Abstract
OBJECTIVE
Many genetic variants have been associated with glucose homeostasis and type 2 diabetes in genome-wide association studies. Zinc is an essential micronutrient that is important for β-cell function and glucose homeostasis. We tested the hypothesis that zinc intake could influence the glucose-raising effect of specific variants.
RESEARCH DESIGN AND METHODS
We conducted a 14-cohort meta-analysis to assess the interaction of 20 genetic variants known to be related to glycemic traits and zinc metabolism with dietary zinc intake (food sources) and a 5-cohort meta-analysis to assess the interaction with total zinc intake (food sources and supplements) on fasting glucose levels among individuals of European ancestry without diabetes.
RESULTS
We observed a significant association of total zinc intake with lower fasting glucose levels (β-coefficient ± SE per 1 mg/day of zinc intake: −0.0012 ± 0.0003 mmol/L, summary P value = 0.0003), while the association of dietary zinc intake was not significant. We identified a nominally significant interaction between total zinc intake and the SLC30A8 rs11558471 variant on fasting glucose levels (β-coefficient ± SE per A allele for 1 mg/day of greater total zinc intake: −0.0017 ± 0.0006 mmol/L, summary interaction P value = 0.005); this result suggests a stronger inverse association between total zinc intake and fasting glucose in individuals carrying the glucose-raising A allele compared with individuals who do not carry it. None of the other interaction tests were statistically significant.
CONCLUSIONS
Our results suggest that higher total zinc intake may attenuate the glucose-raising effect of the rs11558471 SLC30A8 (zinc transporter) variant. Our findings also support evidence for the association of higher total zinc intake with lower fasting glucose levels.
Chronic elevations in fasting or postprandial glucose levels are the cardinal features of type 2 diabetes (T2D), a common complex disease caused by the interplay of genetic and lifestyle factors. Genome-wide association studies (GWAS) have identified genetic loci reproducibly associated with glycemic traits or T2D (1,2). These studies improved our understanding of the mechanisms underlying impaired glucose homeostasis and T2D, potentially aiding the development of novel and individualized medical therapies (3,4).
Modifiable lifestyle factors, such as diet and physical activity, influence glucose homeostasis and thus represent important targets for T2D prevention and management (5). Zinc is an essential trace element found in most foods that facilitates catalytic, structural, and transcriptional actions within cells (6). Zinc is a critical component of the catalytic site of >300 enzymes, including pancreatic carboxypeptidases and RNA polymerases; coordinates with protein domains; facilitates protein folding; produces structures such as zinc fingers; and regulates the expression of metallothioneins (7–9). Zinc is necessary in β-cells for insulin crystallization in hexamers (10). Moreover, it is cosecreted with insulin and exerts insulinomimetic and antioxidant actions and participates in the regulation of β-cell mass (11,12). Zinc homeostasis is impaired in animal models of diabetes and in humans with diabetes (13,14). Indeed, zinc supplementation studies in animal models support a protective effect of zinc against T2D, and in humans plasma levels of zinc are inversely associated with the risk of T2D (13). Nevertheless, a causal link between zinc and T2D in humans is not well established. Data from population-based studies indicate that dietary and total zinc intake (food sources and supplements) may reduce T2D risk (15–17), but the few intervention studies investigating the effect of zinc supplementation on glucose metabolism, insulin homeostasis, or T2D risk have yielded inconsistent results (13,18). Therefore, although zinc supplementation is a potential therapeutic and preventive target for T2D, additional studies are needed before population-wide recommendations regarding zinc intake can be advocated.
Knowledge of gene-environment interactions may enhance our understanding of disease etiology and pathogenesis, as well as help personalize interventions. We recently have demonstrated a favorable association of whole-grain intake with fasting glucose and insulin and observed a potential interaction between the rs780094 GCKR variant and whole-grain intake on fasting insulin concentrations (19). Zinc intake previously has been shown to interact with genetic variants in relation to chronic diseases and inflammatory biomarkers (8,20–22) but not in the context of glycemic traits.
To address these gaps in the literature, we conducted a meta-analysis that included 14 cohorts, totaling up to 45,821 participants, to test the hypothesis that zinc intake modifies the cross-sectional association between fasting glucose levels and genetic variants known to be related to glycemia and zinc metabolism (2) in individuals of European descent without T2D.
RESEARCH DESIGN AND METHODS
The study sample for the current cross-sectional meta-analysis was combined from 14 cohort studies (Supplementary Table 1), the majority of which are included in the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium (23) and/or the Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC). Ethical approval was obtained by local bioethical committees, and all participants provided signed informed consent. Participants included in the current analyses did not have diabetes (defined as diagnosed self-reported diabetes and/or fasting glucose levels ≥7 mmol/L and/or use of antidiabetes medications) and had dietary data that met quality-control criteria (Supplementary Table 1).
Anthropometric and blood glucose measurements.
We calculated BMI as measured weight divided by the square of measured height (kg/m2) in all cohorts. We determined blood glucose levels in fasting plasma samples, with the exceptions of participants in the Family Heart Study (FamHS), the Cardiovascular Health Study (CHS), the Rotterdam Study, and the Atherosclerosis Risk in Communities (ARIC) study, for whom we used fasting serum samples, and the Malmö Diet and Cancer (MDC) study, in which we used fasting whole-blood samples (Supplementary Table 1).
Dietary assessment and zinc intake estimation.
Dietary data were collected (Supplementary Table 1) and processed to estimate the mean daily dietary zinc intake using an appropriate nutrient/food composition database for each cohort (Supplementary Table 1). Supplemental zinc intake (singly or in a multiple-nutrient supplement) was recorded in five cohorts and was quantified in milligrams per day. Total zinc intake was calculated as the sum of dietary and supplemental intake.
Genotyping and imputation.
Twenty single nucleotide polymorphisms (SNPs) were included in the current meta-analysis (Tables 2 and 3 and Supplementary Data). Among these, 18 SNPs were recently associated with fasting glucose and/or fasting insulin levels in a large GWAS and meta-analysis study reported by the MAGIC (2). Two SNPs (rs10493846 and rs11167682) were selected for their potential role in zinc metabolism (24,25). A detailed description of the genotyping methods used for each study is provided in Supplementary Table 1.
TABLE 2.
Total zinc intake and the genetic variants meta-analyzed interactions on fasting glucose levels*
| SNP | Chromosome | Nearest gene | Effect/other allele | Effect allele frequency | n | Cohorts | Interaction (total zinc × SNPs) [β (SE)] | Interaction P | I2 (%) (95% CI) | Q test P |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose-related | ||||||||||
| rs340874 | 1 | PROX1 | C/T | 0.52 | 34,037 | 5 | −0.0012 (0.0005) | 0.03 | 0 (0–79) | 0.99 |
| rs780094 | 2 | GCKR | C/T | 0.58 | 34,307 | 5 | 0.0004 (0.0005) | 0.35 | 0 (0–79) | 0.97 |
| rs560887 | 2 | G6PC2 | C/T | 0.71 | 34,061 | 5 | 3 × 10−5 (0.0005) | 0.95 | 0 (0–79) | 0.84 |
| rs11708067 | 3 | ADCY5 | A/G | 0.76 | 34,111 | 5 | −0.0011 (0.0006) | 0.05 | 25 (0–70) | 0.25 |
| rs11920090 | 3 | SLC2A2 | T/A | 0.83 | 34,033 | 5 | 0.0003 (0.0007) | 0.70 | 42 (0–79) | 0.14 |
| rs2191349 | 7 | DGKB/TMEM195 | T/G | 0.54 | 34,108 | 5 | −0.0002 (0.0005) | 0.71 | 0 (0–79) | 0.47 |
| rs4607517 | 7 | GCK | A/G | 0.21 | 34,333 | 5 | −0.0003 (0.0006) | 0.58 | 34 (0–75) | 0.19 |
| rs11558471 | 8 | SLC30A8 | A/G | 0.70 | 34,150 | 5 | −0.0017 (0.0006) | 0.005 | 0 (0–79) | 0.78 |
| rs7034200 | 9 | GLIS3 | A/C | 0.49 | 33,874 | 5 | 2 × 10−5 (0.0005) | 0.97 | 0 (0–79) | 0.76 |
| rs10885122 | 10 | ADRA2A | G/T | 0.85 | 33,933 | 5 | 0.0002 (0.0008) | 0.75 | 25 (0–70) | 0.25 |
| rs4506565 | 10 | TCF7L2 | T/A | 0.32 | 18,235 | 4 | 0.0002 (0.0005) | 0.71 | 57 (0–86) | 0.07 |
| rs11605924 | 11 | CRY2 | A/C | 0.47 | 34,137 | 5 | 0.0007 (0.0005) | 0.16 | 30 (0–73) | 0.22 |
| rs7944584 | 11 | MADD | A/T | 0.69 | 33,921 | 5 | −0.0004 (0.0006) | 0.44 | 8 (0–81) | 0.36 |
| rs174550 | 11 | FADS1 | T/C | 0.66 | 34,172 | 5 | −0.0005 (0.0005) | 0.33 | 0 (0–79) | 0.82 |
| rs10830963 | 11 | MTNR1B | G/C | 0.28 | 34,009 | 5 | 0.0001 (0.0006) | 0.91 | 48 (0–81) | 0.11 |
| rs11071657 | 15 | C2CD4B | A/G | 0.61 | 33,962 | 5 | 0.0003 (0.0005) | 0.55 | 9 (0–81) | 0.36 |
| Insulin-related | ||||||||||
| rs4675095 | 2 | IRS1 | T/A | 0.06 | 18,241 | 4 | 0.0003 (0.0008) | 0.74 | 0 (0–85) | 0.76 |
| rs35767 | 12 | IGF1 | G/A | 0.81 | 33,888 | 4 | 0.0001 (0.0007) | 0.92 | 0 (0–79) | 0.82 |
| Zinc-related | ||||||||||
| rs10493846 | 1 | SEC63D1 | G/T | 0.74 | 18,158 | 4 | −0.0005 (0.0006) | 0.37 | 0 (0–79) | 0.61 |
| rs11167682 | 5 | SAP30 L | G/T | 0.76 | 18,249 | 5 | 0.0001 (0.0006) | 0.91 | 0 (0–85) | 0.91 |
I2, Higgins heterogeneity index; Q test, Cochran heterogeneity test.
*Estimates for the interaction between total zinc intake (per 1 mg/day from food sources and supplements) and SNPs (per effect allele) on fasting glucose levels (millimoles per liter), adjusted for age and sex, field center (in the CHS and the ARIC study), and family structure by principal components (in the FHS). Boldface values represent P values significant at the conventional level of 0.05.
TABLE 3.
Dietary zinc intake and the genetic variants meta-analyzed interactions on fasting glucose levels*
| SNP | Chromosome | Nearest gene | Effect/other allele | Effect allele frequency | n | Cohorts | Interaction (total zinc × SNPs) [β (SE)] | Interaction P | I2 (%) (95% CI) | Q test P |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose-related | ||||||||||
| rs340874 | 1 | PROX1 | C/T | 0.52 | 45,525 | 14 | −0.0007 (0.0009) | 0.42 | 0 (0–55) | 0.93 |
| rs780094 | 2 | GCKR | C/T | 0.60 | 45,795 | 14 | −0.0006 (0.0009) | 0.52 | 19 (0–56) | 0.25 |
| rs560887 | 2 | G6PC2 | C/T | 0.70 | 45,549 | 14 | 0.0001 (0.001) | 0.90 | 0 (0–55) | 0.79 |
| rs11708067 | 3 | ADCY5 | A/G | 0.70 | 45,599 | 14 | −0.0005 (0.0011) | 0.65 | 0 (0–55) | 0.45 |
| rs11920090 | 3 | SLC2A2 | T/A | 0.76 | 45,521 | 14 | −0.0004 (0.0013) | 0.78 | 30 (0–63) | 0.13 |
| rs2191349 | 7 | DGKB/TMEM195 | T/G | 0.54 | 45,596 | 14 | −0.0021 (0.0008) | 0.01 | 17 (0–55) | 0.27 |
| rs4607517 | 7 | GCK | A/G | 0.25 | 45,821 | 14 | 0.0005 (0.0011) | 0.68 | 23 (0–59) | 0.21 |
| rs11558471 | 8 | SLC30A8 | A/G | 0.70 | 41,994 | 10 | −0.0009 (0.0011) | 0.39 | 0 (0–62) | 0.58 |
| rs7034200 | 9 | GLIS3 | A/C | 0.48 | 45,362 | 14 | 0.0003 (0.0009) | 0.68 | 0 (0–55) | 0.59 |
| rs10885122 | 10 | ADRA2A | G/T | 0.81 | 45,421 | 14 | −0.0016 (0.0012) | 0.17 | 14 (0–52) | 0.31 |
| rs4506565 | 10 | TCF7L2 | T/A | 0.32 | 27,010 | 10 | −0.0004 (0.0010) | 0.68 | 0 (0–62) | 0.73 |
| rs11605924 | 11 | CRY2 | A/C | 0.47 | 45,625 | 14 | 0.0002 (0.0009) | 0.78 | 49 (6–73) | 0.019 |
| rs7944584 | 11 | MADD | A/T | 0.65 | 45,409 | 14 | 0.0008 (0.0010) | 0.44 | 24 (0–60) | 0.20 |
| rs174550 | 11 | FADS1 | T/C | 0.63 | 45,660 | 14 | −0.0019 (0.0009) | 0.04 | 26 (0–61) | 0.18 |
| rs10830963 | 11 | MTNR1B | G/C | 0.28 | 45,497 | 14 | 0.0008 (0.0010) | 0.47 | 34 (0–65) | 0.10 |
| rs11071657 | 15 | C2CD4B | A/G | 0.59 | 45,450 | 14 | −0.0002 (0.0009) | 0.86 | 0 (0–55) | 0.83 |
| Insulin-related | ||||||||||
| rs4675095 | 2 | IRS1 | T/A | 0.07 | 29,729 | 13 | −0.0006 (0.0019) | 0.76 | 42 (0–70) | 0.06 |
| rs35767 | 12 | IGF1 | G/A | 0.74 | 45,376 | 14 | −0.0003 (0.0013) | 0.83 | 0 (0–55) | 0.47 |
| Zinc-related | ||||||||||
| rs10493846 | 1 | SEC63D1 | G/T | 0.74 | 29,646 | 13 | −0.0008 (0.0011) | 0.49 | 21 (0–59) | 0.23 |
| rs11167682 | 5 | SAP30 L | G/T | 076 | 29,737 | 13 | −0.0003 (0.0011) | 0.81 | 0 (0–57) | 0.50 |
I2, Higgins heterogeneity index; Q test, Cochran heterogeneity test.
*Estimates for the interaction between dietary zinc intake (per 1 mg/day from food sources) and SNPs (per effect allele) on fasting glucose levels (millimoles per liter), adjusted for age and sex, field center (in the CHS, the InCHIANTI study, the ARIC study, and the Health ABC study), and family structure by principal components (in the FHS and the FamHS). Boldface values represent P values significant at the conventional level of 0.05.
Statistical analysis.
All cohort-specific statistical analyses followed a uniform analytical plan. Linear regression models were applied to estimate the magnitude of the cross-sectional association of dietary and total zinc intake with fasting glucose levels, as well as the magnitude of the first-order SNP interactions (assuming an additive model) with dietary and total zinc intake on fasting glucose levels. All models were adjusted for age, sex, field center (in the ARIC study, the CHS, the FamHS, the Health Aging and Body Composition [Health ABC] study, and the Aging In the CHIANTI area [InCHIANTI] study), and family structure by principal components (in the Framingham Heart Study [FHS] and the FamHS). Associations were further adjusted for BMI levels to limit potentially confounding effects of adiposity.
Meta-analysis.
The sample size for the association analysis of dietary zinc intake (food sources) with fasting glucose was 46,021 participants. The sample size for the interaction analysis between dietary zinc intake and SNPs ranged from 27,010 to 45,821 participants. Corresponding association and interaction analyses of total zinc intake (food sources and supplements) included 34,533 and 18,158 to 34,333 participants, respectively. We performed power calculations using Quanto version 1.2 (http://hydra.usc.edu/gxe). Accordingly, at 80% power, P < 0.05 detected a difference of 0.0013 mmol/L on fasting glucose levels per 1 mg/day higher dietary zinc intake and 0.0008 mmol/L in fasting glucose levels per 1 mg/day higher total zinc intake. At the same power and critical α, our study was large enough to detect a minimal interaction effect of 0.0031 mmol/L between dietary zinc intake (per 1 mg/day) and SNPs (per effect allele) on fasting blood glucose levels. Our study had 80% power to detect a minimal interaction effect of 0.0012 mmol/L between total zinc intake (per 1 mg/day) and SNPs (per effect allele) on fasting glucose levels.
We conducted an inverse variance-weighted, fixed–effect, meta-analyses on summary statistics provided by each cohort (METAL software, www.sph.umich.edu/csg/abecasis/metal/, for SNPs and zinc intake-interaction effects; SPSS 18.0, SPSS, Chicago, IL, for zinc-intake effects). Heterogeneity was estimated by Cochran’s Q statistic and quantified by the I2 statistic (26). Statistical significance was defined as a P value ≤0.0025 (0.05 per 20 tests), after Bonferroni correction for multiple testing.
RESULTS
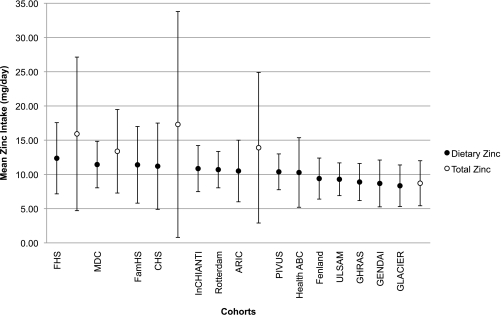
The descriptive characteristics of the 14 participating cohorts are summarized in Table 1. Mean dietary zinc intake (food sources) was comparable across cohorts (Fig. 1), ranging from 8.3 to 12.4 mg/day, with an average dietary zinc intake of 10.3 mg/day. Mean total zinc intake (food sources and supplements) ranged from 8.7 to 17.3 mg/day, resulting in an average total zinc intake of 13.8 mg/day.
TABLE 1.
Descriptive characteristics of the 14 participating cohorts
| Cohorts | N | Age (years) | Women (%) | Fasting glucose (mmol/L)* | Dietary zinc intake (mg/day) | Total zinc intake (mg/day) | Zinc supplement users (%) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| FHS | 5,835 | 46.1 (11.5) | 54.7 | 5.2 (0.5) | 12.4 (5.2) | 15.9 (11.2) | 21.8 | 26.7 (5.0) |
| MDC | 4,867 | 57.5 (5.9) | 60.0 | 5.5 (0.5) | 11.4 (3.4) | 13.4 (6.1) | 18.1 | 25.4 (3.8) |
| FamHS | 2,094 | 50.1 (13.1) | 55.5 | 5.2 (0.5) | 11.4 (5.6) | — | — | 27.3 (5.1) |
| CHS | 1,755 | 71.2 (4.5) | 63.8 | 5.1 (0.6) | 11.2 (6.3) | 17.3 (16.5) | 26.0 | 26.4 (4.3) |
| InCHIANTI | 1,071 | 67.7 (15.8) | 56.3 | 4.8 (0.6) | 10.9 (3.4) | — | 0 | 27.0 (4.1) |
| Rotterdam Study | 2,345 | 65.4 (6.6) | 58.3 | 5.5 (0.5) | 10.7 (2.7) | — | 0 | 26.5 (4.0) |
| ARIC | 6,088 | 60.2 (5.6) | 54.4 | 5.5 (0.6) | 10.5 (4.5) | 13.9 (11.0) | 18.5 | 27.6 (5.0) |
| PIVUS† | 770 | 70.2 (0.2) | 51.0 | 5.0 (0.6) | 10.4 (2.6) | — | — | 26.8 (4.1) |
| Health ABC | 1,263 | 74.8 (2.9) | 50.6 | 5.1 (0.6) | 10.3 (5.1) | — | — | 26.2 (4.0) |
| Fenland Study | 1,071 | 45.0 (7.3) | 56.1 | 4.9 (0.5) | 9.4 (3.0) | — | — | 27.0 (4.9) |
| ULSAM‡ | 931 | 71.0 (0.6) | 0.0 | 5.4 (0.6) | 9.3 (2.4) | — | — | 26.0 (3.2) |
| GHRAS§ | 856 | 71.8 (7.5) | 71.2 | 5.8 (1.6) | 8.9 (2.7) | — | 0 | 29.7 (4.8) |
| GENDAI‖ | 1,087 | 11.2 (0.7) | 53.2 | 4.8 (0.5) | 8.7 (3.4) | — | 0 | 20.0 (3.4) |
| GLACIER¶ | 15,988 | 52.3 (8.8) | 60.2 | 5.4 (0.6) | 8.3 (3.0) | 8.7 (3.3) | 8.3 | 25.9 (4.1) |
Data are means (SD). —, not available.
*Fasting glucose was measured at the baseline examination (when dietary assessment was conducted) in all cohorts, except for the MDC study and Rotterdam Study, in which fasting glucose was measured ~7 months and 6 years, respectively, after the dietary assessment and for the ARIC study, in which examination “three” dietary/supplement data and glucose measurements were used.
†Prospective Investigation of the Vasculature in Uppsala Seniors;
‡Uppsala Longitudinal Study of Adult Men;
§Greek Health Randomized Aging Study;
‖Gene-Diet Investigation on Childhood Obesity;
¶Gene-Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk.
FIG. 1.
Mean dietary and total zinc intake across cohorts. Values are presented as means (SD) and expressed as milligrams per day of dietary (food sources) and total (food sources and supplements) zinc intake. ●, dietary zinc; ○, total zinc.
Association of zinc intake and genetic variants with fasting glucose levels.
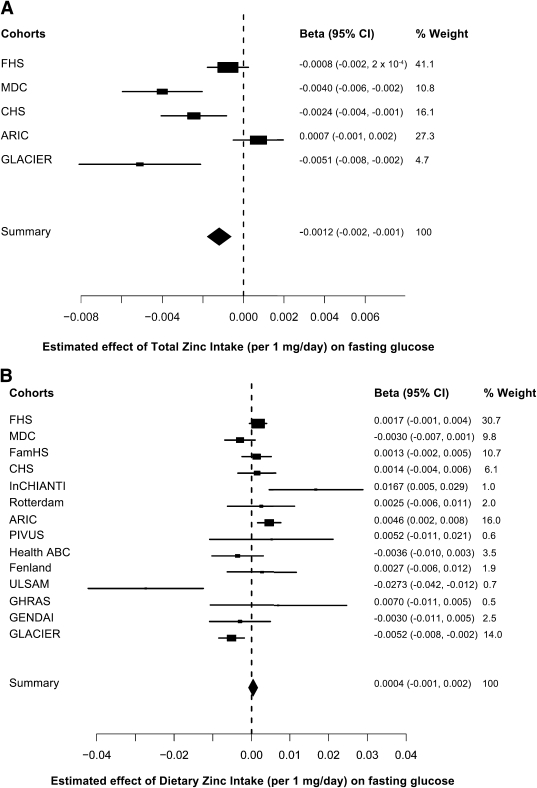
We observed a significant association of total zinc intake with lower fasting glucose levels (summary P value = 0.0003), with an estimated 0.0012 mmol/L lower fasting glucose concentration per 1 mg greater daily total zinc intake (Fig. 2A). When we additionally adjusted for BMI levels, the magnitude of the association was slightly attenuated (β-coefficient ± SE: −0.0009 ± 0.0003 mmol/L fasting glucose per 1 mg/day greater total zinc intake, summary P value = 0.0037). In contrast, we did not observe a significant association of dietary zinc intake with fasting glucose levels (Fig. 2B); however, we observed that the magnitude and direction of cohort-specific associations varied notably. After additional adjustments for BMI levels, the association between dietary zinc intake and fasting glucose levels remained nonsignificant (β-coefficient ± SE: −0.0005 ± 0.0006, summary P value = 0.43).
FIG. 2.
Forest plots of the effect associations of zinc intake with fasting glucose. A: The effect association of total zinc (food sources and supplements) intake (per 1 mg/day) (summary P value = 0.0003, n = 34,533). B: Effect association of dietary zinc (food sources) intake (per 1 mg/day) (summary P value = 0.52, N = 46,021). Fasting blood glucose is expressed in micromoles per liter, and all associations are adjusted for age and sex, field center (in the CHS, the InCHIANTI study, the ARIC study, and the Health ABC study), and family structure by principal components (in the FHS and the FamHS).
In the meta-analysis, 16 of 20 SNPs were significantly associated with fasting glucose levels (Supplementary Table 2). This was consistent with previous published data from the MAGIC (2) that included, as a subsample, roughly one-half of all participants included in the current meta-analysis (Supplementary Table 1).
Interaction of zinc intake and genetic variants on associations with fasting glucose levels.
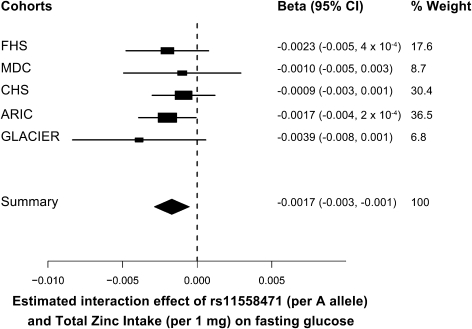
We investigated the interactions between the genetic variants and total zinc intake (available in five cohorts; Supplementary Table 3), and the strongest interaction we observed was for rs11558471 in SLC30A8 (β-coefficient ± SE per A allele of 1 mg/day greater total zinc intake: −0.0017 ± 0.0006; summary interaction P value = 0.005) (Table 2 and Fig. 3). This interaction coefficient indicates that the glucose-raising effect of the risk allele (A) of rs11558471 diminishes as total zinc intake increases (per 1 mg/day), with the strongest inverse association between glucose and total zinc intake seen in individuals carrying both copies of the risk allele. When we applied additional adjustment for BMI, the interaction between rs11558471 and total zinc intake with fasting glucose was slightly attenuated (β-coefficient ± SE: −0.0014 ± 0.0006; summary interaction P value = 0.01).
FIG. 3.
Forest plot of the interaction between SLC30A8 rs11558471 and total zinc intake on fasting glucose. The rs11558471 effect is expressed per A allele, total zinc (food sources and supplements) intake per 1 mg/day, and fasting blood glucose in micromoles per liter. Associations are adjusted for age and sex, field center (in the CHS and the ARIC study), and family structure by principal components (in the FHS) (summary P value = 0.0051, N = 34,150).
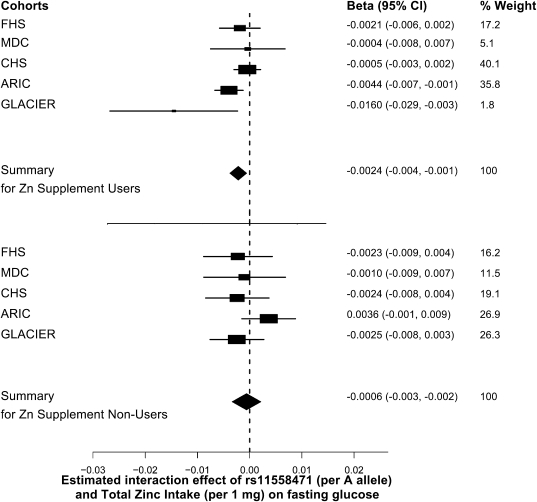
Because the interaction effects reported above may be influenced by the total amount and source of zinc (i.e., dietary or supplemental), we proceeded by stratifying the interaction analyses by zinc supplement use (i.e., zinc supplement users and nonusers) (Fig. 4). As hypothesized, the magnitude of the interaction between total zinc intake and SLC30A8 variant was weaker among zinc-supplement nonusers (β-coefficient ± SE: −0.0006 ± 0.0014; summary P value = 0.65) compared with zinc-supplement users (β-coefficient ± SE: −0.0024 ± 0.0009; summary P value = 0.008).
FIG. 4.
Forest plots of the SLC30A8 rs11558471 interaction with zinc intake on glucose, by zinc supplement use. The rs11558471 effect is expressed per A allele, zinc intake per 1 mg/day, and fasting blood glucose in micromoles per liter. Associations are adjusted for age and sex, field center (in the CHS and the ARIC study), and family structure by principal components (in the FHS) (summary P value = 0.0077, N = 4,986 for zinc supplement users and summary P value = 0.65, n = 29,164 for zinc supplement nonusers).
Few of the additional tests of gene-times-zinc interactions yielded noteworthy results (Tables 2 and 3). Of these, the most statistically significant tests of interaction were observed for the rs340874 PROX1 (for total zinc intake) (Table 2), rs2191349 DGKB/TMEM195, and rs174550 FADS1 variants (for dietary zinc intake) (Table 3 and Supplementary Fig. 2; cohort-specific estimates shown in Supplementary Table 4), although none of these P values withstood correction for multiple testing.
DISCUSSION
Given the important role of zinc in β-cell function and insulin homeostasis (12), we hypothesized that zinc intake might modify the effects of previously discovered glucose-raising genetic loci (2), many of which are thought to influence β-cell function. To test this hypothesis, we conducted a large-scale meta-analysis of up to 14 cohorts in which we assessed the interaction between zinc intake and glucose-associated genetic variants on fasting glucose levels among individuals without T2D.
The strongest interaction effect was detected for the SLC30A8 rs11558471 variant and total zinc intake (food sources and supplements) on fasting glucose levels. We estimated that, for individuals with the A/G genotype (i.e., one glucose-raising allele), an average daily total zinc intake of 14 mg (observed in our study) is associated with a 0.024 mmol/L lower glucose concentration than seen in individuals with the G/G genotype (i.e., two non–glucose-raising alleles). Concordantly, in individuals with both copies of the risk allele (i.e., A/A genotype), the magnitude of this association was doubled (i.e., 0.048 mmol/L). An average daily total zinc intake of 14 mg could be achieved by a nutrient supplement (~10 mg zinc) plus an average serving of red meat or fish/seafood (~3 ounces) or three average servings of dairy products (~2 cups of milk per yogurt and 3 ounces of cheese). Further investigation of this interaction effect showed that it was more evident among zinc supplement users than among nonusers. We also observed a significant association of total zinc intake, but not dietary zinc intake alone, with lower fasting glucose levels. None of the other investigated interactions between zinc intake (dietary or total) and variants were statistically significant after multiple-testing adjustment.
Current findings in the context of gene-environment interaction investigations.
The SLC30A8 gene encodes the newly characterized ZnT8 zinc transporter (27). Zinc homeostasis depends on two families of transporters: the ZIP (SLC39) family regulating cellular zinc influx and the ZnT (SLC30) family regulating efflux (28). It has been shown that the ZnT8 transporter is primarily expressed in β-cells and colocalizes with insulin-containing secretory granules (29). Alterations in ZnT8 expression strongly modulate insulin secretion (29,30). Moreover, it has recently been shown that ZnT8 β-cell–specific knockout (Znt8KO) mice are glucose intolerant have reduced β-cell zinc accumulation, have atypical insulin granules, have reduced first-phase glucose-stimulated insulin secretion, have reduced insulin-processing enzyme transcripts, and have increased proinsulin levels (14). Furthermore, a genetic variant (rs13266634) in SLC30A8 has been reliably associated with fasting glucose levels and T2D risk in several GWAS (31–34). Of interest, observations suggest that this variant impairs islet ZnT8 expression, insulin secretion, or glucose homeostasis (35–37). In addition, this variant is associated with the production of a less active zinc transporter protein, suggesting less efficiency of zinc accumulation and insulin crystallization (38). It recently has been demonstrated that the same variant does not affect insulin secretion from human islets as well as islet expressions of SLC30A8 (39). It is noteworthy that rs13266634 is in strong linkage disequilibrium with rs11558471 (r2 = 0.96), included in the current study. Additional studies on these topics are important to provide evidence that variation at SLC30A8 influences the regulation of zinc transporter activity or the modulation of islet zinc content; this would support the biological plausibility of the statistical interaction we report here.
Current findings in the context of dietary epidemiological investigations.
Our meta-analysis revealed a significant inverse association between total zinc intake and fasting glucose levels. Of interest, we did not observe this association when only zinc derived from food sources was considered. This observation may be attributable to a number of factors, including differences in the bioavailability of dietary zinc compared with zinc supplements (40), a threshold effect of zinc intake on fasting glucose levels, or because when dietary and supplemental zinc are combined the wider trait variance affords greater statistical power to detect associations and interactions. The latter also is based on the power calculations we performed (research design and methods). Moreover, the different dietary assessment tools used to assess dietary zinc intake across the participating cohorts may have varied in precision to a greater extent than when used to assess supplemental zinc intake. However, potential confounding lifestyle characteristics associated with supplement use were not considered in the current study.
Our findings are in line with results from a large 24-year prospective study among women, demonstrating a significant association between total zinc intake and lower risk of T2D (17). In the same study, but in contrast to our results, dietary zinc intake also was associated with a lower risk of T2D after multiple adjustments for dietary and nondietary factors (17). In a cross-sectional study of an Asian-Indian population (16), dietary zinc intake was associated with a lower prevalence of T2D and the metabolic syndrome. However, another study of Chinese adults did not observe a relationship between dietary zinc intake and risk of hyperglycemia (15). However, it is worth mentioning that in the current study we focused on fasting glucose levels in subjects without T2D, as opposed to the prevalence of T2D in the referenced studies.
Strengths and limitations.
The strengths of the current work include the large samples, the availability of standardized exposure and outcome data, and the use of a uniform analytical plan prior to meta-analysis. The advantages of meta-analyses like ours over individual studies or literature-based meta-analysis include improved power to detect interaction effects and the minimization of recall bias and publication bias (41,42).
We selected the majority of the genetic variants included in our meta-analysis on the basis of the results of GWAS for genetic main effects on insulin or glucose traits (2). This approach is convenient, and one can be confident that the genetic variants are reliably associated with the traits of interest, which is rarely the case with conventional biologic candidate-gene studies. Nevertheless, it may be that the variants that reach genome-wide significance in main-effect GWAS are less likely to interact with environmental factors because such interactions tend to weaken the statistical significance of the main effects (43). Although the effect estimates observed here are indeed small in magnitude, it is important to bear in mind that these effects are likely to be underestimated, given that the observed genetic loci may not be causal and because the methods of determining usual zinc intake are imprecise. Our approach does not preclude that other genetic variants not previously known to be associated with glycemic traits might interact with zinc intake.
As with all epidemiological studies assessing dietary intake, systematic measurement error in the diet exposure could have biased our results. However, there currently is no satisfactory biomarker for the assessment of dietary zinc intake, and even though plasma zinc concentration seems to be a reliable marker of zinc status, it has limited sensitivity in the normal range of zinc intake (44,45). The issues of dietary assessment validity versus cost-effectiveness in population-based studies have been widely discussed elsewhere (46,47), but it is unlikely that a glucose-associated genotype is associated with a tendency to misreport dietary intake. Furthermore, we did not assess interactions of zinc with other nutrients; such interactions might influence the bioavailability of zinc (40). Systematic measurement error in the estimation of dietary exposures in gene-environment interaction studies could bias the estimation of the dietary main effect and lead to underestimates of the interaction between dietary factors and genetic variants, potentially raising type II error rates (48).
We conducted a large-scale, gene-diet interaction meta-analysis in which we investigated the association between zinc intake (dietary and total) and fasting glucose levels and the interactions between zinc intake and glucose-, insulin-, and zinc-related genetic variants on fasting glucose levels. We showed that total zinc intake (food sources and supplements), but not zinc from foods alone, is associated with lower fasting glucose levels in individuals without T2D. Our findings suggest that total zinc intake has a stronger inverse association with fasting glucose levels in individuals carrying the glucose-raising A allele of rs11558471 SLC30A8 (a β-cell zinc transporter), compared with individuals carrying the G allele. The current study indicates that gene-environment interaction analyses can help elucidate our understanding of the biological pathways involved in micronutrient influences on systemic glucose homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
S.K., J.A.N., M.-F.H., Z.Y., F.J.A.v.R., J.B.M., P.W.F., and G.V.D. drafted the manuscript. S.K., J.A.N., Z.Y., F.J.A.v.R., D.S., E.S., J.S.N., M.K.W., R.N.L., S.G., J.S.A., T.T., G.H., G.S., F.R., L.A.C., J.D., and P.W.F. performed the data analyses. S.K., M.-F.H., F.J.A.v.R., G.H., A.J.B., C.M.v.D., J.C.F., C.S.F., D.K.H., F.B.H., P.F.J., I.J., L.L., Y.L., N.M., J.O., A.-C.S., A.G.U., M.Y., I.P., S.B., L.F., E.I., and P.W.F. performed the experiments. C.M.D., A.H., A.G.U., N.J.W., S.B., N.G.F., L.A.C., R.J.L., G.H., J.D., C.L., L.F., S.B.K., M.I.M., E.I., I.B.B., J.C.M.W., M.O.-M., D.S.S., J.B.M., P.W.F., and G.V.D. contributed the reagents, materials, and analysis tools. E.S., M.K.W., R.N.L., S.G., C.M.D., J.C.F., A.H., R.C.H., D.K.H., F.B.H., P.F.J., L.L., N.M., J.S.P., E.J.G.S., A.G.U., M.C.Z., L.A.C., J.D., C.L., E.I., J.C.M.W., M.O.-M., and D.S.S. reviewed and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0176/-/DC1.
*A complete list of the MAGIC Investigators and institutions is provided in the Supplementary Data.
REFERENCES
- 1.McCarthy MI, Zeggini E. Genome-wide association studies in type 2 diabetes. Curr Diab Rep 2009;9:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupuis J, Langenberg C, Prokopenko I, et al. ; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC Investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staiger H, Machicao F, Fritsche A, Häring HU. Pathomechanisms of type 2 diabetes genes. Endocr Rev 2009;30:557–585 [DOI] [PubMed] [Google Scholar]
- 4.Wolfs MG, Hofker MH, Wijmenga C, van Haeften TW. Type 2 diabetes mellitus: new genetic insights will lead to new therapeutics. Curr Genomics 2009;10:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bantle JP, Wylie-Rosett J, Albright AL, et al. ; American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 6.Prasad AS. Zinc: an overview. Nutrition 1995;11(Suppl.):93–99 [PubMed] [Google Scholar]
- 7.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 1996;271:1081–1085 [DOI] [PubMed] [Google Scholar]
- 8.Mocchegiani E, Malavolta M. Zinc-gene interaction related to inflammatory/immune response in ageing. Genes Nutr 2008;3:61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev 1993;73:79–118 [DOI] [PubMed] [Google Scholar]
- 10.Scott DA. Crystalline insulin. Biochem J 1934;28:1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rungby J. Zinc, zinc transporters and diabetes. Diabetologia 2010;53:1549–1551 [DOI] [PubMed] [Google Scholar]
- 12.Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab 2009;11(Suppl. 4):202–214 [DOI] [PubMed] [Google Scholar]
- 13.Jansen J, Karges W, Rink L. Zinc and diabetes: clinical links and molecular mechanisms. J Nutr Biochem 2009;20:399–417 [DOI] [PubMed] [Google Scholar]
- 14.Wijesekara N, Dai FF, Hardy AB, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010;53:1656–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Z, Yuan B, Qi L, Dai Y, Zuo H, Zhou M. Zinc intake and the risk of hyperglycemia among Chinese adults: the prospective Jiangsu Nutrition Study (JIN). J Nutr Health Aging 2010;14:332–335 [DOI] [PubMed] [Google Scholar]
- 16.Singh RB, Niaz MA, Rastogi SS, Bajaj S, Gaoli Z, Shoumin Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr 1998;17:564–570 [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 2009;32:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase H, Overbeck S, Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol 2008;43:394–408 [DOI] [PubMed] [Google Scholar]
- 19.Nettleton JA, McKeown NM, Kanoni S, et al. Interactions of dietary whole-grain intake with fasting glucose- and insulin-related genetic loci in individuals of European descent: a meta-analysis of 14 cohort studies. Diabetes Care 2010;33:2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanoni S, Dedoussis GV, Herbein G, et al. Assessment of gene-nutrient interactions on inflammatory status of the elderly with the use of a zinc diet score: ZINCAGE study. J Nutr Biochem 2010;21:526–531 [DOI] [PubMed] [Google Scholar]
- 21.Mariani E, Neri S, Cattini L, et al. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp Gerontol 2008;43:462–471 [DOI] [PubMed] [Google Scholar]
- 22.Mocchegiani E, Giacconi R, Costarelli L, et al. Zinc deficiency and IL-6 -174G/C polymorphism in old people from different European countries: effect of zinc supplementation: ZINCAGE study. Exp Gerontol 2008;43:433–444 [DOI] [PubMed] [Google Scholar]
- 23.Psaty BM, O’Donnell CJ, Gudnason V, et al. ; CHARGE Consortium Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2009;2:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Miyamoto N, Shouguchi-Miyata J, Ikeda JE. HFM1, the human homologue of yeast Mer3, encodes a putative DNA helicase expressed specifically in germ-line cells. DNA Seq 2006;17:242–246 [DOI] [PubMed] [Google Scholar]
- 25.Viiri KM, Jänis J, Siggers T, et al. DNA-binding and -bending activities of SAP30L and SAP30 are mediated by a zinc-dependent module and monophosphoinositides. Mol Cell Biol 2009;29:342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 27.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004;53:2330–2337 [DOI] [PubMed] [Google Scholar]
- 28.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 2006;281:24085–24089 [DOI] [PubMed] [Google Scholar]
- 29.Chimienti F, Devergnas S, Pattou F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 2006;119:4199–4206 [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, Tian W, Pratt EB, et al. Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PLoS ONE 2009;4:e5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena R, Voight BF, Lyssenko V, et al. ; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 32.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 34.Zeggini E, Weedon MN, Lindgren CM, et al. ; Wellcome Trust Case Control Consortium (WTCCC) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007;316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirchhoff K, Machicao F, Haupt A, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 2008;51:597–601 [DOI] [PubMed] [Google Scholar]
- 36.Palmer ND, Goodarzi MO, Langefeld CD, et al. Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes 2008;57:1093–1100 [DOI] [PubMed] [Google Scholar]
- 37.Staiger H, Machicao F, Stefan N, et al. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS ONE 2007;2:e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes–associated variants. Diabetes 2009;58:2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cauchi S, Del Guerra S, Choquet H, et al. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab 2010;100:77–82 [DOI] [PubMed] [Google Scholar]
- 40.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr 2010;91:1478S–1483S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet 2005;6:287–298 [DOI] [PubMed] [Google Scholar]
- 42.Palla L, Higgins JP, Wareham NJ, Sharp SJ. Challenges in the use of literature-based meta-analysis to examine gene-environment interactions. Am J Epidemiol 2010;171:1225–1232 [DOI] [PubMed] [Google Scholar]
- 43.Murcray CE, Lewinger JP, Gauderman WJ. Gene-environment interaction in genome-wide association studies. Am J Epidemiol 2009;169:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr 2008;99(Suppl. 3):S14–S23 [DOI] [PubMed] [Google Scholar]
- 45.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr 2009;89:2040S–2051S [DOI] [PubMed] [Google Scholar]
- 46.Tucker KL. Assessment of usual dietary intake in population studies of gene-diet interaction. Nutr Metab Cardiovasc Dis 2007;17:74–81 [DOI] [PubMed] [Google Scholar]
- 47.Serra-Majem L, Pfrimer K, Doreste-Alonso J, et al. Dietary assessment methods for intakes of iron, calcium, selenium, zinc and iodine. Br J Nutr 2009;102(Suppl. 1):S38–S55 [DOI] [PubMed] [Google Scholar]
- 48.Greenwood DC, Gilthorpe MS, Cade JE. The impact of imprecisely measured covariates on estimating gene-environment interactions. BMC Med Res Methodol 2006;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.