Abstract
OBJECTIVE
Ghrelin reportedly restricts insulin release in islet β-cells via the Gαi2 subtype of G-proteins and thereby regulates glucose homeostasis. This study explored whether ghrelin regulates cAMP signaling and whether this regulation induces insulinostatic cascade in islet β-cells.
RESEARCH DESIGN AND METHODS
Insulin release was measured in rat perfused pancreas and isolated islets and cAMP production in isolated islets. Cytosolic cAMP concentrations ([cAMP]i) were monitored in mouse MIN6 cells using evanescent-wave fluorescence imaging. In rat single β-cells, cytosolic protein kinase-A activity ([PKA]i) and Ca2+ concentration ([Ca2+]i) were measured by DR-II and fura-2 microfluorometry, respectively, and whole cell currents by patch-clamp technique.
RESULTS
Ghrelin suppressed glucose (8.3 mmol/L)-induced insulin release in rat perfused pancreas and isolated islets, and these effects of ghrelin were blunted in the presence of cAMP analogs or adenylate cyclase inhibitor. Glucose-induced cAMP production in isolated islets was attenuated by ghrelin and enhanced by ghrelin receptor antagonist and anti-ghrelin antiserum, which counteract endogenous islet-derived ghrelin. Ghrelin inhibited the glucose-induced [cAMP]i elevation and [PKA]i activation in MIN6 and rat β-cells, respectively. Furthermore, ghrelin potentiated voltage-dependent K+ (Kv) channel currents without altering Ca2+ channel currents and attenuated glucose-induced [Ca2+]i increases in rat β-cells in a PKA-dependent manner.
CONCLUSIONS
Ghrelin directly interacts with islet β-cells to attenuate glucose-induced cAMP production and PKA activation, which lead to activation of Kv channels and suppression of glucose-induced [Ca2+]i increase and insulin release.
Ghrelin, an acylated 28-amino acid peptide, is the endogenous ligand for the growth hormone secretagogue receptor (GHS-R) (1,2). Ghrelin is produced predominantly in the stomach and stimulates growth hormone release and feeding and exhibits positive cardiovascular effects, suggesting its possible clinical application (3). Ghrelin and GHS-R are located in the pancreatic islets (4–6). Furthermore, ghrelin O-acyltransferase (GOAT), which has been identified as the enzyme that promotes the acylation of the third serine residue of ghrelin, is highly expressed in the pancreatic islets (7–9). Administration of ghrelin at 10−9–10−8 mol/L, the concentrations higher than the circulating levels of 10−10–10−9 mol/L, attenuates insulin release and deteriorates glucose tolerance in rodents and humans, whereas desacyl ghrelin has no effects (5,10,11). This effective concentration of ghrelin that is approximately one log order higher than the circulating level is considered physiological in the pancreatic islets for the following reasons: 1) ghrelin is produced in the pancreatic islets (5), and the ghrelin level in the pancreatic vein is one log order higher than that in the pancreatic artery (12), indicative of release of ghrelin from the pancreas; 2) ghrelin immunoneutralization and GHS-R antagonists augment glucose-induced insulin release from perfused pancreas and isolated islets (5,12); and 3) ghrelin knockout mice display enhanced glucose-induced insulin release from isolated islets without altering islet density and size, insulin content, or insulin mRNA levels, indicating increased secretory activity in the knockout mouse islets (12). Furthermore, glucose intolerance in high-fat diet–induced obese (DIO) mice is prevented in the ghrelin knockout mice as a result of enhanced insulin secretory response to glucose (12). These findings suggest that the islet-derived ghrelin regulates insulin release in a paracrine and/or autocrine manner and that manipulation of the ghrelin action could provide a novel tool to optimize insulin release (13,14).
It is currently thought that GHS-R is coupled primarily to G11-phospholipase C signaling (2). Intriguingly, our previous data indicated that the insulinostatic ghrelin signaling is produced via pertussis toxin (PTX)-sensitive G-protein Gαi2 in β-cells; ghrelin PTX-sensitively activates voltage-dependent K+ (Kv) channels, attenuates membrane excitability, and suppresses glucose-induced Ca2+ concentration ([Ca2+]i) increases in β-cells to attenuate insulin release (15). However, the mechanism that links GHS-R and Gαi2 to these activities in β-cells remains to be clarified. It is known that PTX-sensitive Gi-proteins inhibit adenylate cyclase, which produces cyclic AMP in the cells. In pancreatic β-cells, intracellular cAMP signals are generated by nutrient secretagogues and play a critical role in regulating insulin secretion (16–18). However, implication of cAMP signaling in the insulinostatic function of ghrelin has been unclear. In this study, we aimed to clarify whether ghrelin regulates cAMP pathway in islet β-cells and whether this regulation leads to insulinostatic cascade in islet β-cells. We here show a novel signaling mechanism for ghrelin that operates in islet β-cells; GHS-R–mediated attenuation of cAMP and protein kinase-A (PKA) signaling leads to activation of Kv channels and suppression of glucose-induced [Ca2+]i increase and insulin release.
RESEARCH DESIGN AND METHODS
Male Wistar rats (Japan SLC, Hamamatsu, Japan) were housed in accordance with our institutional guidelines and with the Japanese Physiological Society’s guidelines for animal care. PTX (5 μg/kg; Sigma-Aldrich, St. Louis, MO) or saline was intravenously injected into rats. Three days after injections, animals were used.
In vitro perfusion of the pancreas
The rat isolated pancreas was perfused with HEPES-added Krebs-Ringer bicarbonate buffer (HKRB) solution (in millimoles per liter): 129 NaCl, 5.0 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 2.0 CaCl2, 1.2 MgSO4, and 10 HEPES at pH 7.4 with NaOH containing 2.8 mmol/L glucose, 0.5% BSA, and 4% Dextran T70 at 37°C, as previously reported (12). After a 20-min preincubation period, each pancreas was perfused for 10 min with 2.8 mmol/L glucose with or without test reagents. Pancreas was then perfused for 30 min with 8.3 mmol/L glucose with or without test reagents. Fractions, collected at 1-min intervals, were assayed for immunoreactive insulin.
Preparation of pancreatic islets and single β-cells
Animals were anesthetized by pentobarbitone (80 mg/kg i.p.), followed by injection of collagenase at 1.05 mg/mL (Sigma-Aldrich) into the common bile duct as previously described (5,15). Collagenase was dissolved in 5 mmol/L Ca2+-containing HKRB with 0.1% BSA. The pancreas was dissected out and incubated at 37°C for 16 min. Islets were collected and used for either insulin release experiments or cAMP measurements. For β-cell experiments, islets were dispersed into single cells in Ca2+-free HKRB, and the single cells were plated sparsely on coverslips and maintained for 1 day at 37°C in an atmosphere of 5% CO2 and 95% air in Eagle’s minimal essential medium containing 5.6 mmol/L glucose supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 units/mL penicillin.
Measurements of insulin release and cAMP productions in rat islets
For measurements of islet insulin release, groups of 12–15 islets were incubated for 1 h at 37°C in HKRB with 2.8 mmol/L glucose for stabilization, followed by test incubation for 1 h in HKRB with 2.8 or 8.3 mmol/L glucose. In cAMP measurements, islets were incubated for 1 h in HKRB with 500 μmol/L 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase (PDE) inhibitor (Sigma-Aldrich), to avoid degradation of cAMP in the samples. Ghrelin (Peptide Institute, Osaka, Japan) with or without dibutyryl-cAMP (Sigma-Aldrich), 6-Phe-cAMP (BIOLOG, Bremen, Germany), and MDL-12330A (Sigma-Aldrich) was present throughout the incubation. Insulin release and total cellular cAMP content in islets were determined by ELISA (Morinaga, Yokohama, Japan) and EIA kit (GE Healthcare, Buckinghamshire, U.K.).
Real-time cytosolic cAMP concentration monitoring in mouse β-cell line
Mouse MIN6 β-cells were transfected with a fluorescent translocation biosensor comprised of a truncated and membrane-anchored PKA regulatory subunit tagged with cyan-fluorescent protein (ΔRIIβ-CFP-CAAX) and a catalytic subunit labeled with yellow-fluorescent protein (Cα-YFP) (19). Emission wavelengths were detected with interference filters (485/25 nm for cyan fluorescent protein [CFP] excited by 442 nm and 560/40 nm for yellow fluorescent protein [YFP] excited by 514 nm line). Holoenzyme dissociation caused by elevation of cytosolic cAMP concentrations ([cAMP]i) results in translocation of Cα-YFP to the cytoplasm, recorded with evanescent-wave microscopy as selective loss of YFP fluorescence with rise of the CFP-to-YFP fluorescence ratio.
Measurements of cytosolic PKA activity and [Ca2+]i in single β-cells
Dissociated single β-cells on coverslips were mounted in an open chamber and superfused in HKRB. Cytosolic PKA activity ([PKA]i) in rat single β-cells was measured using the fluorescence probe DR-II (Dojindo, Kumamoto, Japan) as previously described (20) with slight modification using two-photon confocal microscopy. The DR-II–loaded islet cells were superfused in HKRB, and DR-II fluorescence was excited at 780 nm by mode-locked Ti:sapphire laser every 10 s; the emission signal at 475 nm was detected with a photomultiplier tube of the multiphoton laser-scanning microscope (FluoView FV300-TP; Olympus, Tokyo, Japan). [Ca2+]i in single β-cells were measured by dual-wavelength fura-2 microfluorometry with excitation at 340/380 nm and emission at 510 nm using a cooled charge-coupled device camera (15). The ratio image was produced on an Aquacosmos system (Hamamatsu Photonics, Hamamatsu, Japan). After the [PKA]i and [Ca2+]i measurements, cells were fixed with 4% paraformaldehyde and β-cells were identified by insulin immunostaining. Data were taken exclusively from the insulin-positive cells.
Patch-clamp experiments in rat single β-cells
Perforated whole-cell currents were recorded using a pipette solution containing nystatin (150 μg/mL) dissolved in 0.1% DMSO as previously described (5). Membrane currents were recorded using an amplifier (Axopatch 200B; Molecular Devices, Foster, CA) in a computer using pCLAMP10.2 software. For conventional whole-cell clamp experiments measuring Ca2+-channel currents, pipette solution contained (in millimoles per liter) 90 aspartate, 10 HEPES, 5 MgCl2, 10 EGTA, 5 ATP-Mg, 20 TEA-Cl, and 90 CsOH at pH 7.2 with CsOH, and cells were superfused with 10 mmol/L Ca2+-containing HKRB. β-Cells were voltage clamped at a holding potential of −70 mV and then shifted to the test potentials from −60 to 40 mV in 10 mV steps with the pulses of either 100-ms duration for Kv channel currents or 50-ms duration for Ca2+ channel currents at room temperature (25°C).
Statistical analysis
Data are means ± SEM. Statistical analyses were performed using Student t test or one-way ANOVA followed by Bonferroni multiple comparison tests. P values < 0.05 were considered statistically significant.
RESULTS
Ghrelin attenuates glucose-induced insulin release in a cAMP signaling-dependent manner
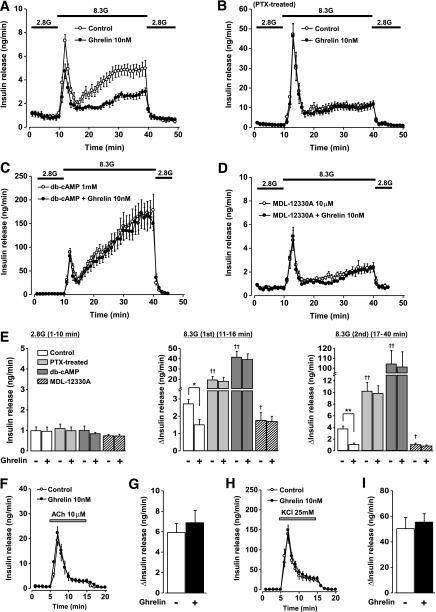
In rat perfused pancreas, the first and second phases of glucose (8.3 mmol/L)-induced insulin release were both significantly suppressed by exogenous ghrelin (10 nmol/L) that was administered 10 min prior to 8.3 mmol/L glucose challenge and present through the end of experiments, whereas the basal insulin release at 2.8 mmol/L glucose was not altered (Fig. 1A and E), confirming a previous report (15). In perfused pancreas of PTX-treated rats, both phases of glucose-induced insulin release were markedly enhanced, and ghrelin (10 nmol/L) failed to affect them (Fig. 1B and E). A membrane-permeable cAMP analog, dibutyryl-cAMP (db-cAMP), at 1 mmol/L markedly enhanced both phases of glucose-induced insulin release, and in the presence of db-cAMP ghrelin failed to suppress both phases of insulin release (Fig. 1C and E). In pancreas preincubated with an irreversible adenylate cyclase inhibitor, MDL-12330A (10 μmol/L), both phases of glucose-induced insulin release were attenuated and were not further altered by administration of ghrelin (10 nmol/L) (Fig. 1D and E). None of these treatments affected basal levels of insulin release at 2.8 mmol/L glucose. Ghrelin (10 nmol/L) affected neither acetylcholine (ACh) (10 μmol/L)-induced (Fig. 1F and G) nor high K+ (25 mmol/L KCl)-induced insulin release in rat perfused pancreas at 2.8 mmol/L glucose (Fig. 1H and I). These results suggest that ghrelin selectively attenuates glucose-induced insulin release via PTX-sensitive G-protein that is coupled to modulation of cAMP signaling.
FIG. 1.
Ghrelin attenuates glucose-induced insulin release in perfused rat pancreas in a cAMP pathway–dependent manner. Ghrelin was added 10 min prior to 8.3 mmol/L glucose challenge. A: Ghrelin (10 nmol/L) suppressed 8.3 mmol/L glucose (8.3G)-induced insulin release in perfused rat pancreas (n = 6). B: Ghrelin (10 nmol/L) did not alter glucose-induced insulin release from the perfused pancreas of PTX-treated rats (n = 6). C: In the presence of a 1 mmol/L dibutyryl-cAMP (db-cAMP), the glucose-induced insulin release was markedly enhanced and ghrelin failed to suppress it (n = 4–6). D: Following preincubation with an irreversible adenylate cyclase inhibitor MDL-12330A (10 μmol/L) for 30 min, the glucose-induced insulin release was attenuated and ghrelin (10 nmol/L) failed to suppress it (n = 3). E: Ghrelin (10 nmol/L) suppressed both the first and second phases of glucose-induced insulin release. In the pancreata from PTX-treated rats and those treated with a db-cAMP, both phases of glucose-induced insulin release were markedly enhanced and ghrelin failed to alter them. In the presence of MDL-12330A, both phases of the insulin release were suppressed, and ghrelin did not further suppress them. None of these treatments affected basal levels of insulin release at 2.8 mmol/L glucose (2.8G). *P < 0.05, **P < 0.01 vs. control; †P < 0.05, ††P < 0.01 vs. 8.3 mmol/L glucose alone (n = 3–6). Ghrelin (10 nmol/L) did not alter 10 μmol/L ACh-evoked (F and G) or 25 mmol/L KCl-evoked insulin release at 2.8 mmol/L glucose (H and I) (n = 4 for each condition).
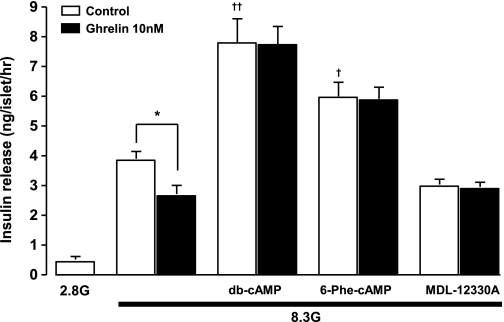
Next, in rat isolated islets, 8.3 mmol/L glucose-induced insulin release was inhibited by exogenous ghrelin (Fig. 2). The glucose-induced insulin release was enhanced by db-cAMP (1 mmol/L). Moreover, 6-Phe-cAMP (10 μmol/L), a membrane-permeable specific PKA activator, enhanced the glucose-induced insulin release. Ghrelin (10 nmol/L) failed to attenuate the insulin release in the presence of these cAMP analogs (Fig. 2). Conversely, the glucose-induced insulin release was significantly suppressed by adenylate cyclase inhibitor MDL-12330A (10 μmol/L), and ghrelin did not affect the insulin release in the MDL-12330A-treated islets (Fig. 2).
FIG. 2.
Ghrelin attenuates glucose-induced insulin release in a cAMP pathway-dependent manner in rat isolated islets. Ghrelin (10 nmol/L) suppressed glucose (8.3 mmol/L) (8.3G)-induced insulin release in islets isolated from rats. Db-cAMP (1 mmol/L) and a PKA activator 6-Phe-cAMP (10 μmol/L) enhanced and an adenylate cyclase inhibitor MDL-12330A (10 μmol/L) suppressed glucose-induced insulin release and blunted the effect of ghrelin on it. *P < 0.05 vs. control; †P < 0.05, ††P < 0.01 vs. 8.3 mmol/L glucose alone (n = 8).
Ghrelin inhibits glucose-induced cAMP production in rat isolated islets
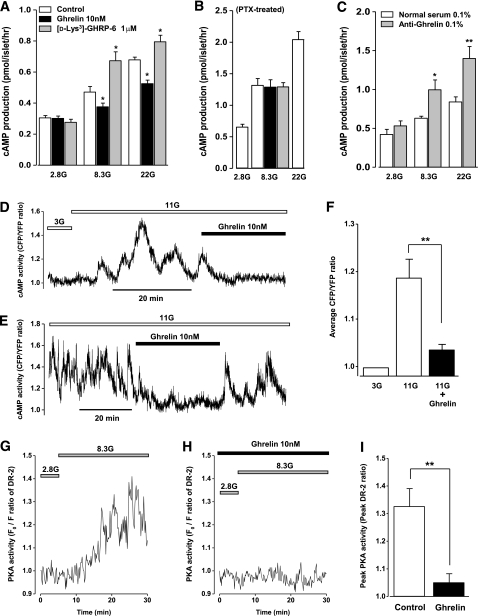
In the presence of PDE inhibitor IBMX (500 μmol/L), static incubation of islets with 8.3 mmol/L glucose induced modest cAMP productions in islets compared with those with 2.8 mmol/L glucose (P < 0.05) (Fig. 3A). Incubation of islets with higher concentration (22 mmol/L) of glucose induced further increases in the cAMP levels. The glucose (8.3 and 22 mmol/L)-induced cAMP responses were significantly inhibited by exogenous ghrelin (10 nmol/L) and augmented by a GHS-R antagonist [D-Lys3]-GHRP-6 (1 μmol/L) (Fig. 3A). These effects of exogenous and endogenous ghrelin were blunted in islets isolated from PTX-treated rats; neither exogenous ghrelin (10 nmol/L) nor [D-Lys3]-GHRP-6 (1 μmol/L) affected the 8.3 mmol/L glucose-induced cAMP productions, whereas 22 mmol/L glucose induced further cAMP productions (Fig. 3B). Moreover, immunoneutralization of endogenous ghrelin with an antighrelin antiserum (0.1%) significantly enhanced the glucose-induced cAMP productions compared with the control with a normal rabbit serum (0.1%) (Fig. 3C).
FIG. 3.
Ghrelin suppresses glucose-induced cAMP-PKA signaling in islet β-cells. A: Glucose at 8.3 (8.3G) and 22 mmol/L (22G) induced cAMP productions in islets in the presence of phosphodiesterase inhibitor IBMX (500 μmol/L). Exogenous ghrelin (10 nmol/L) inhibited the glucose-induced cAMP productions, whereas a GHS-R antagonist, [D-Lys3]-GHRP-6 (1 μmol/L), enhanced them. *P < 0.05 vs. control (n = 12). B: Neither ghrelin (10 nmol/L) nor [D-Lys3]-GHRP-6 (1 μmol/L) altered the glucose (8.3 mmol/L)-induced cAMP productions in islets isolated from PTX-treated rats. Glucose at 22 mmol/L (22G) enhanced larger amount of cAMP productions as a positive control (n = 10). C: Immunoneutralization of endogenous ghrelin with an anti-ghrelin antiserum (0.1%) significantly enhanced the glucose (8.3 and 22 mmol/L)-induced cAMP productions compared with the control, a normal rabbit serum (0.1%). *P < 0.05, **P < 0.01 vs. normal rabbit serum (n = 12). D and E: Representative recordings of glucose-induced changes of [cAMP]i in MIN6 β-cells expressed as CFP-to-YFP ratio by evanescent-wave microscopy. Ghrelin (10 nmol/L) inhibited the glucose-induced elevation of [cAMP]i in a reversible manner. F: Time-average CFP-to-YFP ratio (AUC/time) at 11 mmol/L glucose (11G) was suppressed by ghrelin (10 nmol/L). The basal CFP-to-YFP ratio at 3 mmol/L glucose (3G) was normalized to 1. **P < 0.01 vs. 11 mmol/L glucose alone (n = 8). G: Typical trace of glucose (8.3 mmol/L)-induced cytosolic PKA activation expressed as the ratio of the initial DR-II fluorescence intensity (F0) to that at each time point (F) in rat β-cells. Increase in F0-to-F ratio indicates PKA activation in cells. H: In the presence of ghrelin (10 nmol/L), the glucose (8.3 mmol/L)-elicited PKA response was diminished in 12 of 13 cells examined. After recording, cells were identified as β-cells by immunocytochemical staining with anti-insulin antiboby. I: Peak amplitude of the glucose (8.3 mmol/L)-induced PKA response was markedly suppressed by ghrelin (10 nmol/L). The data were taken only from insulin-positive cells. **P < 0.01 vs. control (n = 12–13).
Ghrelin suppresses glucose-induced [cAMP]i elevations in MIN6 β-cells
To determine the direct effect of ghrelin on the glucose-induced cAMP production, [cAMP]i were monitored in mouse β-cell line MIN6 cells transfected with a fluorescent-translocation biosensor using evanescent-wave microscopy. Raising the glucose concentration from 3 to 11 mmol/L induced a rise in [cAMP]i in an oscillatory manner (Fig. 3D). Ghrelin markedly suppressed the glucose-induced oscillatory rise in [cAMP]i (Fig. 3D). Furthermore, in a MIN6 cell with pronounced oscillations of [cAMP]i during an 11 mmol/L glucose challenge, the [cAMP]i oscillations were inhibited by administration of ghrelin and recovered by its washout (Fig. 3E), showing the reversible effect of ghrelin. Average CFP-to-YFP ratio (AUC/time) revealed that 11 mmol/L glucose increased CFP-to-YFP ratio by 19 ± 4% and ghrelin significantly suppressed it (Fig. 3F), indicating that ghrelin directly inhibits glucose-induced cAMP signaling in β-cells.
Ghrelin suppresses glucose-induced PKA activation in rat β-cells
The activity of PKA, a downstream effector of cAMP elevations, was measured in rat single β-cells using PKA-sensitive DR-II fluorescence with confocal microscopy. DR-II fluorescence is inversely related to PKA activity (21). Fluorescence intensity decreased by only 2% during scanning for 30 min, indicating that photo bleaching of DR-II signal was negligible in this experimental condition (data not shown). A rise in the perfusate glucose concentration from 2.8 to 8.3 mmol/L decreased DR-II fluorescence, as shown by an increase in F0-to-F ratio reflecting an increase in PKA activity in rat single β-cells (Fig. 3G). The glucose (8.3 mmol/L)-induced increase in the PKA activity was significantly inhibited by treatment with ghrelin in the majority of β-cells examined (Fig. 3H and I).
Ghrelin activates Kv channels via PKA-dependent signaling pathway but does not alter L-type Ca2+ channels in β-cells
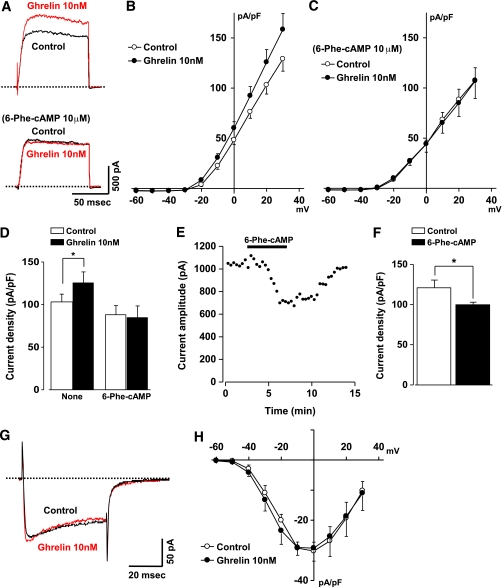
The Kv channel currents in rat β-cells under nystatin-perforated whole-cell clamp were measured in the presence of 100 μmol/L tolbutamide to inhibit the ATP-sensitive K+ (KATP) channel and thereby exclude involvement of this channel in the currents. In the presence of 8.3 mmol/L glucose, outward K+ currents evoked by depolarizing pulses from a holding potential of −70 to 20 mV were increased by exposure to 10 nmol/L ghrelin (Fig. 4A), confirming previous reports (5,15). The current-voltage relationships demonstrated that ghrelin significantly increased the current densities through Kv channels at potentials positive to −30 mV (Fig. 4B). At a holding potential of 20 mV, ghrelin significantly increased the current densities to 125.8 ± 12.6 pA/pF from 103.2 ± 9.1 pA/pF (P < 0.05, n = 8) (Fig. 4D). In the presence of 6-Phe-cAMP (10 μmol/L), the Kv channel currents evoked by depolarizing pulse to 20 mV were not increased by ghrelin (Fig. 4A) and ghrelin failed to enhance the current densities in the entire range of potentials (Fig. 4C and D). The Kv channel currents in the 6-Phe-cAMP-treated cells tended to decrease compared with control currents in nontreated cells (Fig. 4B and C), though the difference was not statistically significant (Fig. 4D). We next examined the effect of 6-Phe-cAMP by itself on the β-cells. A constant depolarizing pulse from −70 to 20 mV every 20 s evoked Kv currents in the external solution containing 5.6 mmol/L glucose (Fig. 4E). The currents markedly decreased upon exposure to 6-Phe-cAMP (10 μmol/L) and recovered by washout of it (Fig. 4E and F). In control experiments without the PKA activator, Kv currents were kept constant for >15 min (data not shown).
FIG. 4.
Ghrelin enhances Kv channel currents in rat islet β-cells via PKA-dependent signaling pathway. A: Current traces evoked by a step pulse to 20 mV from a holding potential of −70 mV were measured in a single β-cell under perforated whole-cell clamp mode. Data were recorded in the presence of 8.3 mmol/L glucose and 100 μmol/L tolbutamide (control [black trace]) and during exposure to 10 nmol/L ghrelin (red trace). The dotted lines indicate zero current levels. Upper panel: exposure to ghrelin increased the amplitudes of delayed outward currents. Lower panel: in the presence of PKA activator 6-Phe-cAMP at 10 μmol/L, Kv channel currents were not increased by exposure to 10 nmol/L ghrelin. B: Current levels measured at the end of test pulses were plotted as a current density (pA/pF) against membrane potentials in β-cells (n = 8). C: Ghrelin failed to potentiate Kv channel currents in the presence of 6-Phe-cAMP (n = 7). D: Peak amplitudes of current density at 20 mV of a membrane potential. Ghrelin (10 nmol/L) induced significant increase in the Kv channel currents, which was not observed in the presence of 6-Phe-cAMP. *P < 0.05 by paired t tests (n = 7–8). E: Amplitude of the Kv channel currents in a single β-cell evoked by a step pulse to 20 mV from a holding potential of −70 mV in the presence of 5.6 mmol/L glucose was decreased by 6-Phe-cAMP in a reversible manner. F: 6-Phe-cAMP (10 μmol/L) significantly decreased peak amplitude of the current density in β-cells (n = 5). *P < 0.05. G: Whole-cell Ca2+ channel current evoked by a step pulse to 0 mV from a holding potential of −70 mV was recorded in a single β-cell in the presence of 8.3 mmol/L glucose (control [black trace]) and during exposure to 10 nmol/L ghrelin (red trace). The dotted line indicates zero current level. H: Ghrelin (10 nmol/L) did not alter the current-voltage relationship for the peak Ca2+-channel current density (n = 3).
The effect of ghrelin on the voltage-dependent Ca2+ channel was determined in rat single β-cells. In the control external solution containing 8.3 mmol/L glucose, a depolarizing pulse from holding potential of −70 to 0 mV evoked a long-lasting inward current in rat β-cells (Fig. 4G), and this current was markedly inhibited by an L-type Ca2+ channel blocker, nifedipine (10 μmol/L) (data not shown). Exposure of cells to ghrelin (10 nmol/L) did not alter the current amplitude or current-voltage relationships (Fig. 4G and H), indicating that ghrelin does not directly affect L-type Ca2+ channels in β-cells.
Ghrelin attenuates glucose-induced [Ca2+]i increases in β-cells in a PKA pathway-dependent manner
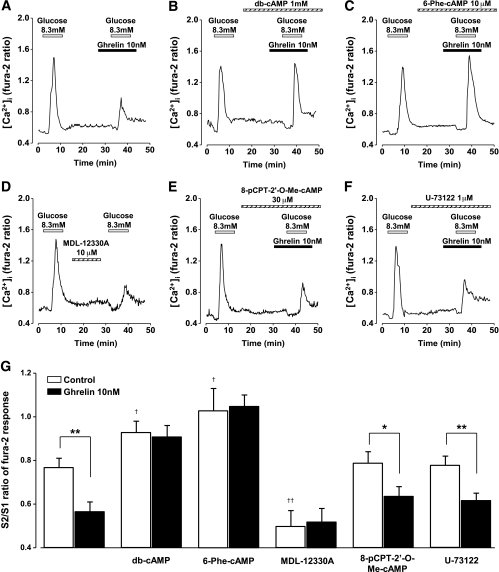
A repetitive glucose (8.3 mmol/L) stimulation induced repeated [Ca2+]i increases in rat single β-cells. The ratio of the peak [Ca2+]i response to the second glucose stimulation (S2) to that to the first stimulation (S1), S2/S1, was 0.77 ± 0.04 (n = 93) in the control cells. Ghrelin (10 nmol/L), added to perfused solution 5 min prior to the second glucose stimulation, suppressed [Ca2+]i responses, decreasing S2-to-S1 ratio to 0.57 ± 0.04 (P < 0.01, n = 91) (Fig. 5A and G). The effect of ghrelin on [Ca2+]i responses was abolished by treatment with db-cAMP (1 mmol/L) (Fig. 5B and G) and 6-Phe-cAMP (10 μmol/L) (Fig. 5C and G). These cAMP analogs alone potentiated the glucose-induced [Ca2+]i responses in β-cells, resulting in increases in S2-to-S1 ratio (Fig. 5G). Pretreatment of cells with MDL-12330A (10 μmol/L) significantly suppressed the glucose-induced [Ca2+]i increases (Fig. 5D and G), suggesting that activation of adenylate cyclase is involved in the glucose-induced [Ca2+]i increases in β-cells. In MDL-12330A-treated cells, ghrelin did not attenuate [Ca2+]i responses to glucose (Fig. 5G). The inhibitory effect of ghrelin on [Ca2+]i responses was unaltered in the presence of Epac activator 8-pCPT-2′-O-Me-cAMP at 30 μmol/L (Fig. 5E and G) or phospholipase-C inhibitor U-73122 (1 μmol/L) (Fig. 5F and G). Neither 8-pCPT-2′-O-Me-cAMP (30 μmol/L) nor U-73122 (1 μmol/L) by itself had effect on [Ca2+]i. These results suggest that ghrelin acts on the cAMP signaling route linked primarily to PKA but not Epac and thereby suppresses the glucose-induced [Ca2+]i increases in β-cells.
FIG. 5.
Ghrelin attenuates glucose-induced [Ca2+]i increases in β-cells in a cAMP and PKA pathway-dependent manner. A–F: Representative traces of [Ca2+]i in rat β-cells are expressed by dual-wavelength fura-2 fluorescence ratio (F340 to F380). A: Repeated administration of 8.3 mmol/L glucose induced [Ca2+]i increases repetitively and ghrelin (10 nmol/L) added 5 min prior to the second glucose stimulation attenuated the [Ca2+]i increases. The inhibitory effects of ghrelin were abolished by treatment with db-cAMP (1 mmol/L) (B) and with 6-Phe-cAMP (10 μmol/L) (C). D: Pretreatment of cells with MDL-12330A (10 μmol/L) suppressed [Ca2+]i response to glucose challenge. The inhibitory effect of ghrelin on [Ca2+]i responses was observed in the presence of 8-pCPT-2'-O-Me-cAMP (30 μmol/L) (E) and U-73122 (1 μmol/L) (F). G: The S2-to-S1 ratio of the peak [Ca2+]i response to the second 8.3 mmol/L glucose stimulation (S2) to that to the first glucose stimulation (S1). The data were taken only from insulin-positive cells. *P < 0.05, **P < 0.01 vs. control; †P < 0.05, ††P < 0.01 vs. 8.3 mmol/L glucose alone (n = 66–93).
DISCUSSION
In this study, we have demonstrated that ghrelin suppresses glucose-induced insulin secretion from pancreatic islets in a cAMP-dependent manner. Moreover, ghrelin decreases cAMP levels in islets and β-cells, and the decreased cAMP mediates the electrical and [Ca2+]i responses to ghrelin.
In rat perfused pancreas and isolated islets, the insulinostatic ability of ghrelin was blunted when cellular cAMP level was clamped upward with a cAMP analog and downward with an adenylate cyclase inhibitor. The adenylate cyclase inhibitor itself mimicked the insulinostatic ghrelin action. Ghrelin, moreover, reduced the glucose-induced cAMP production in isolated islets, whereas blockade of endogenous ghrelin with a ghrelin receptor antagonist and an antiserum enhanced it. Since cAMP was measured in the presence of PDE inhibitor, it is likely that ghrelin affects cAMP levels primarily via interacting with the step of cAMP synthesis rather than degradation. Furthermore, evanescent-wave microscopy and two-photon confocal microscopy demonstrated that ghrelin directly interacts with MIN6 and rat β-cells to decrease glucose-induced increases in [cAMP]i and [PKA]i.
In pancreatic β-cells, Kv channels repolarize cells and attenuate glucose-stimulated action potentials, limiting Ca2+ entry through voltage-dependent Ca2+ channels to suppress insulin secretion (22). We previously reported that ghrelin activates Kv channels via PTX-sensitive mechanisms to cause rapid repolarization and shortening of bursting action potentials, thereby attenuating glucose-induced [Ca2+]i increases and insulin secretion (15). In the current study, we found that the action of ghrelin to suppress glucose-induced [Ca2+]i increases was blunted in the β-cells whose cAMP levels were clamped upward by a cAMP analog and downward by an adenylate cyclase inhibitor (Fig. 5). It is known that cAMP potentiates insulin secretion by both PKA-dependent and Epac-dependent mechanisms. 6-Phe-cAMP is a selective activator of PKA (23), and 8-pCPT-2′-O-Me-cAMP relatively activates Epac with high affinity (24). Ghrelin failed to suppress glucose-induced [Ca2+]i increases in β-cells and insulin release in isolated islets in the presence of 6-Phe-cAMP (10 μmol/L), whereas 8-pCPT-2′-O-Me-cAMP (30 μmol/L) did not alter the effect of ghrelin on [Ca2+]i in β-cells. In consideration of the reports that higher concentrations of cAMP are required for activating Epac pathway (25–27), it is likely that the glucose-induced elevation of cAMP to a moderate level in β-cells is capable of activating PKA but not Epac. Ghrelin, therefore, may attenuate [Ca2+]i increases in β-cells at least partly by counteracting the glucose-stimulated cAMP and PKA signaling. However, our data with cAMP analogs cannot exclude an additional involvement of Epac in the ghrelin signaling because Epac activator used in the current study reportedly potentiates glucose-induced insulin release in a PKA-dependent manner (28). Furthermore, potentiation of the β-cell Kv channel currents by ghrelin was blunted when PKA activity was clamped at high levels with a 6-Phe-cAMP. The PKA activator itself inhibited the channel currents (Fig. 4E). These data suggest that ghrelin activates Kv channels by counteracting the cAMP-PKA signaling. In agreement with this finding, it has been shown that incretin hormones GLP-1, glucose-dependent insulinotropic polypeptide, and pituitary adenylate cyclase-activating polypeptide promote cAMP generation in pancreatic β-cells to potentiate insulin secretion (29–31) and that GLP-1 receptor agonist exendin-4 suppresses Kv channel currents in rat β-cells via PKA-dependent and Epac-independent mechanisms (32). Possible additional inhibitory action of ghrelin on the cAMP-dependent exocytotic machinery remains to be established. It was reported that several hormone receptors of the G-protein Gi/o-family regulate the KATP channel activity and insulin exocytosis (33–35). Whether the ghrelin action could also involve these processes remains to be studied.
We found that ghrelin selectively attenuated glucose-induced insulin release without altering ACh-induced insulin release at 2.8 mmol/L glucose in the perfused rat pancreas. In an apparent discrepancy, ghrelin is reported to suppress carbachol-induced insulin secretion at 5.5 mmol/L glucose, a concentration over the threshold for insulin release, in the perfused rat pancreas (36). ACh stimulates β-cells exclusively via muscarinic M3 receptor (37,38) and induces [Ca2+]i increases and insulin secretion in a glucose-dependent manner (39,40). Collectively, it is likely that ghrelin suppresses M3 receptor-mediated glucose-dependent insulin release by interfering with glucose signaling but not with muscarinic receptor signaling.
It has been reported that GHS-R is coupled to G11-phospholipase C signaling, leading to production of inositol 1,4,5-trisphosphate (IP3) and Ca2+ release from IP3-sensitive stores (2), and that ghrelin activates phospholipase-C pathway for stimulating growth hormone release in pituitary cells (41,42). In contrast, the mechanisms by which GHS-R activates PTX-sensitive Gi-proteins and decreases cAMP in β-cells are unclear. PTX-sensitive ghrelin signaling is reported in the guinea pig femoral artery smooth muscle cells (43). G-protein–coupled receptors often activate more than one type of G-protein (44). Hence, the ghrelin interaction with GHS-R could activate distinct classes of G-protein signaling including G11- and Gi-mediated pathways in a tissue-specific manner. Alternatively, the isoforms of GHS-R (45,46) or heterodimer of GHS-R with other receptors (47) could access the Gi-protein complex. The ghrelin receptor in β-cells remains to be further characterized.
In conclusion, ghrelin attenuates the glucose-induced cAMP production and PKA activation, which drives activation of Kv channels and suppression of the glucose-induced [Ca2+]i increase and insulin release. This is a novel ghrelin signaling that operates in islet β-cells, being distinct from that reported in other tissues including pituitary growth hormone cells. It has been shown that ghrelin receptor antagonists and GOAT inhibitor increase the insulin release and decrease blood glucose in glucose tolerance tests, thereby acting as antidiabetic (5,12,15,48–50). The current study demonstrated that the ghrelin’s signaling cascade and consequent inhibition of insulin release are mediated by attenuation of the cAMP pathway. Hence, counteracting the attenuation of cAMP could provide a new strategy to counteract insulinostatic ghrelin action and thereby to treat type 2 diabetes.
ACKNOWLEDGMENTS
This publication was subsidized by JKA through its promotion funds from KEIRIN RACE. This work was supported by grants-in-aid for Scientific Research (to K.D., M.K., and T.Y.) and that on Priority Areas (to K.D.) from the Japan Society for the Promotion of Science, and by grants from the Salt Science Research Foundation (09C5 and 10C5) (to K.D.), The Pharmacological Research Foundation, Tokyo (to K.D.), Takeda Science Foundation (to T.Y.), The Swedish Research Council, Swedish Diabetes Association, Novo Nordisk Foundation, European Foundation for the Study of Diabetes/Merck Sharp and Dohme (EFSD/MSD), Family Ernfors Foundation (to A.T.), and O.E. and Edla Johanssons Scientific Foundation (to O.D.). No other potential conflicts of interest relevant to this article were reported.
K.D. designed and performed the experiments, contributed to discussion of the results, and wrote the manuscript. B.D. and H.S. performed the experiments. O.D. and A.T. performed the experiments and contributed to discussion of the results. E.G. contributed to discussion of the results. T.K. and M.Y. performed the experiments. M.K. performed the experiments and contributed to discussion of the results. T.Y. designed the experiments, contributed to discussion of the results, and wrote the manuscript.
The authors thank S. Ookuma, M. Warashina, and M. Motoshima for technical assistance at Jichi Medical University.
REFERENCES
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 2.Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996;273:974–977 [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Kangawa K. Drug insight: the functions of ghrelin and its potential as a multitherapeutic hormone. Nat Clin Pract Endocrinol Metab 2006;2:80–88 [DOI] [PubMed] [Google Scholar]
- 4.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002;87:2988–2991 [DOI] [PubMed] [Google Scholar]
- 5.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes 2004;53:3142–3151 [DOI] [PubMed] [Google Scholar]
- 6.Kageyama H, Funahashi H, Hirayama M, et al. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul Pept 2005;126:67–71 [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 2008;105:6320–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387–396 [DOI] [PubMed] [Google Scholar]
- 9.An W, Li Y, Xu G, et al. Modulation of ghrelin O-acyltransferase expression in pancreatic islets. Cell Physiol Biochem 2010;26:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003;144:916–921 [DOI] [PubMed] [Google Scholar]
- 11.Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006;55:3486–3493 [DOI] [PubMed] [Google Scholar]
- 13.Yada T, Dezaki K, Sone H, et al. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr Diabetes Rev 2008;4:18–23 [DOI] [PubMed] [Google Scholar]
- 14.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 2008;118:239–249 [DOI] [PubMed] [Google Scholar]
- 15.Dezaki K, Kakei M, Yada T. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 2007;56:2319–2327 [DOI] [PubMed] [Google Scholar]
- 16.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000;49:1751–1760 [DOI] [PubMed] [Google Scholar]
- 17.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 1987;67:1185–1248 [DOI] [PubMed] [Google Scholar]
- 18.Dyachok O, Idevall-Hagren O, Sågetorp J, et al. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 2008;8:26–37 [DOI] [PubMed] [Google Scholar]
- 19.Dyachok O, Isakov Y, Sågetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature 2006;439:349–352 [DOI] [PubMed] [Google Scholar]
- 20.Hashiguchi S, Yada T, Arima T. A new hypoglycemic agent, JTT-608, evokes protein kinase A-mediated Ca2+ signaling in rat islet β-cells: strict regulation by glucose, link to insulin release, and cooperation with glucagon-like peptide-1(7-36)amide and pituitary adenylate cyclase-activating polypeptide. J Pharmacol Exp Ther 2001;296:22–30 [PubMed] [Google Scholar]
- 21.Higashi H, Sato K, Ohtake A, Omori A, Yoshida S, Kudo Y. Imaging of cAMP-dependent protein kinase activity in living neural cells using a novel fluorescent substrate. FEBS Lett 1997;414:55–60 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald PE, Wheeler MB. Voltage-dependent K+ channels in pancreatic β cells: role, regulation and potential as therapeutic targets. Diabetologia 2003;46:1046–1062 [DOI] [PubMed] [Google Scholar]
- 23.Christensen AE, Selheim F, de Rooij J, et al. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 2003;278:35394–35402 [DOI] [PubMed] [Google Scholar]
- 24.Enserink JM, Christensen AE, de Rooij J, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 2002;4:901–906 [DOI] [PubMed] [Google Scholar]
- 25.Bubis J, Neitzel JJ, Saraswat LD, Taylor SS. A point mutation abolishes binding of cAMP to site A in the regulatory subunit of cAMP-dependent protein kinase. J Biol Chem 1988;263:9668–9673 [PubMed] [Google Scholar]
- 26.Ringheim GE, Taylor SS. Effects of cAMP-binding site mutations on intradomain cross-communication in the regulatory subunit of cAMP-dependent protein kinase I. J Biol Chem 1990;265:19472–19478 [PubMed] [Google Scholar]
- 27.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 2000;275:20829–20836 [DOI] [PubMed] [Google Scholar]
- 28.Chepurny OG, Kelley GG, Dzhura I, et al. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab 2010;298:E622–E633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drucker DJ. Glucagon-like peptides. Diabetes 1998;47:159–169 [DOI] [PubMed] [Google Scholar]
- 30.Gremlich S, Porret A, Hani EH, et al. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes 1995;44:1202–1208 [DOI] [PubMed] [Google Scholar]
- 31.Klinteberg KA, Karlsson S, Ahrén B. Signaling mechanisms underlying the insulinotropic effect of pituitary adenylate cyclase-activating polypeptide in HIT-T15 cells. Endocrinology 1996;137:2791–2798 [DOI] [PubMed] [Google Scholar]
- 32.MacDonald PE, Wang X, Xia F, et al. Antagonism of rat β-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 2003;278:52446–52453 [DOI] [PubMed] [Google Scholar]
- 33.Dunne MJ, Bullett MJ, Li GD, Wollheim CB, Petersen OH. Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. EMBO J 1989;8:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol 1996;271:C1781–C1799 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Park S, Bajpayee NS, et al. Augmented glucose-induced insulin release in mice lacking Go2, but not Go1 or Gi proteins. Proc Natl Acad Sci USA 2011;108:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 2002;146:241–244 [DOI] [PubMed] [Google Scholar]
- 37.Henquin JC, Nenquin M. The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett 1988;236:89–92 [DOI] [PubMed] [Google Scholar]
- 38.Gautam D, Han SJ, Hamdan FF, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 2006;3:449–461 [DOI] [PubMed] [Google Scholar]
- 39.Hermans MP, Henquin JC. Relative importance of extracellular and intracellular Ca2+ for acetylcholine stimulation of insulin release in mouse islets. Diabetes 1989;38:198–204 [DOI] [PubMed] [Google Scholar]
- 40.Yada T, Hamakawa N, Yaekura K. Two distinct modes of Ca2+ signalling by ACh in rat pancreatic β-cells: concentration, glucose dependence and Ca2+ origin. J Physiol 1995;488:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glavaski-Joksimovic A, Jeftinija K, Scanes CG, Anderson LL, Jeftinija S. Stimulatory effect of ghrelin on isolated porcine somatotropes. Neuroendocrinology 2003;77:367–379 [DOI] [PubMed] [Google Scholar]
- 42.Malagón MM, Luque RM, Ruiz-Guerrero E, et al. Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology 2003;144:5372–5380 [DOI] [PubMed] [Google Scholar]
- 43.Mladenov MI, Hristov KL, Dimitrova DZ, et al. Ghrelin signalling in guinea-pig femoral artery smooth muscle cells. Acta Physiol (Oxf) 2008;194:195–206 [DOI] [PubMed] [Google Scholar]
- 44.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther 2003;99:25–44 [DOI] [PubMed] [Google Scholar]
- 45.Geelissen SM, Beck IM, Darras VM, Kühn ER, Van der Geyten S. Distribution and regulation of chicken growth hormone secretagogue receptor isoforms. Gen Comp Endocrinol 2003;134:167–174 [DOI] [PubMed] [Google Scholar]
- 46.Kineman RD, Gahete MD, Luque RM. Identification of a mouse ghrelin gene transcript that contains intron 2 and is regulated in the pituitary and hypothalamus in response to metabolic stress. J Mol Endocrinol 2007;38:511–521 [DOI] [PubMed] [Google Scholar]
- 47.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 2006;20:1772–1785 [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006;3:379–386 [DOI] [PubMed] [Google Scholar]
- 49.Esler WP, Rudolph J, Claus TH, et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology 2007;148:5175–5185 [DOI] [PubMed] [Google Scholar]
- 50.Barnett BP, Hwang Y, Taylor MS, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 2010;330:1689–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]