Abstract
Clem, W. H. (University of Washington, Seattle), and S. J. Klebanoff. Inhibitory effect of saliva on glutamic acid accumulation by Lactobacillus acidophilus and the role of the lactoperoxidase-thiocyanate system. J. Bacteriol. 91:1848–1853. 1966.—Saliva contains an antimicrobial system which inhibits the growth of Lactobacillus acidophilus, as well as a number of other organisms, in complete growth medium. This antimicrobial system consists of the salivary peroxidase (lactoperoxidase) and thiocyanate ions, and requires the presence of H2O2. Saliva inhibits the accumulation of glutamic acid and certain other amino acids by resting cells. This effect of saliva is decreased by dialysis, and thiocyanate ions restore the inhibitory effect of dialyzed saliva. The inhibitory effect of saliva is decreased by heat (100 C, 10 min), and lactoperoxidase restores the inhibitory effect of heated saliva. Thus, the inhibition of glutamic acid accumulation by saliva appears to be due in part to the lactoperoxidase-thiocyanate antimicrobial system. H2O2 increases the inhibitory effect of both saliva and the lactoperoxidase-thiocyanate system on glutamic acid accumulation. The inhibition of glutamic acid accumulation is not preceded by a loss in microbial viability. The glutamic acid accumulated by L. acidophilus under the conditions employed remains largely (over 90%) as free glutamic acid. This suggests that saliva and the lactoperoxidase-thiocyanate-H2O2 system inhibit the net transport of glutamic acid into the cell.
Full text
PDF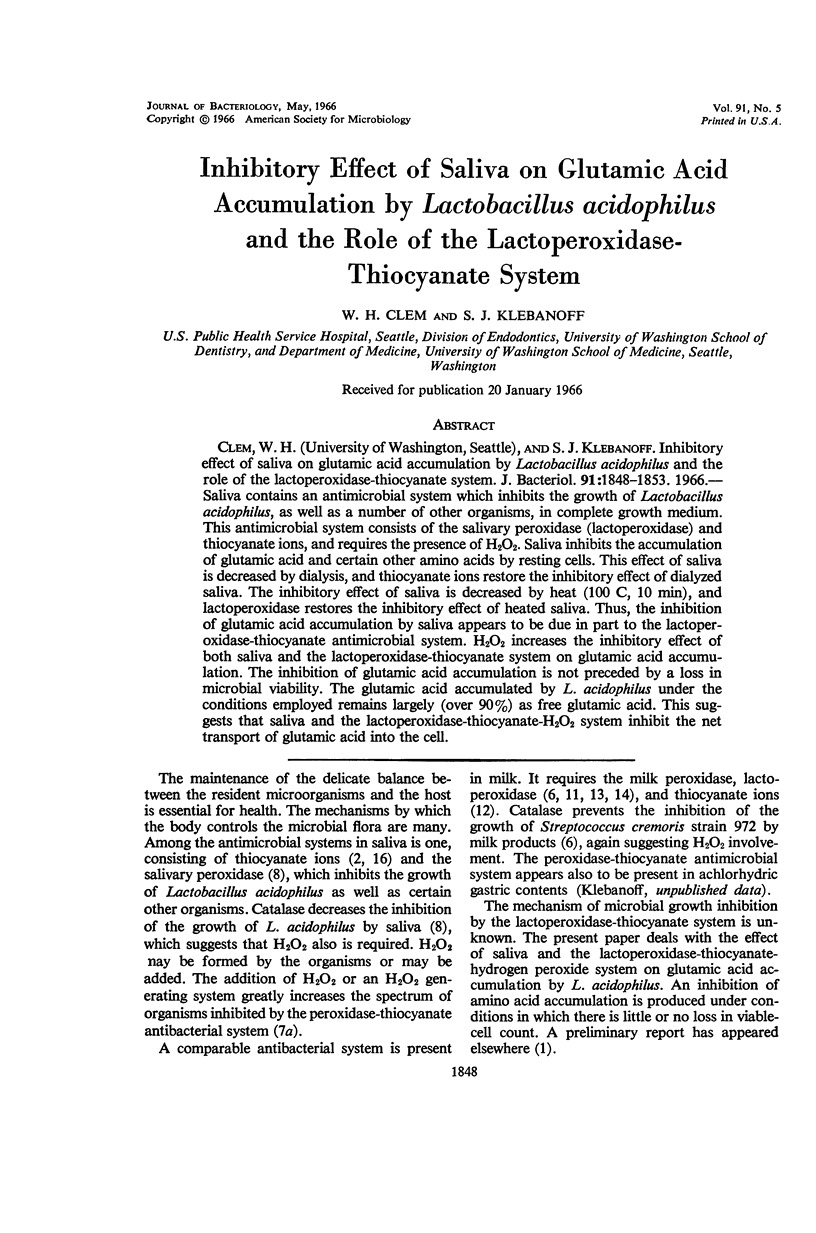



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOGON I. L., KERR A. C., AMDUR B. H. Characterization of an antibacterial factor in human parotid secretions, active against Lactobacillus casei. Arch Oral Biol. 1962 Jan-Feb;7:81–90. doi: 10.1016/0003-9969(62)90051-1. [DOI] [PubMed] [Google Scholar]
- DREIZEN S., MOSNY J. J., GILLEY E. J., SPIES T. D. The amino acid requirements of oral acidogenic microorganisms associated with human dental caries. J Dent Res. 1954 Jun;33(3):339–345. doi: 10.1177/00220345540330030701. [DOI] [PubMed] [Google Scholar]
- HANCOCK R. Accumulation of pool amino acids in Staphylococcus aureus following inhibition of protein synthesis. Biochim Biophys Acta. 1960 Jan 1;37:47–55. doi: 10.1016/0006-3002(60)90077-9. [DOI] [PubMed] [Google Scholar]
- JAGO G. R., MORRISON M. Anti-streptococcal activity of lactoperoxidase III. Proc Soc Exp Biol Med. 1962 Dec;111:585–588. doi: 10.3181/00379727-111-27862. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- ZELDOW B. J. Studies on the antibacterial action of human saliva. III. Cofactor requirements of Lactobacillus bactericidin. J Immunol. 1963 Jan;90:12–16. [PubMed] [Google Scholar]


