes in urinary NAG activity, a marker of tubular damage. This provides unprecedented evidence that signaling though the CB2 receptor is important in controlling glomerular permeability to albumin.
AM1241 administration did not affect body weight in either diabetic or control mice. This is in agreement with a report showing that CB2 agonism does not alter body weight in mice fed a standard diet (37). Glycemic control and systolic blood pressure were similar in treated and untreated diabetic mice, consistent with the antiproteinuric effect of CB2 activation observed in these mice being independent of both metabolic and hemodynamic factors.
Treatment with AM1241 significantly reduced diabetes-induced downregulation of the podocyte proteins nephrin and ZO-1, and prevention of podocyte protein loss is a potential mechanism of the antiproteinuric effect of CB2 activation. Indeed, slit diaphragm and slit diaphragm–associated proteins play a key role in the pathogenesis of proteinuria (38), and nephrin downregulation is an early feature of human DN (39). It is unlikely that rescue of podocyte proteins by AM1241 was secondary to prevention of podocyte damage as no abnormalities in either podocyte number or ultrastructure are present in this animal model after 14 weeks of diabetes, as we have previously reported (8).
Previous studies have shown that CB2 agonists have antifibrotic effects in animal models of dermal and liver fibrosis (10,15,40). However, in our study, treatment with AM1241 did not prevent diabetes-induced overexpression of fibronectin and TGF-β1. The relatively modest profibrotic potential of podocytes may explain AM1241 failure. However, we cannot exclude the possibility that the extent to which CB2 was modulated pharmacologically by AM1241 was not of sufficient magnitude to elicit a protective response on renal fibrogenesis.
In diabetic mice, treatment with AM1241 significantly reduced glomerular infiltration of monocytes. Likewise, a recent study has shown that CB2 activation attenuates cisplatin-induced renal inflammatory cell infiltration (41). Reduced monocyte recruitment is a potential mechanism whereby AM1241 can prevent podocyte protein loss. Indeed, nephrin gene promoter activity is suppressed in podocytes cocultured with activated monocytes (42), and conditioned medium from activated monocytes downregulates nephrin in both cultured podocytes and isolated glomeruli (43).
Our finding in both control and diabetic mice that AM1241 strongly downregulated CCR2 without affecting MCP-1 expression supports the hypothesis that CB2 activation affects monocyte recruitment by lowering CCR2 expression and hence MCP-1 signaling. A previous in vitro study has reported reduced CCR2 expression in monocytes exposed to CB2 agonists (44), but our results provide the first in vivo evidence linking CB2 activation to CCR2 downregulation.
The CCR2 receptor is also expressed both in vitro and in vivo by podocytes (5), and our data indicate that podocytes are the predominant glomerular cell type expressing CB2 receptors. This prompted us to test in vitro if CB2 modulates CCR2 expression in podocytes. We found that cultured podocytes expressed both CB2 and CB1 receptors. In addition, CB2 activation downregulated CCR2 via an Akt-dependent mechanism, while both CB2 knockout and blockade upregulated CCR2. These data provide the first evidence that in podocytes CCR2 expression is under CB2 control. They also suggest that the protective effect of AM1241 on nephrin loss and albuminuria may be, at least in part, due to podocyte CCR2 downregulation. Indeed, in this cell type, MCP-1 binding to CCR2 reduces nephrin expression via a ρ-kinase–dependent mechanism (5). Consistently with this hypothesis, in human endothelial cells, CB2 agonists attenuate not only the inflammatory response to TNF-α but also ρ activation (13).
A recent study has demonstrated a cross-talk between the two major endocannabinoid receptors in monocytes (45). However, in our study, CB2 deprivation did not alter CB1 expression, and CB1 silencing did not affect the CB2–CCR2 pathway. The latter finding makes it unlikely that the protective effects of CB1 blockade, which we have previously reported in experimental DN (8), occurred through modulation of the podocyte CB2–CCR2 pathway.
In conclusion, our findings may have important implications for DN. The beneficial effect of AM1241 in a preclinical model of DN makes CB2 agonism an attractive new strategy for the treatment of DN. CB2 activation may also positively affect other diabetes-related complications as CB2 agonists may, under certain conditions, delay progression of atherosclerotic lesions (14) and ameliorate diabetes-induced neuropathic pain (46,47) in rodents. The observation that CB2 receptors are also expressed by podocytes of human origin and undergo downregulation in patients with advanced DN suggests that our results may also have clinical relevance in humans. In this regard, it is noteworthy that CB2 agonists are devoid of the psychoactive properties of CB1 antagonists and are currently being evaluated in phase I and II clinical trials for the treatment of pain, inflammation, and autoimmune disorders. Our study may thus pave the way for future clinical trials on CB2 agonists in human DN. However, further animal studies showing evidence of efficacy in secondary intervention are warranted prior to testing these compounds in humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Compagnia di San Paolo, the Italian Society of Diabetes, the University of Turin (ex-60% grant), and the Piedmont Region Research Grant.
No potential conflicts of interest relevant to this article were reported.
F.B. researched data and wrote the manuscript. F.P. and S.P. researched data. G.B. reviewed and edited the manuscript. R.G. researched data. M.P.R. reviewed and edited the manuscript. G.S. researched data. V.D.M. researched data and reviewed and edited the manuscript. P.C.P. reviewed and edited the manuscript. G.G. researched data and wrote the manuscript.
The authors thank Peter Mundel (University of Miami, Miami, Florida) for the generous gift of the mouse podocyte cell line.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1809/-/DC1.
REFERENCES
- 1.Molitch ME, DeFronzo RA, Franz MJ, et al. ; American Diabetes Association Nephropathy in diabetes. Diabetes Care 2004;27(Suppl. 1):S79–S83 [DOI] [PubMed] [Google Scholar]
- 2.Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001;44:1957–1972 [DOI] [PubMed] [Google Scholar]
- 3.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 2006;69:73–80 [DOI] [PubMed] [Google Scholar]
- 4.Giunti S, Barutta F, Perin PC, Gruden G. Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr Vasc Pharmacol 2010;8:849–860 [DOI] [PubMed] [Google Scholar]
- 5.Tarabra E, Giunti S, Barutta F, et al. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes 2009;58:2109–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giunti S, Tesch GH, Pinach S, et al. Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 2008;51:198–207 [DOI] [PubMed] [Google Scholar]
- 7.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 2009;89:309–380 [DOI] [PubMed] [Google Scholar]
- 8.Barutta F, Corbelli A, Mastrocola R, et al. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 2010;59:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol 2008;160:1–24 [DOI] [PubMed] [Google Scholar]
- 10.Julien B, Grenard P, Teixeira-Clerc F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005;128:742–755 [DOI] [PubMed] [Google Scholar]
- 11.Shmist YA, Goncharov I, Eichler M, et al. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem 2006;283:75–83 [DOI] [PubMed] [Google Scholar]
- 12.Rajesh M, Mukhopadhyay P, Haskó G, Huffman JW, Mackie K, Pacher P. CB2 cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol 2008;153:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajesh M, Mukhopadhyay P, Bátkai S, et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol 2007;293:H2210–H2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steffens S, Veillard NR, Arnaud C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005;434:782–786 [DOI] [PubMed] [Google Scholar]
- 15.Muñoz-Luque J, Ros J, Fernández-Varo G, et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther 2008;324:475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossing P, Astrup AS, Smidt UM, Parving HH. Monitoring kidney function in diabetic nephropathy. Diabetologia 1994;37:708–712 [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA 2003;100:10529–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross RA, Brockie HC, Stevenson LA, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol 1999;126:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 2007;101:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 2004;65:1959–1967 [DOI] [PubMed] [Google Scholar]
- 21.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002;161:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weibel ER. Stereological methods. In Practical Methods for Biological Morphometry. London, U.K., Academic, 1979, p. 51–57 [Google Scholar]
- 23.Saito D, Maeshima Y, Nasu T, et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am J Physiol Renal Physiol 2011;300:F873–F886 [DOI] [PubMed] [Google Scholar]
- 24.Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int 2007;72:26–36 [DOI] [PubMed] [Google Scholar]
- 25.Burt D, Salvidio G, Tarabra E, et al. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol 2007;171:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logie L, Ruiz-Alcaraz AJ, Keane M, et al. Characterization of a protein kinase B inhibitor in vitro and in insulin-treated liver cells. Diabetes 2007;56:2218–2227 [DOI] [PubMed] [Google Scholar]
- 27.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol 2000;57:1045–1050 [PubMed] [Google Scholar]
- 28.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006;211:609–618 [DOI] [PubMed] [Google Scholar]
- 29.Gardner B, Zu LX, Sharma S, et al. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem Biophys Res Commun 2002;290:91–96 [DOI] [PubMed] [Google Scholar]
- 30.Furuta T, Saito T, Ootaka T, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis 1993;21:480–485 [DOI] [PubMed] [Google Scholar]
- 31.D’Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes 2008;57:1262–1268 [DOI] [PubMed] [Google Scholar]
- 32.Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci 2008;29:229–233 [DOI] [PubMed] [Google Scholar]
- 33.Annuzzi G, Piscitelli F, Di Marino L, et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis 2010;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matias I, Wang JW, Moriello AS, Nieves A, Woodward DF, Di Marzo V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot Essent Fatty Acids 2006;75:413–418 [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini-Giampietro DE, Mannaioni G, Bagetta G. Post-ischemic brain damage: the endocannabinoid system in the mechanisms of neuronal death. FEBS J 2009;276:2–12 [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay P, Pan H, Rajesh M, et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 2010;160:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deveaux V, Cadoudal T, Ichigotani Y, et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009;4:e5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 2009;5:463–468 [DOI] [PubMed] [Google Scholar]
- 39.Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 2003;52:1023–1030 [DOI] [PubMed] [Google Scholar]
- 40.Akhmetshina A, Dees C, Busch N, et al. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum 2009;60:1129–1136 [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay P, Rajesh M, Pan H, et al. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 2010;48:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takano Y, Yamauchi K, Hayakawa K, et al. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett 2007;581:421–426 [DOI] [PubMed] [Google Scholar]
- 43.Ikezumi Y, Suzuki T, Karasawa T, Kawachi H, Nikolic-Paterson DJ, Uchiyama M. Activated macrophages down-regulate podocyte nephrin and podocin expression via stress-activated protein kinases. Biochem Biophys Res Commun 2008;376:706–711 [DOI] [PubMed] [Google Scholar]
- 44.Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol 2008;294:H1145–H1155 [DOI] [PubMed] [Google Scholar]
- 45.Han KH, Lim S, Ryu J, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res 2009;84:378–386 [DOI] [PubMed] [Google Scholar]
- 46.Toth CC, Jedrzejewski NM, Ellis CL, Frey WH., 2nd Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain 2010;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bujalska M. Effect of cannabinoid receptor agonists on streptozotocin-induced hyperalgesia in diabetic neuropathy. Pharmacology 2008;82:193–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.