Abstract
OBJECTIVE
The incretin hormone GIP (glucose-dependent insulinotropic polypeptide) promotes pancreatic β-cell function by potentiating insulin secretion and β-cell proliferation. Recently, a combined analysis of several genome-wide association studies (Meta-analysis of Glucose and Insulin-Related Traits Consortium [MAGIC]) showed association to postprandial insulin at the GIP receptor (GIPR) locus. Here we explored mechanisms that could explain the protective effects of GIP on islet function.
RESEARCH DESIGN AND METHODS
Associations of GIPR rs10423928 with metabolic and anthropometric phenotypes in both nondiabetic (N = 53,730) and type 2 diabetic individuals (N = 2,731) were explored by combining data from 11 studies. Insulin secretion was measured both in vivo in nondiabetic subjects and in vitro in islets from cadaver donors. Insulin secretion was also measured in response to exogenous GIP. The in vitro measurements included protein and gene expression as well as measurements of β-cell viability and proliferation.
RESULTS
The A allele of GIPR rs10423928 was associated with impaired glucose- and GIP-stimulated insulin secretion and a decrease in BMI, lean body mass, and waist circumference. The decrease in BMI almost completely neutralized the effect of impaired insulin secretion on risk of type 2 diabetes. Expression of GIPR mRNA was decreased in human islets from carriers of the A allele or patients with type 2 diabetes. GIP stimulated osteopontin (OPN) mRNA and protein expression. OPN expression was lower in carriers of the A allele. Both GIP and OPN prevented cytokine-induced reduction in cell viability (apoptosis). In addition, OPN stimulated cell proliferation in insulin-secreting cells.
CONCLUSIONS
These findings support β-cell proliferative and antiapoptotic roles for GIP in addition to its action as an incretin hormone. Identification of a link between GIP and OPN may shed new light on the role of GIP in preservation of functional β-cell mass in humans.
More than 35 genetic loci have been shown to influence risk of type 2 diabetes or plasma glucose or insulin levels in genome-wide association studies (GWAS) (1–3). For most of these loci we lack insight into the mechanisms by which they increase risk of type 2 diabetes. Recently, a combined analysis of several GWAS (Meta-analysis of Glucose and Insulin-Related Traits Consortium [MAGIC]) showed association to postprandial insulin at the GIP (glucose-dependent insulinotropic polypeptide) receptor (GIPR) locus (SNP rs10423928) on chromosome 19q13.3 (4). Carriers of the risk genotype showed impaired insulin secretion, but this was surprisingly not translated into a similar increased risk of type 2 diabetes as seen for other variants with similar effects on insulin secretion in the DIAbetes Genetics Replication and Meta-analysis (DIAGRAM +) study (2,4). The human GIPR gene contains 14 exons with a protein coding region of 12.5 kb (5). GIP is released after food ingestion from intestinal K cells to stimulate insulin and, to a lesser extent, glucagon secretion from pancreatic β- and α-cells, respectively. GIP has also been ascribed long-term positive effects on β-cell function by stimulating cell proliferation and inhibiting apoptosis (6). A similar insulinotropic effect is achieved by glucagon-like peptide 1 (GLP-1), which is secreted from intestinal L cells, but in contrast to GIP, GLP-1 inhibits glucagon secretion. Both GLP-1 and GIP are rapidly degraded by the enzyme dipeptidyl peptidase IV, inhibition of which is a novel approach enhancing incretin levels for treatment of type 2 diabetes (7).
Circulating concentrations of the cytokine osteopontin (OPN) are elevated in patients with type 2 diabetes, and OPN has been suggested to promote the development of atherosclerosis and diabetes complications (8–10). In islets, however, OPN has been shown to inhibit cytokine-induced apoptosis via reduction of NO and iNOS levels (11) and to stimulate β-cell proliferation (12).
Since GIP and OPN have similar effects in many tissues, including proapoptotic effects on β-cell survival in islets (11–16) and regulation of adipocyte metabolism in fat tissue (17,18), we advanced the hypothesis that the effect of GIP on apoptosis and β-cell proliferation involves OPN. The aim of the current study was to explore metabolic effects by which a variant in the GIPR gene contributes to altered islet function in humans and why this impairment in β-cell function was not translated into a similarly increased risk of type 2 diabetes as seen for other variants with similar effects on insulin secretion. We further examined mechanisms that could explain the effects of GIP in different tissues and whether GIP could stimulate osteopontin in human islets and whether this was influenced by the GIPR gene variant.
RESEARCH DESIGN AND METHODS
All human and animal protocols were approved by the local ethics committees and performed in accordance with local institutional and national regulations.
Study participants.
We explored associations of GIPR rs10423928 with metabolic phenotypes in both nondiabetic (N = 53,730) and type 2 diabetic individuals (N = 2,731) from 11 studies: Botnia Prospective Study (BPS) (19,20), Prevalence, Prediction, and Prevention of Diabetes (PPP)-Botnia (21), Steno Incretin Clamps (22), Malmö Preventive Project (MPP) (20,23), Malmö Diet and Cancer Study (24), the METabolic Syndrome In Men (METSIM) (25), Genetics, Physiopathology and Evolution of Type 2 Diabetes (GENFIEV: www.genfiev.it), Verona Newly Diagnosed Type 2 Diabetes Study (26,27), Low Birth Weight Cohort (22,28,29), Steno Twins (30,31), and European network on Functional Genomics of Type 2 Diabetes (EUGENE) (32,33).
In vivo experiments, measurements, and calculations.
Weight, height, waist and hip circumference, lean body mass, and blood pressure were measured in each cohort. Fat mass and lean body mass were measured with the bioelectric impedance method. Blood samples were drawn at baseline at 0, 30 (40 in MPP), and 120 min of the 75-g oral glucose tolerance test (OGTT) for measurements of blood glucose and serum insulin concentrations; in addition, plasma GIP concentrations were measured in the PPP-Botnia study.
Forty-seven young healthy men from the Steno Low Birth Weight Cohort underwent hyperglycemic clamps (7 mmol/L; 2 h) with infusion of GLP-1 or GIP on separate days (22). Glucose infusion was initiated at t = −30 min and terminated at t = 120 min. At t = −2 min, a bolus of either GIP or GLP-1 was infused to increase the plasma concentration to approximately 120 and 1000 pmol/L, respectively. At t = 0 min, a continuous infusion of GIP or GLP-1 (60 or 240 pmol/kg ⋅ h, respectively) was initiated and terminated at t = 120 min. P-glucose and p-insulin were determined as previously described (22,34). Intact, biologically active GIP was measured using an assay specific for the intact NH2 terminus of GIP (35). Plasma samples were assayed for GLP-1 immunoreactivity using a radioimmunoassay specific for amidated COOH terminus of the GLP-1 molecule; this assay measures the sum of the intact peptide plus the primary metabolite, GLP-1 (9–36) amide, which is formed by the actions of the enzyme DPP-4. The results of this assay therefore provide an estimate of the secretion of GLP-1 (36). The first-phase insulin response to GIP or GLP-1 infusions was defined as AUCinsulin 0–20 min, and the second-phase response as AUCinsulin 20–120 min, where AUC is area under the curve.
Insulin secretion during OGTT was assessed as corrected incremental insulin response to glucose (CIR = [100 × insulin 30 min]/[glucose 30 min] × [glucose 30 min − 3.89]) (37) or as disposition index, i.e., insulin secretion adjusted for insulin sensitivity (CIR × ISI) (38). Insulin sensitivity index (ISI) was calculated from the OGTT as 10,000/√([fasting glucose × fasting insulin][mean OGTTglucose × mean OGTTinsulin]) (39).
Genotyping.
Genotyping of rs10423928 was performed using matrix-assisted laser desorption ionization time of flight mass spectrometry on the Sequenom MassARRAY platform (San Diego, CA) for PPP-Botnia and METSIM studies and using an allelic discrimination method with a TaqMan assay on the ABI 7900 platform (Applied Biosystems, Foster City, CA) for MPP, BPS, Steno (incretin clamps and twins), human islets, Verona, and GINFIEV. We obtained an average genotyping success rate of >95.5%, and the average concordance rate in all studies was >99.9%. Hardy-Weinberg equilibrium was fulfilled in all studied populations (P > 0.50).
Human islets from cadaver donors.
Islets from 50 human cadaver donors (mean ± SEM: nondiabetic N = 43, sex M/F 24/19, age 55.9 ± 1.8 years, BMI 25.2 ± 0.5 kg/m2, HbA1c % 5.5 ± 0.09; diabetic N = 7, sex M/F 3/4, age 55.9 ± 4.5 years, BMI 27.3 ± 1.3 kg/m2, HbA1c % 7.3 ± 0.3) were provided by the Nordic Network for Clinical Islets Transplantation by the courtesy of Olle Korsgren (Uppsala University, Uppsala, Sweden). The experimental protocol for isolation of islets was approved by the ethics committee of Uppsala University and performed in accordance with local institutional and Swedish national regulations. Further characterization of islets was performed at Lund University Diabetes Center (LUDC) Human Tissue Laboratory. Glucose- and GIP- (Bachem, Bubendorf, Switzerland) stimulated insulin secretion were measured using radioinmmunoassays (Euro-Diagnostica, Malmö, Sweden).
Measurements of GIPR and OPN mRNA expression.
Real-time quantitative RT-PCR (TaqMan Gene Expression Assays, Applied Biosystems) was used to measure mRNA levels in human and mouse islets. RNA was isolated using AllPrep DNA/RNA Mini Kit or RNeasy Plus Mini Kit for human islets (both from Qiagen, Valencia, CA). Concentration and purity were measured using NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). No sign of degradation was observed using agarose gel electrophoresis and Experion DNA 1 K gel chips (Bio-Rad, Hercules, CA). 0.2–0.5-μg RNA was used for cDNA synthesis with RevertAid First Strand cDNA Synthesis Kit (Fermentas Life Sciences, St. Leon-Rot, Germany). TaqMan gene expression assays were purchased from Applied Biosystems. For human tissue, the following assay identification numbers were used: GIPR, Hs00164732_m1; OPN (SPP1) Hs00959010_m1; and PDX1, (pancreatic and duodenal homeobox 1) Hs00236830_m1. The corresponding assay identification number used for mouse OPN was Mm00436767_m1. Q-PCR reactions were run in triplicate (total volume of PCR reactions was 10 μl) using 5–10 ng cDNA, depending on the tissue on the ABI 7900 HT (Applied Biosystems). Human GIPR, OPN, and PDX1 mRNA levels were normalized to three housekeeping genes, HPRT1 (HGPRT, article no. 4326321E), PPIA (cyclophilin A, article no. 4326316E), and polymerase (RNA) II (DNA-directed) polypeptide A, 220 kDa (POLR2A) (Hs00172187_m1), and mouse OPN mRNA to two housekeeping genes, PPIb (cyclophilin B) (Mm00478295_m1) and GAPDH (article no. 4352339E) using Genorm v. 3.5 software. GIPR mRNA levels in human islets were corrected for PDX1 gene expression to adjust for potential differences in β-cell mass.
Detection of GIPR and OPN protein by immunohistochemistry.
Human pancreatic islet specimens (n = 5) taken during pancreatic surgery and pancreatic islets from SD rats (n = 10) and C57Bl/6J mice (n = 10, Taconic, Copenhagen, Denmark) were used.
Immunofluorescence.
Pancreatic tissue was stained as previously described (40). Primary antibodies were diluted in PBS containing 0.25% bovine serum albumin and 0.25% Triton X-100 and applied overnight at 4°C. Rabbit polyclonal anti-GIPr (1:1,600) (41), guinea pig polyclonal antiproinsulin (1:5,120; code 9003, Euro-Diagnostica, Malmö, Sweden), guinea pig polyclonal antiglucagon (1:5,120; code 8708, Euro-Diagnostica), goat polyclonal antisomatostatin (1:800; code SC7819, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and either mouse monoclonal for human and rat OPN or rabbit polyclonal for mouse OPN (1:500; Developmental Studies Hybridoma Bank [Iowa City, IA] and IBL [Hamburg, Germany], respectively) were used. Secondary antibodies specific for rabbit, guinea pig, or goat IgG and coupled to either fluorescein isothiocyanate (FITC) or Texas red (Jackson, West Grove, PA) were applied for 1 h at room temperature. Immunofluorescence was examined in an epifluorescence microscope (BX60; Olympus, Lund, Sweden). Images were taken with a digital camera (DS-2Mv; Nikon, Lund, Sweden).
In vitro stimulation experiments and OPN protein measurements
Islets and pancreatic β-cells.
Islets were isolated from female NMRI mice (Bolmholtgaard, Ry, Denmark) by collagenase digestion as described elsewhere (42). The procedure for sacrificing the animals was approved by the ethical committee in Lund. Islets were used directly or after culture for 24 or 48 h in plastic petri dishes containing RPMI 1640 medium with 10% (vol/vol) fetal calf serum, 100 μg/mL streptomycin, and 100 IU/mL penicillin and supplemented with 5 or 16.7 mmol/L glucose (Sigma, Malmö, Sweden) in the presence or absence of GIP or GLP-1 (0.1, 1, or 100 nmol/L; Bachem). Approximately 100–200 islets were used for each condition and experiments were repeated 6–16 times. After stimulation, islets were used for measurements of OPN mRNA by real-time quantitative RT-PCR and/or protein expression by immunofluorescence confocal microscopy and Western blotting. For protein measurements, cells were isolated from islets and double stained for insulin and OPN as previously described (36). Images were obtained at 63× magnification on a Zeiss LSM 5 laser scanning confocal microscope (Carl Zeiss, Inc., Jena, Germany). For quantification, mean fluorescence intensity of OPN in insulin-positive β-cells (range 0–255 grayscale values) after background subtraction was calculated by using the Zeiss LSM 5 Pascal Analysis software. For Western blotting, primary anti-OPN antibody was used (1:500 dilution; IBL) with a horseradish peroxidase–conjugated secondary antibody (Cell Signaling, Danvers, MA). Bands were detected with chemiluminescence (Supersignal West Dura; Pierce Biotechnology, Rockford, IL), and Western blotting of β-actin (1:3,000 dilution; GenScript Corporation, Piscataway, NJ) was used as loading control.
Assessment of β-cell viability.
Pancreatic β-cell viability was performed using a CellTiter 96 AQueous One Solution Cell Proliferation Assay Reagent (Promega, Stockholm, Sweden) according to the manufacturer’s instructions. The actual performance is based on the spectrophotometric detection of a colored formazan product converted from an (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) compound by NADPH or NADH via metabolically active cells. After a culture period of 24 h at 5.5 mmol/L glucose in the presence and absence of a cytokine cocktail containing interleukin (IL)-1β (50 ng/mL), interferon (INF)-γ (75 ng/mL), and tumor necrosis factor (TNF)-α (75 ng/mL) with or without GIP (100 nM), the dispersed β-cells were washed three times with fresh culture medium. Thereafter, the cells were incubated for 2 h in CellTiter 96 Aqueous One Solution Reagent before measuring absorbance at 490 nm with a 96-well plate reader.
Proliferation assay.
INS-1 832/13 cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2-mercaptoethanol (50 μmol/L), penicillin (100 IU/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. 50,000 or 100,000 cells/well were seeded in 96-well plates in standard cell culture medium or in medium containing only 2% fetal calf serum for 48 h in the presence of PBS (control) or osteopontin (as indicated; R&D Systems, Abingdon, U.K.). To measure DNA synthesis, the cells were pulsed with 1 μCi [methyl-3H]thymidine (Amersham Biosciences, Uppsala, Sweden) during the last 20 h of the experiment. Macromolecular material was then harvested onto glass fiber filters using a Printed Filtermat A (1450–421; Wallac Oy, Turku, Finland). The filters were air dried, and the bound radioactivity was measured in a β-counter (Wallac 1450; MicroBeta, Ramsey, MN).
Statistical analyses.
Linear regression analyses were used to test genotype–phenotype correlations adjusted for age, sex, and BMI (apart from BMI and weight) and for within-family dependence (BPS) or for birth weight and sampling period (Steno Low Birth Weight Cohort). Nonnormally distributed variables were logarithmically (natural) transformed before analysis. The odds ratios for risk of developing type 2 diabetes were calculated using logistic regression analyses adjusted for age, sex, and BMI. Analyses were performed using SPSS version 17.0, PLINK, or STATA version 10. For in vitro studies, results were expressed as mean ± SEM, where applicable. Statistical analyses were performed using GraphPad (Prism 4.0) or Origin (Originlab), and significance was determined using one-way ANOVA followed by Bonferroni or Tukey–Kramer tests, or unpaired two-tailed Student t test.
RESULTS
The GIPR variant is associated with glucose- and GIP-stimulated insulin secretion.
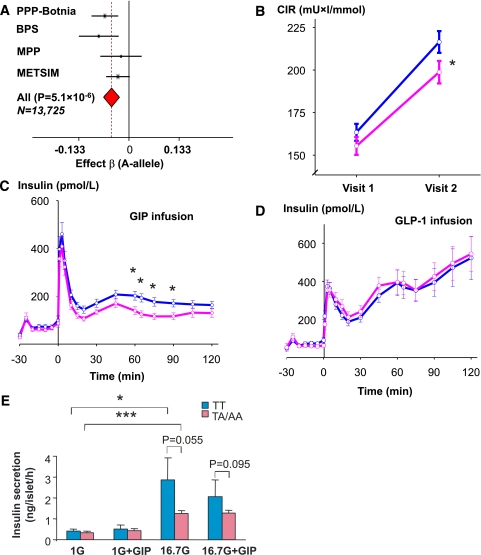
Figure 1A shows that the A allele of GIPR rs10423928 (4) is associated with impaired glucose-stimulated insulin secretion adjusted for BMI during an OGTT in a meta-analysis of 13,725 nondiabetic individuals (Pmeta = 5.1 × 10−6) (Fig. 1A). In addition, the A allele was associated with impaired β-cell function in patients with type 2 diabetes (Supplementary Table 1, studies VII and VIII). Carriers of the TA/AA genotypes increased their insulin secretion during a mean 7.8-year follow-up period less than carriers of the wild-type TT genotype (P < 0.01; Fig. 1B). In contrast to the impairment in insulin response to oral glucose, there was no impairment in the insulin response to intravenous glucose, supporting the presence of an incretin defect (Supplementary Table 1, study II). To demonstrate that the impaired incretin effect was due to impaired GIP action, we also assessed the insulin response to an exogenous GIP infusion in nondiabetic subjects. Despite similar GIP concentrations, the TA/AA genotype carriers showed reduced GIP-stimulated (P < 0.05), but not GLP-1–stimulated, insulin secretion compared with TT genotype carriers (Fig. 1C and D). The glucagon response to GIP or OGTT was not influenced by the GIPR variant (Supplementary Table 1, studies I and IX).
FIG. 1.
Effects of GIPR rs10423928 on insulin secretion in vitro and in vivo. A: Meta-analysis of the effect of GIPR rs10423928 on CIR estimated from the glucose-stimulated insulin secretion measured at 30 min during OGTT in the PPP-Botnia (N = 4,358), BPS (N = 2,255), MPP (N = 1,547), and METSIM (N = 5,563) studies. Effect β is for the risk A allele. B: Change in insulin secretion (CIR, corrected early insulin response to glucose at 30 min adjusted for BMI) over mean 7.8-year follow-up time in nondiabetic individuals (BPS, N = 2,255) in carriers of nonrisk TT (blue) and risk TA/AA (pink) genotypes of GIPR rs10423928 (*P < 0.05). C: Insulin response to GIP infusions was lower in TA/AA than in TT genotype carriers (N = 47; *P < 0.05). D: Insulin response to GLP-1 infusion was not affected by genotype (N = 47). E: Insulin release from nondiabetic donors. Insulin secretion was measured from islets from cadaver donors with nonrisk (TT) or risk (TA/AA) genotype. Islets were preincubated with 1 mmol/L glucose prior to incubation for 1 h in either 1 or 16.7 mmol/L glucose with or without the addition of 100 nmol/L GIP as indicated. The number of donors (N) in each group ranged between 11 and 23. *P < 0.05. ***P < 0.001 vs. 1 mmol/L glucose.
Also, in islets from 17 human cadaver donors with the TA/AA genotypes, a trend toward decreased glucose- and GIP-stimulated insulin secretion was observed when compared with islets from 20 TT genotype carriers (Fig. 1E). Together, these data demonstrate that the GIPR variant is associated with both GIP- and glucose-stimulated insulin secretion.
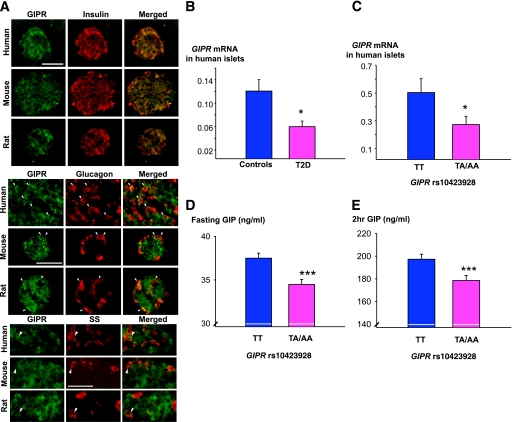
GIPR expression in human, mouse, and rat islets.
GIPR protein as detected by immunofluorescence microscopy of pancreatic sections was evident in β-cells from human, mouse, and rat islets, but less so in α- and δ-cells (Fig. 2A). GIPR mRNA was lower in islets from diabetic (N = 7) than from nondiabetic donors (N = 43) (P = 0.017; Fig. 2B). It was also lower in islets from nondiabetic donors with the TA/AA genotypes (N = 20) compared with donors with the TT genotype (N = 22) (P = 0.0127; Fig. 2C).
FIG. 2.
Expression of GIPR in islets. A: Human, mouse, and rat islet sections double immunostained for GIPR (green) and insulin (red), glucagon (red), and somatostatin (red) showing GIPR expression in β-, α-, and δ-cells (yellow in the merged images). Scale bars, 50 μm. Arrowheads indicate GIPR-immunoreactive α- and δ-cells. B: GIPR mRNA levels were lower in human pancreatic islets from diabetic (n = 7) as compared with nondiabetic donors (n = 43) (P = 0.017). C: GIPR mRNA levels were lower in nondiabetic carriers of the TA/AA (n = 20) than in TT genotypes (n = 22) (P = 0.0127). D: Fasting GIP levels were lower in carriers of the TA/AA than in TT genotypes in nondiabetic subjects from the PPP-Botnia study (N = 3,011; P = 3.1 × 10−6). E: GIP levels at 2 h during the OGTT were lower in carriers of the TA/AA than in TT genotypes in nondiabetic subjects from the PPP-Botnia study (N = 2,958; P = 8.3 × 10−7). Carriers of TA/AA genotypes are shown in pink and TT genotypes in blue. Bars represent mean ± SEM. *P < 0.05 and ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
Opposite to the expected increase in GIP concentrations when the receptor is down-regulated, TA/AA genotype carriers with a presumed reduced function of the receptor had lower GIP concentrations, both at fasting (P = 3.1 × 10−6) and after the glucose load (P = 8.3 × 10−7) than TT genotype carriers (Fig. 2D and E). Of note, the association of the TA/AA genotypes with reduced insulin secretion remained unchanged after adjustment for GIP levels, supporting the view that the effect of a presumed reduced GIPR function was independent of decreased circulating GIP levels (Supplementary Table 1, study I).
The GIPR variant results in decreased BMI, which neutralizes the effect of the SNP on risk of type 2 diabetes.
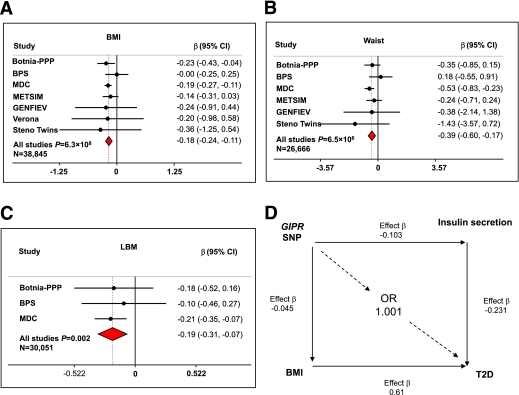
Although the effect of the A allele on insulin secretion was of similar magnitude as observed by SNPs in other genes, resulting in impaired islet function and increased risk of type 2 diabetes (2), the A allele was not associated with a similarly increased risk of type 2 diabetes as seen for the other variants (odds ratio 1.03; 95% CI 0.95–1.12; P = 0.51) in our two large prospective cohorts with >20,000 individuals, 2,200 of whom developed diabetes (Supplementary Table 1, studies II and IV).
One potential explanation for the lack of effect of the A allele on risk of type 2 diabetes could be the concomitant effect of the SNP on body composition. In a meta-analysis of 38,845 subjects, the A allele was associated with a decrease in BMI of ∼0.18 kg/m2 (β, 95% CI −0.18 [−0.24 to −0.11]; Pmeta= 6.3 × 108; Fig. 3A) as well as a decrease in waist circumference of 0.39 cm (−0.39 [−0.60 to −0.17]; Pmeta= 6.5 × 106; Fig. 3B). In addition, the A allele was associated with a decrease in lean body mass (P = 0.002; Fig. 3C). BMI is an established strong predictor of future type 2 diabetes (20), which, in the prospective MPP study, increased risk of type 2 diabetes by an odds ratio (OR) of 1.84 (P = 2.1 × 10−153) (20). When in the MPP study we take into account the decrease in BMI associated with the A allele, the effect of decreased BMI neutralizes the effect of impaired insulin secretion on type 2 diabetes risk (Fig. 3D).
FIG. 3.
GIPR and type 2 diabetes risk reduction attributable to BMI. A: Meta-analysis of the effect of GIPR rs10423928 on BMI in the PPP-Botnia (N = 4,531), BPS (N = 2,250), MDC (N = 24,883), METSIM (N = 5,591), GENFIEV (N = 814), Verona (N = 491), and Steno Twins (N = 285) studies. B: Meta-analysis of the effect of GIPR rs10423928 on waist in the PPP-Botnia (N = 4,508), BPS (N = 1,807), MDC (N = 24,883), METSIM (N = 5,586), GENFIEV (N = 798), and Steno Twins (N = 239) studies. C: Meta-analysis of the effect of GIPR rs10423928 on lean body mass (LBM) in the PPP-Botnia (N = 2,859), BPS (N = 2,309), and MDC (N = 24,883) studies. D: We have also calculated type 2 diabetes risk reduction attributable to BMI and impaired insulin secretion. We have previously shown that every SD unit change in BMI increases risk for type 2 diabetes by 1.84-fold (β= 0.61) and every SD unit decrease in insulin secretion (CIR) increases risk for type 2 diabetes by 1.26-fold (β= −0.23) in the MPP study (20). The A allele of GIPR rs10423928 is associated with a decrease in BMI by −0.045 SD units (−0.148 SE units). Thus, the predicted BMI-attributable risk of type 2 diabetes conferred by the SNP rs10423928 would neutralize the risk associated with insulin secretion: 0.61 * (−0.045) * 0.231 = −0.001 or exp(−0.001) = odds ratio of 1.001. (A high-quality color representation of this figure is available in the online issue.)
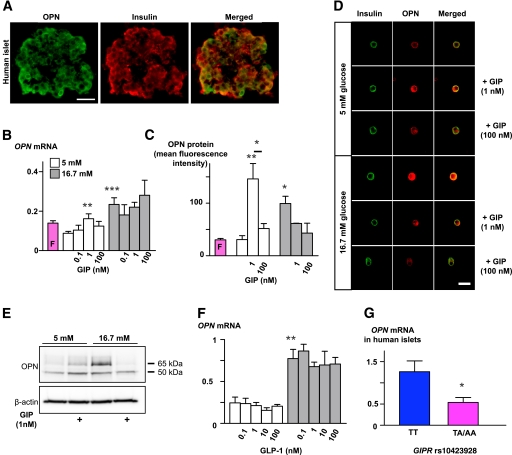
GIP influences osteopontin expression in islets in a dose- and glucose-dependent fashion.
Next, in search for the mechanisms that could explain effects of GIP in different tissues, we explored whether GIP effects on islet function involved OPN. In line with earlier findings in rodents (11), we observed clear OPN expression in human β-cells (Fig. 4A and G). To explore a possible link between GIP and OPN, we measured changes in OPN expression upon stimulation of mouse islets with various concentrations of GIP. Under basal glucose concentrations (5 mmol/L), 1 nmol/L GIP significantly increased OPN at both mRNA and protein level, the last one assessed both by quantitative confocal immunofluorescence microscopy and Western blot (Fig. 4B–E). The dose-response of GIP on OPN expression was bell shaped as both lower (0.1 nmol/L) and higher (100 nmol/L) concentrations did not increase OPN expression to the same extent as 1 nmol/L GIP (Fig. 4B and C). High glucose (16.7 mmol/L) per se effectively increased OPN expression and blunted the stimulatory effect of GIP. The effect of GIP on OPN expression was specific for GIP, as GLP-1 had no impact on OPN expression regardless of the glucose concentrations (Fig. 4F). Furthermore, OPN expression was lower in human islets from carriers of the TA/AA compared with TT genotypes (P < 0.05) (Fig. 4G).
FIG. 4.
Regulation of OPN expression by glucose and GIP in pancreatic islets. A: Immunofluorescence images demonstrating OPN expression (green) in β-cells (red) of human isolated islets. Scale bar = 50 μm. B: Changes in OPN mRNA expression in mouse islets upon incubation in normal glucose (5 mmol/L) or high glucose (16.7 mmol/L) with or without GIP (0.1, 1, or 100 nmol/L) for 24–48 h. **P < 0.01 and ***P < 0.001 vs. 5 mmol/L glucose without GIP. Real-time RT-PCR was performed in triplicate. Experiments were performed 6–16 times, with 100–200 islets in each condition. C: Summarized data from confocal immunofluorescence experiments showing changes in OPN protein expression in β-cells isolated after incubation of mouse islets as described in B. Results show increased OPN expression upon GIP (1 nmol/L) and high glucose stimulation. **P < 0.01 and *P < 0.05 vs. 5 mmol/L glucose without GIP. Experiments were performed three times, once after 48-h incubation and twice after 24 h. Each time, stimulation was performed in duplicate with ∼100 islets per chamber; 28–44 images were analyzed for each condition. D: Representative confocal immunofluorescence images of mouse β-cells double stained for insulin (green) and OPN (red; right panels depict merged images). Cells were dispersed from islets that had been cultured under various stimulation conditions as explained in B and summarized in C. Scale bar = 20 μm. E: Western blot showing increased expression of OPN protein in mouse islets after 48-h stimulation with GIP (1 nmol/L) in normal (5 mmol/L) or high glucose (16.7 mmol/L). GIP had no stimulatory effect under high glucose condition. Two bands were distinguished, one at 65 and the other at 50 kDa. Expression of β-actin was used as loading control. F: OPN mRNA measurement in experiments performed in the same conditions as in B, but with or without GLP-1 instead of GIP, n = 4. **P < 0.01. G: OPN mRNA levels in human islets were lower in nondiabetic carriers of TA/AA (n = 20) than TT genotypes (n = 21). *P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
Effect of GIP and OPN on cell viability and proliferation.
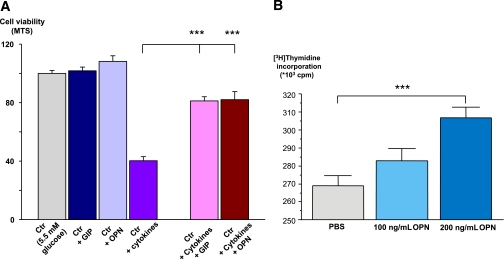
In islets, GIP has been demonstrated not only to stimulate secretion through amplification of exocytosis (43), but also to promote proliferation and inhibit apoptosis of β-cells (13–15). OPN has also been shown to stimulate cell proliferation and inhibit apoptosis in islets by influencing NO production (11,12). Here we show that cytokine stimulation of human islets induced a significant reduction in the number of viable cells, as assessed by an MTS assay, and that this was partially prevented by coincubation of islets with both GIP and OPN, supporting a protective role of OPN and GIP in human islets (Fig. 5A).
FIG. 5.
Effect of GIP and OPN on cell viability and proliferation. A: GIP and OPN partially prevented cytokine-induced reduction in cell viability in human islets. N = 5 donors of human pancreatic islets; six measurements in each experiment for each donor. ***P < 0.0001. Cytokines included were IL-1β (50 ng/mL), INF-γ (75 ng/mL), and TNF-α (75 ng/mL). B: Increased cell proliferation in presence of OPN. [3H]Thymidine incorporation measured in INS-1 832/13 cells incubated for 48 h in PBS alone (control) or including 100 ng/mL or 200 ng/mL OPN as indicated. n = 24; ***P < 0.001 vs. [3H]thymidine incorporation in control group. The values are mean ± SEM.
As mouse and human islets show limited cell division ex vivo, we chose to assess the effect of OPN on β-cell proliferation in clonal rat INS-1 832/13 cells. OPN (200 ng/mL) significantly (P < 0.001) increased [3H]thymidine incorporation in INS-1 832/13 cells (Fig. 5B), demonstrating a proliferative effect of OPN on pancreatic β-cells. As GIPR rs10423928 TA/AA genotype carriers had reduced OPN expression in human pancreatic islets compared with TT carriers (Fig. 4F), it is possible that the protective effect of OPN on cell proliferation and apoptosis (as shown in rat islets [12]) is impaired in carriers of the A allele.
DISCUSSION
The current study provides novel insights into the role of GIP in the pathophysiology of islet function and type 2 diabetes by exploring metabolic effects of a variant (rs10423928) in the GIPR gene in vivo and in vitro, and provides mechanisms that could explain the protective effects of GIP on islet function. We present evidence that GIP influences expression of the inflammatory cytokine OPN in islets, which in turn, has protective effects on β-cell proliferation and potentially apoptosis. Although the GIPR variant was associated with impaired glucose- and GIP-stimulated insulin secretion, this was not translated into a similarly increased risk of type 2 diabetes as seen for other variants with similar effects on insulin secretion (DIAGRAM +), most likely as the variant also resulted in lower BMI including smaller waist and lower lean body mass. This does not exclude a very small effect on risk of type 2 diabetes as the variant in a meta-analyses of 19,091 type 2 diabetes cases versus 38,508 nondiabetic individuals showed modest association with risk of type 2 diabetes (OR 1.07; 95% CI 1.03–1.12; P = 1.8 × 10−4) (4).
The A allele of the rs10423928 in the GIPR gene was associated with decreased GIPR expression in human pancreatic islets, suggesting a possible mechanism for the observed reduced function of the receptor. A novel finding of the current study was that GIP signaling influences OPN expression in islets at both the mRNA and protein level. Consistently, OPN expression was lower in carriers of the A allele in the GIPR gene. Reduced GIPR signaling and OPN expression could result in reduced β-cell mass due to decreased β-cell proliferation and increased apoptosis, given that both GIP and OPN have previously been ascribed β-cell–protective effects (11–15). Our data suggest that the previously reported protective effects of GIP on β-cells could be, at least in part, mediated through regulation of OPN expression. A potential mechanism could involve activation of CREB (cAMP response element-binding) transcription factor, which has been implicated as a mediator of GIP effects in islets (16) and adipose tissue (20) and as a key transcriptional regulator of OPN in the vasculature (44,45). A previous study by Arafat et al. (12) reported that OPN protects β-cells from IL-1β–induced cytotoxicity. Here we provide direct proof of a dose-dependent effect of OPN on β-cell proliferation. Furthermore, we showed that the protective effects of GIP previously demonstrated in murine and porcine islets are also seen in human islets, as shown by GIP’s ability to preserve cell viability in response to inflammatory cytokines. Interestingly, it was recently demonstrated that transgenic pigs with impaired GIP function have 60% reduced β-cell proliferation, resulting in a 58% reduction of β-cell mass (14). Taken together, these data demonstrate that GIP, in addition to its incretin effect, has profound β-cell–protective effects, which could be partially mediated by OPN.
Another novel observation was that the A allele of the GIPR gene was associated with a lean body phenotype including reduced fat and lean body mass. It could be argued that the decrease in body mass is a consequence of the decrease in insulin levels. This is not likely for two reasons. First, associations with BMI adjusted for the decrease in insulin or GIP levels did not abolish the difference between A and T allele carriers with respect to BMI. Secondly, tissue-specific disruption of the GIPR in adipose tissue results in reduced adiposity without any effects on islet function (46). These findings are also supported by a recent meta-analysis by the GIANT consortium demonstrating an association between BMI and a nearby SNP, which is in linkage disequilibrium (r2 = 0.83) with rs10423928 (47). Importantly, the lowering effect of the A allele on BMI seems sufficient to neutralize the effect of the associated impairment in insulin secretion on the risk of type 2 diabetes.
A possible explanation for the observed reduced function of the receptor arises from the fact that the SNP rs10423928 located in intron 12 is in strong linkage disequilibrium (r2 = 0.93) with a nonsynonomous polymorphism, rs1800437, located in exon 10 of GIPR. The minor, at-risk C allele of rs1800437 encodes a glutamine instead of a glutamic acid residue at position 354 in the sixth transmembrane helix (TM6; Supplementary Fig. 2). This region is critical for ligand-mediated activation in the G protein–coupled receptors (GPCR) class B family, to which GIPR belongs (48), thereby likely resulting in decreased activation of the receptor. Recently, it was demonstrated that the coding rs1800437 variant E354Q was associated with decreased basal signaling, possibly as a consequence of reduced cell surface expression (49). The GIPR showed both ligand-dependent and ligand-independent signaling. These recent data therefore support the view that translational changes could contribute to ligand-independent signaling. Although a functional study of the E354Q variant in Chinese hamster fibroblasts did not show differences in GIPR activity measured as cAMP formation at higher GIP levels, there seemed to be differences in the lower physiological range of GIP concentrations (50).
Since most polymorphisms resulting in phenotype expression have developed as a consequence from their exposure to the environment, one can speculate that the variant in the GIPR gene has been associated with certain advantages during evolution. A decrease in insulin secretion coupled with a reduction in body size would be compatible with saving of energy. In support of this, Gipr−/− mice show decreased energy expenditure (46). The T allele is the ancestral allele in chimpanzee, rhesus monkey, dog, and mouse, whereas the A allele is the derived allele in humans, the frequency of which has increased with migration out of Africa (from 0.12 in Africans, 0.18 in Europeans, and 0.20 in Asians; HapMap build 36). Gene variants that show positive selection during evolution often show an increase in the derived allele in Europeans compared with Africans (51). In support of this, the GIP gene has been shown to be under strong adaptive selection during its evolution (52).
In conclusion, our study reinforces the central role of the gut in the pathophysiology of metabolic disorders like type 2 diabetes and obesity and positions GIP as a key anabolic hormone with effects partially mediated through the cytokine OPN. Therapeutic manipulations of GIP and/or OPN might be potential approaches to treat disorders like metabolic syndrome and type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
The studies at Lund University were supported by a Linnaeus grant (349-2008-2974) as well as several other grants from the Swedish Research Council (Grant 521-2007-4037; Exodiab 2009-1039 and 2009-4120) and an equipment grant from the Knut & Alice Wallenberg Foundation (KAW 2009-0243), as well as by grants from the following foundations: Novo Nordisk, the Swedish Diabetes Foundation (2009-060), Åhlén, Åke Wiberg, Magnus Bergwall, Tore Nilsson, Fredrik and Ingrid Thuring, Crafoord, Albert Påhlsson, Lars Hierta Memorial, Royal Physiographic Society, the Foundation of the National Board of Health and Welfare, Malmö University Hospital, the European Foundation for the Study of Diabetes (EFSD) and the EU-7th Framework Programme projects ENGAGE (2007-201413) and CEED3 (2008-223211), IngaBritt och Arne Lundberg’s Research Foundation (Grant 359), and Torsten och Ragnar Söderbergs Stiftelser (MT33/09). M.F.G. and L.E. are senior scientists at the Swedish Heart and Lung Foundations and Swedish Research Council, respectively. Work in Kuopio, Finland, was supported by grants from the Academy of Finland and Finnish Diabetes Research Foundation. Work in Italy was supported by grants from the EC Eurodia project, the 6th Framework Programme (LSHM-CT-2006-518153) to M.B. and P.M., University of Verona to R.B., E.B., and M.M. of the Verona Newly Diagnosed Type 2 Diabetes Study, and from the EFSD/Novartis Programme to R.B. The GENFIEV study was supported by ForiSID-Eli Lilly Italy and from the EFSD/Novartis Programme (R.M. and S.D.P.). No other potential conflicts of interest relevant to this article were reported.
V.L. designed the study DGI GWAS, performed genetic data analysis, and drafted the report. L.E. designed the study, performed in vitro islet experiments, and drafted the report. O.K. and L.M.B. performed in vitro experiments and analysis. K.P., C.B., and P.N. performed incretin clamp and expression in adipose tissue experiments. N.W. performed immunocytochemistry. A.Sa., M.B., J.T., A.B., and P.M. performed in vitro human islet experiments. A.W. and P.D. performed in vitro proliferation experiments. A.J. performed genotyping and analyzed data. Y.Z.D.M. performed in vitro islet experiments. O.H. and P.O. researched data. A.St. and J.K. performed phenotyping and data analysis in the METSIM study. R.S. and E.A. analyzed DGI GWAS. T.J.K. provided GIPR antibody. T.T. and B.I. performed phenotyping in the Botnia study. O.M. and M.O.-M. performed genotyping and phenotyping in the Malmö Diet and Cancer study. E.S. participated in the genetic analyses of the Malmö Diet and Cancer study P.N. performed phenotyping in the Malmö Diet and Cancer study. S.B., R.B., R.M., and S.D.P. performed phenotyping and data analysis in the Verona Newly Diagnosed Type 2 Diabetes Study and GENFIEV study. S.M. and A.V. were the Principal Investigators of the Steno studies. P.P. performed phenotyping in the Steno studies. M.L. was the Principal Investigator of the METSIM study. M.F.G. designed the study, performed in vitro and confocal experiments and analysis, and drafted the report. L.G. designed and supervised all parts of the study and drafted the report. All researchers took part in the revision of the report and approved the final version.
The authors thank Britt-Marie Nilsson, Mona Svärdh, Esa Laurila, Anna Berglund, Malin Neptin, Doris Persson, Anna-Maria Veljanovska-Ramsey (LUDC), and people involved in the different substudies for skillful technical assistance; David Altshuler, (Broad Institute, Boston, MA) for thoughtful comments on the manuscript; and Jonathan Esguerra for performing database searches on OPN expression (LUDC).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1532/-/DC1.
REFERENCES
- 1.Saxena R, Voight BF, Lyssenko V, et al. ; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Scott LJ, Steinthorsdottir V, et al. ; MAGIC investigators; GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeggini E, Scott LJ, Saxena R, et al. ; Wellcome Trust Case Control Consortium Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena R, Hivert MF, Langenberg C, et al. ; GIANT consortium; MAGIC investigators Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Hayami T, Nakamura K, et al. Human gastric inhibitory polypeptide receptor: cloning of the gene (GIPR) and cDNA. Genomics 1995;29:773–776 [DOI] [PubMed] [Google Scholar]
- 6.McIntosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP). Vitam Horm 2009;80:409–471 [DOI] [PubMed] [Google Scholar]
- 7.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–E206 [DOI] [PubMed] [Google Scholar]
- 8.Kase S, Yokoi M, Saito W, et al. Increased osteopontin levels in the vitreous of patients with diabetic retinopathy. Ophthalmic Res 2007;39:143–147 [DOI] [PubMed] [Google Scholar]
- 9.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007;27:2302–2309 [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Igarashi M, Hirata A, et al. Progression of diabetic nephropathy enhances the plasma osteopontin level in type 2 diabetic patients. Endocr J 2004;51:499–504 [DOI] [PubMed] [Google Scholar]
- 11.Katakam AK, Chipitsyna G, Gong Q, Vancha AR, Gabbeta J, Arafat HA. Streptozotocin (STZ) mediates acute upregulation of serum and pancreatic osteopontin (OPN): a novel islet-protective effect of OPN through inhibition of STZ-induced nitric oxide production. J Endocrinol 2005;187:237–247 [DOI] [PubMed] [Google Scholar]
- 12.Arafat HA, Katakam AK, Chipitsyna G, et al. Osteopontin protects the islets and beta-cells from interleukin-1 beta-mediated cytotoxicity through negative feedback regulation of nitric oxide. Endocrinology 2007;148:575–584 [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 2005;280:22297–22307 [DOI] [PubMed] [Google Scholar]
- 14.Renner S, Fehlings C, Herbach N, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes 2010;59:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widenmaier SB, Ao Z, Kim SJ, Warnock G, McIntosh CH. Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J Biol Chem 2009;284:30372–30382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Nian C, Widenmaier S, McIntosh CH. Glucose-dependent insulinotropic polypeptide-mediated up-regulation of beta-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol Cell Biol 2008;28:1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Nian C, McIntosh CH. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. J Lipid Res 2010;51:3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 2007;117:2877–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groop L, Forsblom C, Lehtovirta M, et al. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 1996;45:1585–1593 [DOI] [PubMed] [Google Scholar]
- 20.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008;359:2220–2232 [DOI] [PubMed] [Google Scholar]
- 21.Isomaa B, Forsén B, Lahti K, et al. A family history of diabetes is associated with reduced physical fitness in the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia study. Diabetologia 2010;53:1709–1713 [DOI] [PubMed] [Google Scholar]
- 22.Schou JH, Pilgaard K, Vilsbøll T, et al. Normal secretion and action of the gut incretin hormones glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in young men with low birth weight. J Clin Endocrinol Metab 2005;90:4912–4919 [DOI] [PubMed] [Google Scholar]
- 23.Berglund G, Nilsson P, Eriksson KF, et al. Long-term outcome of the Malmö preventive project: mortality and cardiovascular morbidity. J Intern Med 2000;247:19–29 [DOI] [PubMed] [Google Scholar]
- 24.Berglund G, Elmstähl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med 1993;233:45–51 [DOI] [PubMed] [Google Scholar]
- 25.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonadonna RC, del Prato S, Bonora E, Gulli G, Solini A, DeFronzo RA. Effects of physiological hyperinsulinemia on the intracellular metabolic partition of plasma glucose. Am J Physiol 1993;265:E943–E953 [DOI] [PubMed] [Google Scholar]
- 27.Cretti A, Lehtovirta M, Bonora E, et al. Assessment of beta-cell function during the oral glucose tolerance test by a minimal model of insulin secretion. Eur J Clin Invest 2001;31:405–416 [DOI] [PubMed] [Google Scholar]
- 28.Alibegovic AC, Højbjerre L, Sonne MP, et al. Increased rate of whole body lipolysis before and after nine days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab 2010;298:E555–E564 [DOI] [PubMed]
- 29.Brøns C, Jensen CB, Storgaard H, et al. Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. J Clin Endocrinol Metab 2008;93:3885–3892 [DOI] [PubMed] [Google Scholar]
- 30.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 1999;42:139–145 [DOI] [PubMed] [Google Scholar]
- 31.Poulsen P, Vaag A, Beck-Nielsen H. Does zygosity influence the metabolic profile of twins? A population based cross sectional study. BMJ 1999;319:151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laakso M, Zilinskaite J, Hansen T, et al. ; EUGENE2 Consortium Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008;51:502–511 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Kuusisto J, Vänttinen M, et al. Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia 2007;50:1192–1200 [DOI] [PubMed] [Google Scholar]
- 34.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 35.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000;85:3575–3581 [DOI] [PubMed] [Google Scholar]
- 36.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994;43:535–539 [DOI] [PubMed] [Google Scholar]
- 37.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 2000;151:190–198 [DOI] [PubMed] [Google Scholar]
- 38.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 39.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 40.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002;107:63–69 [DOI] [PubMed] [Google Scholar]
- 41.Lewis JT, Dayanandan B, Habener JF, Kieffer TJ. Glucose-dependent insulinotropic polypeptide confers early phase insulin release to oral glucose in rats: demonstration by a receptor antagonist. Endocrinology 2000;141:3710–3716 [DOI] [PubMed] [Google Scholar]
- 42.Eliasson L, Ma X, Renström E, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 2003;121:181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding WG, Gromada J. Protein kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 1997;46:615–621 [DOI] [PubMed] [Google Scholar]
- 44.Jalvy S, Renault MA, Lam Shang Leen L, et al. CREB mediates UTP-directed arterial smooth muscle cell migration and expression of the chemotactic protein osteopontin via its interaction with activator protein-1 sites. Circ Res 2007;100:1292–1299 [DOI] [PubMed] [Google Scholar]
- 45.Jalvy S, Renault MA, Leen LL, et al. Autocrine expression of osteopontin contributes to PDGF-mediated arterial smooth muscle cell migration. Cardiovasc Res 2007;75:738–747 [DOI] [PubMed] [Google Scholar]
- 46.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002;8:738–742 [DOI] [PubMed] [Google Scholar]
- 47.Speliotes EK, Willer CJ, Berndt SI, et al. ; MAGIC; Procardis Consortium Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 2008;7:339–357 [DOI] [PubMed] [Google Scholar]
- 49.Fortin JP, Schroeder JC, Zhu Y, Beinborn M, Kopin AS. Pharmacological characterization of human incretin receptor missense variants. J Pharmacol Exp Ther 2010;332:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almind K, Ambye L, Urhammer SA, et al. Discovery of amino acid variants in the human glucose-dependent insulinotropic polypeptide (GIP) receptor: the impact on the pancreatic beta cell responses and functional expression studies in Chinese hamster fibroblast cells. Diabetologia 1998;41:1194–1198 [DOI] [PubMed] [Google Scholar]
- 51.Green RE, Krause J, Briggs AW, et al. A draft sequence of the Neandertal genome. Science 2010;328:710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CL, Cai JJ, Lo C, Amigo J, Park JI, Hsu SY. Adaptive selection of an incretin gene in Eurasian populations. Genome Res 2011;21:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.