Abstract
OBJECTIVE
The therapeutic potential of exendin-4, an agonist of the glucagon-like peptide-1 receptor (GLP-1R), on diabetic polyneuropathy (DPN) in streptozotocin (STZ)-induced diabetic mice was investigated.
RESEARCH DESIGN AND METHODS
The presence of the GLP-1R in lumbar dorsal root ganglion (DRG) was evaluated by immunohistochemical analyses. DRG neurons were dissected from C57BL6/J mice and cultured with or without Schwann cell–conditioned media in the presence or absence of GLP-1 (7–37) or exendin-4. Then neurite outgrowth was determined. In animal-model experiments, mice were made diabetic by STZ administration, and after 12 weeks of diabetes, exendin-4 (10 nmol/kg) was intraperitoneally administered once daily for 4 weeks. Peripheral nerve function was determined by the current perception threshold and motor and sensory nerve conduction velocity (MNCV and SNCV, respectively). Sciatic nerve blood flow (SNBF) and intraepidermal nerve fiber densities (IENFDs) also were evaluated.
RESULTS
The expression of the GLP-1R in DRG neurons was confirmed. GLP-1 (7–37) and exendin-4 significantly promoted neurite outgrowth of DRG neurons. Both GLP-1R agonists accelerated the impaired neurite outgrowth of DRG neurons cultured with Schwann cell–conditioned media that mimicked the diabetic condition. At the doses used, exendin-4 had no effect on blood glucose or HbA1c levels. Hypoalgesia and delayed MNCV and SNCV in diabetic mice were improved by exendin-4 without affecting the reduced SNBF. The decreased IENFDs in sole skins of diabetic mice were ameliorated by exendin-4.
CONCLUSIONS
Our findings indicate that exendin-4 ameliorates the severity of DPN, which may be achieved by its direct actions on DRG neurons and their axons.
Diabetes is the most common cause of peripheral neuropathy encompassing both mononeuropathy and polyneuropathy (1,2). In general, diabetic polyneuropathy (DPN) develops symmetrically in a nerve length–dependent fashion, with dying-back degeneration of both myelinated and unmyelinated fibers. Diabetic patients may exhibit various symptoms of DPN, such as spontaneous pain, hyperalgesia, and diminished sensation (3). It has been shown that tight glycemic control is effective in slowing the progression of DPN but cannot completely prevent it (4). We have focused on the role of reduced nerve blood flow in the development and the progression of DPN (5–7). In addition to the hemodynamic deterioration of diabetic nerves, previous studies have described a number of pathogenic mechanisms suggesting favorable treatments of DPN, but these treatments have generally failed in clinical trials (2). Thus, at this time, there are few effective therapies for DPN. Because the etiology of DPN seems to be multifactorial, a multitargeted intervention may be necessary.
An incretin hormone, glucagon-like peptide (GLP)-1, is released from the L cells of the small intestine (8). GLP-1 and a GLP-1 receptor (GLP-1R) agonist, exendin-4, potentiate glucose-stimulated insulin secretion after a meal, and GLP-1R agonists have been used as therapeutic agents for type 2 diabetes (9–11). In addition to this antihyperglycemic effect, GLP-1R agonists have been shown to have several actions, such as slowing gastric emptying (11) and reducing food intake (12), that are independent of insulin secretion (13). Many reports have suggested that GLP-1R agonists have neurotrophic and neuroprotective properties in some neurons and neural cells (14–18). It has been revealed that prolonged neurite extension is induced by mechanisms involving cAMP (19), which also is involved in the cascade mechanisms of insulin secretion induced by GLP-1R agonists. In addition, the therapeutic effects of GLP-1R agonists on stroke, Parkinsonism, and pyridoxine-induced peripheral sensory neuropathy (18–20) using animal models have been reported.
Although several beneficial effects of GLP-1 or the GLP-1R agonist on central and peripheral nervous systems have been reported, their effects under the diabetic condition have not yet been evaluated. Here, we investigated the effects of the GLP-1R agonist exendin-4 on DPN by both in vitro and in vivo experiments.
RESEARCH DESIGN AND METHODS
Schwann cell culture and preparation of Schwann cell–conditioned media.
Immortalized Schwann cells (IMS32), established by long-term culture of adult mouse dorsal root ganglions (DRGs) and peripheral nerves (21), were a gift from Dr. Kazuhiro Watabe. IMS32 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO) containing 5.5 mmol/L d-glucose, penicillin (100 units/mL)-streptomycin (100 mg/mL), and 5% FBS (Moregate Biotech, Bulimba QLD, Australia). When the cells reached ~70% confluency, they were maintained in DMEM with 2% FBS containing 5.5 mmol/L d-glucose (normal glucose [NG]) or 30 mmol/L d-glucose (high glucose [HG]). After a 3-day culture, the cells were maintained in serum-free DMEM containing NG or HG. After 24 h, culture media were collected, concentrated 10 times using 10 kD centrifugal filters (Amicom Ultra-15; Nihon Millipore, Tokyo, Japan), and frozen at −80°C until use. We defined these media as NG-IMS media or HG-IMS media.
Primary culture of DRG neurons and evaluation of neurite outgrowth.
DRG neuron cultures were prepared from 5-week-old male C57BL/6 mice (Chubu Kagaku Shizai, Nagoya, Japan), as previously described (22). In brief, DRGs were collected, dissociated by collagenase (Wako Pure Chemical, Osaka, Japan), and diluted in a medium consisting of F-12 media, 10 mmol/L glucose, and 30 nmol/L selenium. Isolated DRG neurons were seeded on glass coverslips coated with poly-l-lysine. DRG neurons were cultured with or without 10 nmol/L GLP-1 (7–37) (Bachem Bioscience, Torrance, CA) or exendin-4 (Sigma-Aldrich) (0.1, 1, 10, and 100 nmol/L). To evaluate the effects of GLP-1R agonists on impaired neurite outgrowth under the diabetic condition, DRG neurons were cultured in HG-IMS media that was diluted one-tenth with F-12 media.
After a 24-h culture, DRG neurons fixed with 4% paraformaldehyde were immunostained with rabbit polyclonal antineurofilament heavy-chain antibody (1:5,000; Nihon Millipore) and visualized with Alexa Fluor 488–coupled goat anti-rabbit IgG antibody (1:200; Invitrogen, Carlsbad, CA). Coverslips were counterstained with 4′,6-Diamidine-2′-phenylindole dihydrochloride (Merck, Tokyo, Japan). Images were captured by a charge-coupled device camera (DP70; Olympus Optical, Tokyo, Japan) using a fluorescence microscope (BX51; Olympus Optical). Neurite outgrowth was observed in 10–20 neurons per coverslip and evaluated by a computed image analysis system (Angiogenesis Image Analyzer version 2; KURABO Industries, Osaka, Japan).
Animals and induction of diabetes.
Five-week-old male C57BL/6 mice (Chubu Kagaku Shizai) were used. Diabetes was induced by intraperitoneal injection of streptozotocin (STZ) (150 mg/kg; Sigma-Aldrich). Control mice received an equal volume of citric acid buffer. One week after STZ administration, the mice with plasma glucose concentrations >16 mmol/L were selected as diabetic mice. Twelve weeks after the induction of diabetes, mice were treated once daily with exendin-4 (10 nmol/kg in 0.1 mL of water i.p.) or vehicle (saline) over 4 weeks (n = 10 in each group). Before and after exendin-4 treatment, fasting blood glucose levels and HbA1c were examined by a FreeStyle Freedom Glucose Meter (Nipro, Osaka, Japan) and a RAPIDIA Auto HbA1c-L assay kit using latex agglutination (Fujirebio, Tokyo, Japan), respectively. After the exendin-4 treatment, intraperitoneal glucose tolerance tests (IPGTTs) were performed on the mice. Serum insulin and glucagon levels also were measured in fasted mice by an insulin ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan) and a glucagon enzyme immune assay kit (Yanaihara Institute, Fujinomiya, Japan), respectively. The Nagoya University Institutional Animal Care and Use Committee approved the protocols of this experiment.
Measurement of current perception threshold using a neurometer.
To determine a nociceptive threshold, the current perception threshold (CPT) was measured in 12- and 16-week diabetic and age-matched normal mice using a CPT/laboratory neurometer (Neurotron, Denver, CO). The electrodes (SRE-0405–8; Neurotron) for stimulation were attached to plantar surfaces. Each mouse was kept in a Ballman cage (Natsume Seisakusho, Tokyo, Japan) suitable for light restraint to keep awake. Three transcutaneous-sine-wave stimuli with different frequencies (2,000, 250, and 5 Hz) were applied to the plantar surfaces. The intensity of each stimulation was gradually increased automatically (increments of 0.01 mA for 5 and 250 Hz and increments of 0.02 mA for 2,000 Hz). The minimum intensity at which the mouse withdrew its paw was defined as the CPT. Six consecutive measurements were conducted at each frequency.
Nerve conduction velocity.
Mice anesthetized with pentobarbital were placed on a heated pad in a room maintained at 25°C to ensure a constant rectal temperature of 37°C. Motor nerve conduction velocity (MNCV) was determined between the sciatic notch and ankle with a Neuropak NEM-3102 instrument (Nihon-Koden, Osaka, Japan), as previously described (5,6,23). The sensory nerve conduction velocity (SNCV) was measured between the knee and ankle with retrograde stimulation.
Sciatic nerve blood flow.
Sciatic nerve blood flow (SNBF) was measured by laser-Doppler flowmetry (FLO-N1; Omegawave, Tokyo, Japan). The thigh skin of an anesthetized mouse was cut along the femur and then an incision through the fascia was carefully made to expose the sciatic nerve. Five minutes after this procedure, the blood flow was measured by a laser-Doppler probe placed 1 mm above the nerve. During this measurement, the mouse was placed on a heated pad in a room maintained at 25°C to ensure a constant rectal temperature of 37°C.
Tissue collection.
Four weeks after the treatment with exendin-4, mice were killed by an overdose of pentobarbital or perfusion with 50 mL of 4% paraformaldehyde. DRGs and sciatic nerves were obtained from normal and diabetic mice. Some of the tissues were snap frozen in liquid nitrogen, followed by preservation at –80°C until use, and others were transferred to RNAlater solution (Invitrogen), followed by freezing preservation for RT-PCR. For immunohistochemistry, DRGs, pancreas, and sole skin were excised, fixed in 4% paraformaldehyde, and frozen in optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan) after cryoprotection.
GLP-1R mRNA expression in DRGs and sciatic nerves.
RNAs were extracted from frozen samples of DRGs and sciatic nerves using Isogen (Nippon Gene, Toyama, Japan) and were quantified spectrophotometrically. Starting from 1 µg of RNA, cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). TaqMan gene expression assays (Applied Biosystems, Foster City, CA) were used for GLP-1R and 18S rRNA for the endogenous control. Real-time quantitative RT-PCR was performed using the Mx3000P QPCR System (Stratagene, La Jolla, CA). Relative quantity was calculated by the ΔΔCt method with normalization to 18S rRNA (24).
Western blotting.
DRGs and sciatic nerves were used for Western blotting. Samples were lysed in detergent lysis buffer (Cell Lysis Buffer; Cell Signaling Technology, Boston, MA) adding 1 mmol/L phenylmethanesulfonylfluoride (Sigma-Aldrich), following centrifugation. Proteins were quantitated the concentrations with a bicinchoninic acid assay (Sigma Chemical) and were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA) after SDS-PAGE. Membranes were blocked and incubated with rabbit polyclonal anti–GLP-1R antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti–β-actin antibody (1:10,000; Abcam, Cambridge, MA). Antigen detection was performed using ECL Plus Western Blotting Detection Reagents (Amersham Pharmacia Biotech, Piscataway, NJ) with horseradish peroxidase–conjugated anti-rabbit IgG antibody (1:6,000; Cell Signaling Technology). Images were scanned and their densities were determined by ImageJ (National Institutes of Health, Bethesda, MD). The expression of the GLP-1R protein was corrected by β-actin density, and the expression in tissues of normal mice was arbitrarily set at 1.0.
Immunocytochemistry and frozen section staining.
After a 24-h culture, DRG cells, as indicated above, were fixed with 4% paraformaldehyde. The cells were blocked with 3% goat serum, and the following primary antibodies were applied to the glass coverslips at 4°C overnight: rabbit polyclonal anti–GLP-1R antibody (1:200, sc-66911; Santa Cruz Biotechnology); mouse monoclonal anti-neurofilament 70 kDa (NF70) antibody (1:1,000, MAB1615; Millipore); and mouse monoclonal anti-S100b antibody (1:300, S2532; Sigma-Aldrich). After washing, the following secondary antibodies were loaded for 1 h at room temperature in a dark box: Alexa Fluor 488–coupled goat anti-rabbit IgG antibody (1:200; Invitrogen) and Alexa Fluor 594–coupled goat anti-mouse antibody (1:300; Invitrogen).
For immunohistochemistry, after the microwave irradiation in citrate buffer (pH 6.0), cryostat sections were blocked with 5% skim milk (Meiji Milk, Tokyo, Japan) and the following primary antibodies were applied to the sections at 4°C overnight: rabbit polyclonal anti–protein-gene-product 9.5 (PGP 9.5) antibody (1:500; Millipore); guinea-pig polyclonal anti-insulin antibody (1:500, Ab7842-500; Abcam); rabbit polyclonal anti–GLP-1R antibody (1:200, sc-66911, Santa Cruz Biotechnology; LS-A1205 and LS-A1206, MBL International, Woburn, MA); mouse monoclonal NF70 antibody (1:1,000; Millipore); and mouse monoclonal anti-S100b antibody (1:300; Sigma-Aldrich). After washing, the secondary antibodies, as indicated above, were loaded for 1 h at room temperature. Coverslips and tissues were counterstained with diaminido phenyl indol (Merck). Images were captured by a charge-coupled device camera (DP70; Olympus Optical) using a fluorescence microscope (BX51; Olympus Optical).
Measurement of intraepidermal nerve fiber densities.
Nerve fibers stained with anti–PGP 9.5 antibody were counted as previously reported (25). In brief, each individual nerve fiber with branching inside the epidermis was counted as one, and a nerve fiber with branching in the dermis was counted separately. Six fields from each section were randomly selected for the intraepidermal nerve fiber (IENF) densities (IENFDs). IENFDs were derived and expressed as epidermal nerve fiber numbers per length of the epidermal basement membrane (fibers per millimeter).
Statistical analysis.
All the group values were expressed as means ± SD. Statistical analyses were made by one-way ANOVA, with the Bonferroni correction for multiple comparisons. All analyses were performed by personnel who were unaware of the animal identities.
RESULTS
DRG neurons and satellite cells expressed GLP-1Rs.
To confirm the quality of GLP-1R antibody, we stained the islets of glp1r−/− mice. GLP-1R antibody obtained from Santa Cruz (sc-66911) detected the β-cells of wild-type mice but not those of glp1r−/− mice. In contrast, GLP-1R antibodies from MBL International (LS-A1205 and LS-A1206) nonspecifically stained the islets of glp1r−/− mice (Supplementary Fig. 1). In addition to immunohistochemistry, GLP-1R protein in the pancreas of wild-type mice was specifically detected and that of glp1r−/− mice was undetected with the GLP-1R antibody from Santa Cruz by Western blotting methods (Supplementary Fig. 2). Therefore, in this study, we used sc-66911 as a primary antibody to detect the expression of GLP-1R in DRGs. GLP-1R expression was detected in both DRG neurons indicated by NF70 and satellite cells indicated by S100b antibody (Fig. 1A–F). In contrast to these wild-type mice DRGs, GLP-1R expression was not detected in the DRGs of glp1r−/− mice (Fig. 1G). To further evaluate the localization of GLP-1R, we stained enzymatically dissociated DRG cells with GLP-1R antibody in addition to S100b or NF70 antibody. GLP-1R proteins also were expressed in both DRG neurons and satellite cells by this immucytochemical method (Supplementary Fig. 3).
FIG. 1.
Expression of GLP-1R in DRGs and sciatic nerves. A, C, D, and F: Immunohistochemically, GLP-1R (green) in DRG was detected with anti–GLP-1R antibody (sc-66911). Both DRG neurons, indicated by NF70 antibody (red) (E and F), and satellite glia cells, indicated by S100b antibody (red) (B and C), expressed GLP-1R. C, F, and G: Nuclei (blue) were stained with diaminido phenyl indol. A–F: White arrowheads indicate satellite glia cells. White arrows indicate neurons. G: GLP-1R protein (red) was not detected in DRG neurons of glp1r−/− mice. H: The transcript levels of glp1r in DRGs and sciatic nerves of diabetic mice were not significantly different from those of normal mice (DRG: normal mice [N] [n = 6], 1 ± 0.54, threshold cycle value [Ct] of glp1r 34.8 ± 2.3, Ct of 18S rRNA 11.2 ± 1.0 and diabetic mice [DM] [n = 7], 0.65 ± 0.23, Ct of glp1r 34.0 ± 1.5, Ct of 18S rRNA 11.0 ± 1.4, P = 0.544; sciatic nerves: normal mice [n = 4], 1 ± 0.34, Ct of glp1r 33.6 ± 1.6, Ct of 18S rRNA 14.7 ± 1.1 and diabetic mice [n = 5], 1.95 ± 1.29, Ct of glp1r 34.5 ± 1.3, Ct of 18S rRNA 12.5 ± 2.8, P = 0.606). I: There were no significant differences in the protein levels of GLP-1R evaluated by Western blotting analyses between diabetic and normal mice (DRG: normal mice [n = 4], 1 ± 0.09 and diabetic mice [n = 4], 1.02 ± 0.20, P = 0.875; sciatic nerves: normal mice [n = 4], 1 ± 0.17 and diabetic mice [n = 4], 1.09 ± 1.35, P = 0.438). (A high-quality digital representation of this figure is available in the online issue.)
GLP-1R agonists promoted neurite outgrowth of DRG neurons.
In our DRG culture system, GLP-1R protein was expressed in all neurons and in about two-thirds of glia cells (data not shown). Therefore, we used our DRG culture system to evaluate the impact of the GLP-1R agonist on the sensory nervous system, especially sensory neurons.
Neurite outgrowth of DRG neurons was increased in the presence of GLP-1 (7–37) or exendin-4 (Fig. 2A and B). Total length and joint number of neurites were significantly increased by GLP-1 (7–37) or exendin-4 (joint number: control vs. 10 nmol/L exendin-4, P = 0.0002; control vs. 100 nmol/L exendin-4, P = 0.0093; and control vs. 10 nmol/L GLP-1 (7–37), P < 0.0001; total length: control vs. 10 nmol/L exendin-4, P = 0.0003; control vs. 100 nmol/L exendin-4, P < 0.0001; and control vs. 10 nmol/L GLP-1 (7–37), P < 0.0001) (Fig. 2C and D).
FIG. 2.
Neurite outgrowth of DRG neurons by GLP-1 (7–37) and exendin-4 (Ex4). Representative fluorescence micrograph of DRG neurons cultured in the absence (A) or presence (B) of GLP-1 (7–37) (GLP-1) (10 nmol/L). GLP-1 (7–37) (10 nmol/L) or exendin-4 (0.1, 1, 10, and 100 nmol/L) increased the total neurite length (C) and joint number (D) of neurites. Results are means ± SD. CON, control medium. *P < 0.05 vs. control medium (n = 10–20). Control medium, joint number 23.5 ± 17.2 per cell, total length 833 ± 462 µm per cell, 10 nmol/L GLP-1 (7–37); joint number 54.8 ± 58.3, total length 2,786 ± 2,976, 0.1 nmol/L exendin-4; joint number 19.81 ± 29.59, total length 1,056.3 ± 904.5, 1 nmol/L exendin-4; joint number 32.23 ± 29.35, total length 761.9 ± 414.7, 10 nmol/L exendin-4; joint number 78.1 ± 58.4, total length 2,035 ± 1,162, 100 nmol/L exendin-4; and joint number 63.1 ± 33.8, total length 2,329 ± 1,104. (A high-quality digital representation of this figure is available in the online issue.)
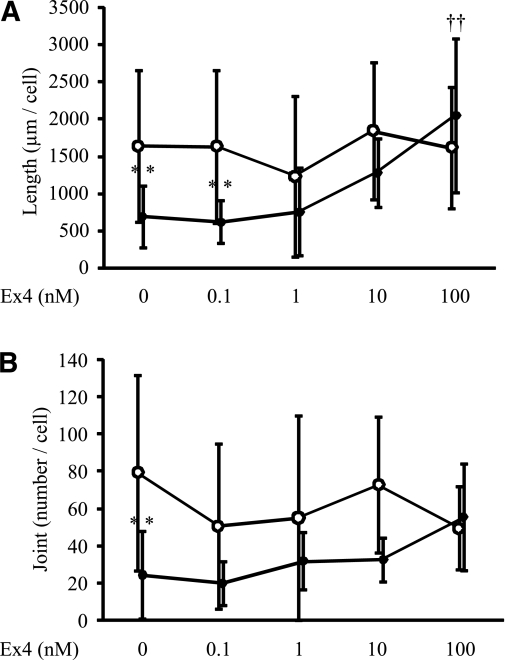
Exendin-4 ameliorated high glucose–induced reduction in neurite outgrowth of DRG neurons.
DRG neurons cultured with HG-IMS media, which mimicked the diabetic state, had shorter neurites and smaller joint numbers compared with those cultured with NG-IMS media, which mimicked the nondiabetic normal state (total length: P = 0.0205, joint number: P = 0.0006) (Fig. 3). The impaired neurite outgrowth of DRG neurons cultured with HG-IMS media was improved by exendin-4 (total length: HG with 10 nmol/L exendin-4, P = 0.1620, and HG with 100 nmol/L exendin-4, P = 0.0012; joint number: HG with 10 nmol/L exendin-4, P = 0.5871, and HG with 100 nmol/L exendin-4, P = 0.0433). In contrast, exendin-4 did not promote the neurite outgrowth of DRG neurons cultured with NG-IMS media.
FIG. 3.
Neurite outgrowth of DRG neurons in IMS media with or without exendin-4 (Ex4). Total length (A) and joint number (B) of DRG neurons cultured in IMS media were measured. Decreased total length and joint number of DRG neurites cultured in HG were ameliorated by Ex4 in a dose-dependent fashion. Results are means ± SD. ●, Neurite cultured in HG-IMS media; ○, neurite cultured in NG-IMS media. NG-IMS media were obtained from IMS cultured in F-12 media with 5.5 mmol/L d-glucose; HG-IMS media were obtained from IMS cultured in F-12 media with 30 mmol/L d-glucose. **P < 0.005 vs. NG-IMS media without exendin-4; ††P < 0.005 vs. HG-IMS media without exendin-4. n = 10–20. Total length: NG 1,635 ± 1,014 μm per cell and HG 684 ± 410; joint number: NG 79.2 ± 52.5 per cell and HG 24.2 ± 23.8. Total length: HG with 10 nmol/L exendin-4 1,278 ± 457 and HG with 100 nmol/L exendin-4 2,045 ± 1,029. Joint number: HG with 10 nmol/L exendin-4 32.3 ± 12.0 and HG with 100 nmol/L exendin-4 55.1 ± 28.8. Total length: NG with 10 nmol/L exendin-4 1,839 ± 915 and NG with 100 nmol/L exendin-4 1,614 ± 821. Joint number: NG with 10 nmol/L exendin-4 72.7 ± 36.3 and NG with 100 nmol/L exendin-4 49.3 ± 22.3.
Levels of GLP-1R in DRGs and sciatic nerves were not impaired in diabetic mice.
To ascertain the levels of glp1r mRNA and protein, we carried out real-time PCR analyses and Western blotting analyses in the DRGs and sciatic nerves of normal and diabetic mice before the exendin-4 treatment. The levels of glp1r transcript in DRGs and sciatic nerves of diabetic mice were not significantly different between those of normal mice (DRG: P = 0.544, sciatic nerves: P = 0.606) (Fig. 1H). Furthermore, there were no significant differences in GLP-1R protein contents between diabetic and normal mice (DRG: P = 0.875, sciatic nerves: P = 0.438) (Fig. 1I).
Body weights, blood glucose levels, and HbA1c.
At 12 weeks, diabetic mice showed severe hyperglycemia (P = 0.0003) and significantly reduced body weight gain (P = 0.003). Random blood glucose levels measured during the experimental period were not significantly different in any group. Exendin-4 treatment for 4 weeks did not alter body weight, blood glucose, or HbA1c levels in the diabetic groups (Table 1).
TABLE 1.
Body weight, blood glucose, and HbA1c levels in normal and diabetic mice
| Normal mice |
Diabetic mice |
|||||
|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment |
Pretreatment | Posttreatment |
|||
| Saline | Exendin-4 | Saline | Exendin-4 | |||
| n | 8 | 5 | 6–8 | 9–10 | 8 | 9 |
| Blood glucose (mmol/L) | 9.1 ± 1.6 | 9.1 ± 1.6 | 8.7 ± 2.4 | 23.1 ± 2.8* | 26.6 ± 2.9* | 23.9 ± 2.3* |
| HbA1c (%) | 4.0 ± 0.1 | 4.1 ± 0.2 | 3.9 ± 0.1 | 7.6 ± 1.2* | 7.7 ± 2.1* | 6.9 ± 2.1* |
| Body weight (g) | 31 ± 3 | 31 ± 3 | 27 ± 2† | 26 ± 3* | 24 ± 4* | 23 ± 2* |
Data are means ± SD.
*P < 0.05 vs. pretreatment normal mice.
†P < 0.05 vs. normal mice treated with saline.
Serum insulin and glucagon levels and IPGTTs.
After the exendin-4 treatment, serum insulin levels were significantly decreased in diabetic mice, and exendin-4 administration provided no significant improvement in both diabetic and normal mice (Supplementary Fig. 4A). In IPGTT, blood glucose levels in diabetic mice after 15 min of glucose injection were significantly elevated compared with those in normal mice. These elevations were not significantly decreased by exendin-4 treatment (Supplementary Fig. 4C).
Although serum glucagon concentrations were not incremented in diabetic mice compared with those in normal mice, the concentrations in diabetic mice had a high propensity to be decreased by exendin-4 treatment (Supplementary Fig. 4B).
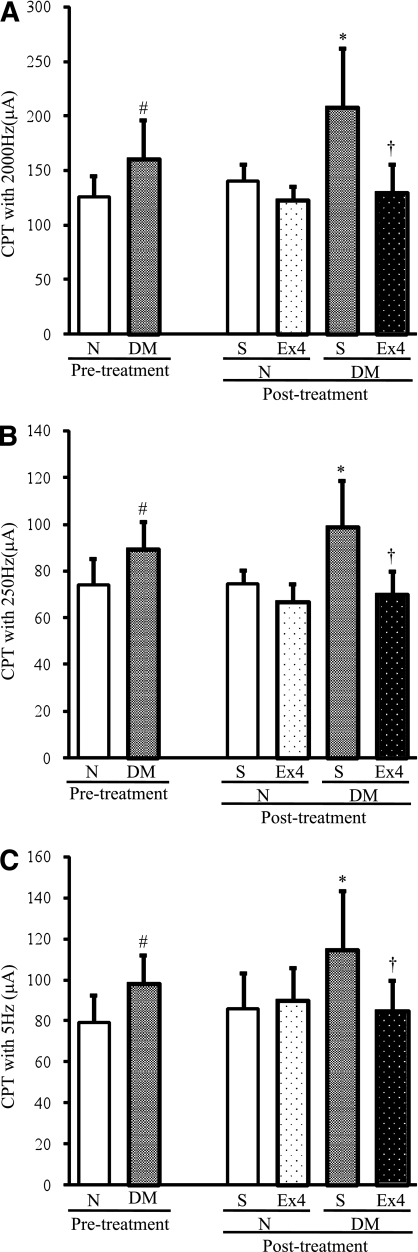
Reduced sensory perception in diabetic mice was ameliorated by exendin-4 administration.
After 12 weeks of diabetes, CPTs at 5, 250, and 2,000 Hz were significantly increased compared with those in normal mice (5 Hz: P = 0.015, 250 Hz: P = 0.019, and 2,000 Hz: P = 0.028), representing hypoalgesia in diabetic mice. After the 4 weeks of exendin-4 administration, these deficits in sensation were significantly improved in diabetic mice compared with saline-treated diabetic controls (5 Hz: P = 0.0161, 250 Hz: P = 0.0012, and 2,000 Hz: P = 0.0011). The injection of exendin-4 into normal mice did not induce significant changes in CPTs (Fig. 4A–C).
FIG. 4.
Evaluation of sensory nerve functions. Measurements of CPTs at 2,000 (A), 250 (B), and 5 (C) Hz by a neurometer were performed before and at the end of exendin-4 (Ex4) administration. CPTs for all pulses were significantly increased in the diabetic group (DM), and these deficits were significantly prevented by exendin-4. N, normal mice; S, saline. Results are means ± SD. #P < 0.05 vs. pretreatment normal mice; *P < 0.05 vs. saline-treated normal mice; †P < 0.05 vs. saline-treated diabetic mice. Saline-treated normal mice, n = 8; exendin-4–treated normal mice, n = 8; saline-treated diabetic mice, n = 9; exendin-4–treated diabetic mice, n = 9.
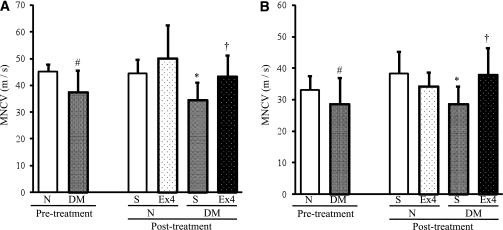
Exendin-4 improved delayed NCVs in diabetic mice.
MNCVs and SNCVs of diabetic mice were significantly delayed compared with those of normal mice (MNCV: P = 0.0341, SNCV: P = 0.0489). The delay in MNCVs and SNCVs was significantly restored by exendin-4 treatment (MNCV: P = 0.0289, SNCV: P = 0.0201) (Fig. 5A and B). However, exendin-4 administration did not alter NCVs in normal mice.
FIG. 5.
NCVs. MNCV (A) and SNCV (B) were measured before and after the treatment with exendin-4 (Ex4). DM, diabetic mice; N, normal mice; S, saline. Before the treatment: MNCV for normal mice 45.2 ± 2.7 m/s and diabetic mice 37.5 ± 8.2, P = 0.0341; SNCV for normal mice 33.0 ± 4.6 and diabetic mice 28.6 ± 7.2, P = 0.0489. After the treatment: MNCV for saline-treated diabetic mice 34.5 ± 8.1 and exendin-4–treated diabetic mice 43.3 ± 7.9, P = 0.0289; SNCV for saline-treated diabetic mice 28.6 ± 5.6 and exendin-4–treated diabetic mice 38.0 ± 8.5, P = 0.0201. Results are means ± SD. #P < 0.05 vs. pretreatment normal mice. *P < 0.05 vs. saline-treated normal mice. †P < 0.05 vs. saline-treated diabetic mice. Saline-treated normal mice, n = 7; exendin-4–treated normal mice, n = 8; saline-treated diabetic mice, n = 8; exendin-4–treated diabetic mice, n = 8.
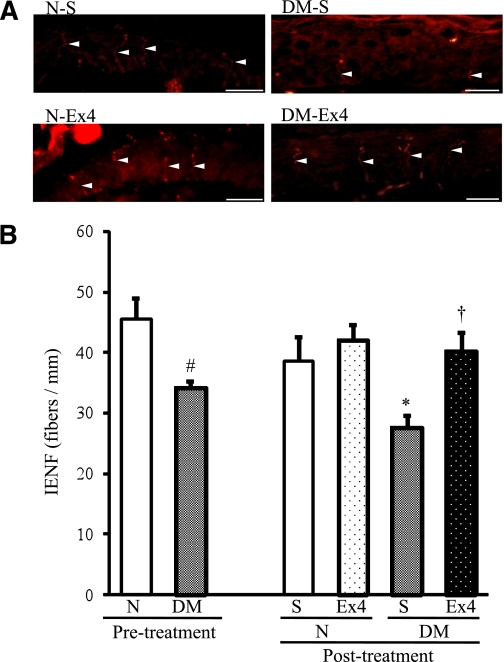
Nerve fibers in epidermis were preserved by exendin-4.
IENFDs were evident in both the epidermis and the dermis of the foot skin by the fluorescent imaging (Fig. 6A). Although IENFDs were decreased in diabetic mice (P = 0.0011), this decrement was significantly ameliorated by exendin-4 (P = 0.0007) (Fig. 6B). Administration of exendin-4 did not change IENFDs in normal mice (P = 0.2212).
FIG. 6.
IENFDs after the treatment with exendin-4 (Ex4). A: IENFs indicated by white arrowheads were detected by immunofluorescence assay with anti–PGP 9.5 antibody (red). B: Quantification of the density revealed a significant decrement in untreated diabetic mice and the significant amelioration by exendin-4 treatment. DM, diabetic mice; N, normal mice; S, saline. Bar: 20 μm. Saline-treated normal mice 38.5 ± 3.9, saline-treated diabetic mice 27.7 ± 1.9, exendin-4–treated normal mice 42.1 ± 2.4, and exendin-4–treated diabetic mice 40.2 ± 3.1 fibers per mm. Results are means ± SD. #P < 0.05 vs. pretreatment normal mice. *P < 0.05 vs. saline-treated normal mice. †P < 0.05 vs. saline-treated diabetic mice. Saline-treated normal mice, n = 4; exendin-4–treated normal mice, n = 3; saline-treated diabetic mice, n = 4; exendin-4–treated diabetic mice, n = 3. (A high-quality digital representation of this figure is available in the online issue.)
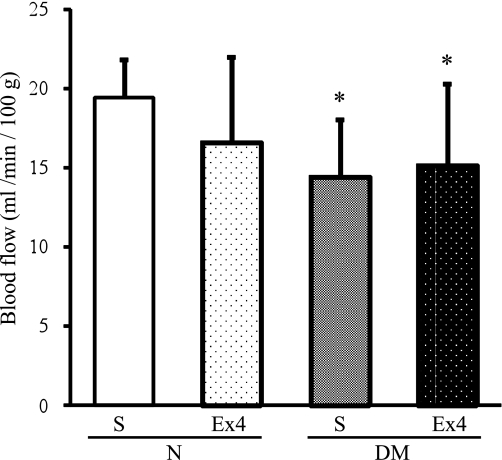
Exendin-4 had no effects on SNBF.
SNBF in diabetic mice was significantly decreased compared with those in normal mice (P = 0.0203), and the decrease was not ameliorated by exendin-4 (P = 0.7407) (Fig. 7).
FIG. 7.
SNBF treated with or without exendin-4 (Ex4) in normal (N) or diabetic (DM) mice. S, saline. Saline-treated normal mice 19.4 ± 2.4, saline-treated diabetic mice 14.4 ± 3.6, and exendin-4–treated diabetic mice 15.2 ± 5.2 mL/min/100 g. Results are means ± SD. *P < 0.05 vs. saline-treated normal mice. Saline-treated normal mice, n = 9; exendin-4–treated normal mice, n = 8; saline-treated diabetic mice, n = 8; exendin-4–treated diabetic mice, n = 9.
DISCUSSION
In this study, we investigated whether GLP-1R agonists have therapeutic effects on DPN. First, we confirmed the expression of the GLP-1R on DRG neurons by immunohistochemical analyses. Second, we observed that both GLP-1 (7–37) and exendin-4 promoted neurite outgrowth of DRG neurons, and exendin-4 ameliorated the impaired neurite outgrowth of DRG neurons in conditioned media obtained from Schwann cell cultures under high-glucose conditions. We then demonstrated that administration of exendin-4 improved the reduced sensory perception of the plantar pedis, delayed NCVs of hindlimbs, and decreased IENFDs of the plantar skin in diabetic mice. However, neither the hyperglycemic state nor decreased SNBF were improved by exendin-4. These results indicate that exendin-4 has direct effects on peripheral nerves that are independent of its antihyperglycemic and hemodynamic effects.
Several antibodies against the GLP-1R are commercially available. GLP-1R antibodies, LS-A1205 and LS-A1206, produced by MBL International, have recently been used in some studies (26–28). To confirm the reliability of these antibodies, we stained islet cells in which the presence of the GLP-1R has repeatedly been demonstrated (29,30). LS-A1205 and LS-A1206 distinctively reacted with β-cells that also were clearly stained with anti-insulin antibody, but these antisera also reacted with β-cells or perhaps α-cells in Glp1r−/− mice. We then tested another antibody for GLP-1R, sc-66911 (Santa Cruz). This antiserum detected β-cells of wild-type mice but did not react with those of glp1r−/− mice (Supplementary Fig. 1). Furthermore, the antiserum detected GLP-1R proteins in the pancreata of wild-type mice but not in that of glp1r−/− mice, using immunoblotting assay. We confirmed the expression of GLP-1R in DRG neurons and glia cells with a GLP-1R antibody, sc-66911.
GLP-1 and exendin-4 previously have been shown to promote neurite outgrowth of rat pheochromocytoma cells (19,31) and to protect rat primary hippocampal neurons from cell death (14). It has been reported that exogenous GLP-1R activation significantly reduces glucose-dependent reactive oxygen species generation in hypothalamus (32). These antioxidative effects of GLP-1R agonists might yield benefits to central and peripheral nervous systems.
The dipeptidyl peptidase (DPP)-IV inhibitor (vildagliptin) recently has been shown to prevent peripheral nerve degeneration in STZ-induced diabetic mice (33). Although GLP-1 is one of the substrates of DPP-IV, several bioactive peptides, such as neuropeptide Y, substance P, glucagon-like peptide-2, and stromal cell–derived factor-1α also have been reported as substrates of DPP-IV (34,35). Among these peptides, neuropeptide Y and substance P are known as neurotransmitters or modulators of peripheral nervous systems (36,37), and it also has been demonstrated that stromal cell–derived factor-1α released from DRG glia regulates leukocyte chemotaxis and modulates neuropathic pain behavior (38). Therefore, the preventive effects of the DPP-IV inhibitor on DPN may be attributed to its protective effects on these neurotrophic peptides and mediated through increased levels of GLP-1.
Although we cannot infer the precise site(s) of action of exendin-4 in our diabetic mice that ameliorated DPN in vivo, our data using cellular models of DPN in vitro implicate a direct role for GLP-1 and the GLP-1R agonist exendin-4. We previously reported that conditioned media obtained from Schwann cell cultures under high-glucose conditions impaired neurite outgrowth of DRG neurons, likely mediated through a decrease in nerve growth factor production (21). In our current study, the impaired neurite outgrowth induced by hyperglycemia-conditioned IMS media was improved by exendin-4. In contrast, under the normal glucose–conditioned IMS media, which mimicked the nondiabetic normal state, exendin-4 did not exert any changes in neurite outgrowth because of basal enhancement of neurite outgrowth by various growth factors, such as nerve growth factor secreted from Schwann cells (21). These results indicate that GLP-1R agonists exert regenerative effects on peripheral sensory neurons under experimental conditions, mimicking diabetes in vitro. Additional studies on the effect on the role of downstream neurotrophic factors, such as nerve growth factor, will be required to evaluate the mechanism of action of GLP-1R agonists.
We also demonstrated the effects of exendin-4 on DPN in STZ-induced diabetic mice. We evaluated sensory nerve functions using a CPT/laboratory neurometer. The neurometer is now widely and clinically used to evaluate the effects of analgesic drugs and peripheral nerve functions in various painful neuropathies, including DPN (39–42). In this study, after 12 weeks of diabetes, hypoalgesia at 2,000, 250, and 5 Hz was observed in the diabetic mice, and injection of exendin-4 improved these abnormalities. In addition, exendin-4 ameliorated the decreased IENFDs in diabetic mice. The restoration of sensory functions by exendin-4 was confirmed by the improvement of IENFDs.
In addition, we measured MNCVs and SNCVs that represent relatively large axonal functions. Both the delayed MNCVs and SNCVs in diabetic mice were improved by exendin-4, indicating that exendin-4 had therapeutic effects on impaired motor and sensory nerve functions. These data also are consistent with previous reports (14,16,18,19) that revealed the plausible effects of GLP-1 or GLP-1R agonists on central and peripheral nerve disorders.
Decreased nerve blood flow has been recognized as one of the most important mechanisms in the development of DPN. Recently, GLP-1R has been detected in blood vessels, and the beneficial effects of GLP-1 or GLP-1R agonists on vascular functions have been reported (28,43,44). In our experimental study, however, exendin-4 did not alter normal nerve blood flow in normal mice nor did it improve the reduced nerve blood flow in diabetic mice. One of the reasons for this discrepancy is that the vasodilatory actions of GLP-1 agonists are likely mediated mainly through a GLP-1R–independent pathway, which depends on a GLP-1 metabolite, GLP-1 (9–36) (28). In our present study, therefore, exendin-4 could not exert vasoregulatory effects. Another reason is that the central-peripheral sympathetic nervous system function would likely be impaired in the diabetic state. It has been reported that GLP-1 in the central nervous system regulates sympathetic outflow, resulting in increases in blood pressure and heart rate independent of the peripheral actions of GLP-1 on glucoregulation (43,44). As mice with a long duration of diabetes in the current study might have developed sympathetic nerve dysfunction, exendin-4 may have been unable to modulate nerve blood flow after prolonged experimental diabetes. Although the precise mechanisms remain unclear, the effects of exendin-4 on DPN may be attributed to its direct actions on DRG neurons and their axons.
Because GLP-1R agonists possess glucose-lowering effects, we used a dosing regimen that did not produce sustained reductions in blood glucose or body weight. Although trends toward reduced levels of HbA1c and glucagon were observed in exendin-4–treated diabetic mice (Table 1 and Supplementary Fig. 4), there were no significant differences in the glucose levels, insulin concentrations, or IPGTTs between exendin-4–treated and untreated mice. In previous studies (45,46), exendin-4 was administrated at the same time as or before STZ injection. On the other hand, we started exendin-4 injections 12 weeks after the onset of diabetes. Because HbA1c in diabetic mice treated with exendin-4 tended to decrease (Table 1), longer treatment or larger doses of exendin-4 might have significantly improved hyperglycemia, indirectly contributing to the amelioration of DPN. In the current study, however, it is conceivable that the effectiveness of exendin-4 on DPN may be independent of the glucose-lowering effects.
Miki et al. (47) previously reported that glucose-responsive neurons in the hypothalamus regulate the secretion of glucagon and govern the glucose homeostasis. In addition, it was shown in a recent study that GLP-1R agonists had a glucagon-lowering effect (48), which is consistent with the present observation that glucagon concentrations tended to be decreased by the administration of exendin-4 in diabetic mice. It remains to be evaluated whether the glucagon levels influence the pathophysiology of DPN through mechanisms independent of hyperglycemia.
In conclusion, we demonstrated the beneficial effects of GLP-1R agonist on DPN. GLP-1 analogs and GLP-1R agonists have been broadly used as antihyperglycemic agents in type 2 diabetic patients. In addition to the established use of these agents to control blood glucose, there currently is little data demonstrating an effect of GLP-1R agonists on peripheral nerve functions. Our data demonstrating the improvement of experimental DPN after administration of GLP-1 and exendin-4 suggest that additional clinical investigation with attention to whether therapy with GLP-1R agonists produce changes in DPN may be warranted.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by a grant-in-aid for scientific research (21592506) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and in part by the “Strategic Research AGU-Platform Formation (2008–2012)” Project for Private Universities: matching fund subsidy from MEXT of Japan.
No potential conflicts of interest relevant to this article were reported.
T.H. researched data. H.K. designed the animal and cell culture studies and contributed to the writing and editing of the manuscript. K.N. contributed to discussion. N.H. was responsible for the maintenance of the animals. N.O. and Yus.S. contributed to discussion. T.S. and M.K. reviewed the manuscript. J.K., T.O., and A.F. researched data. Y.H. contributed to discussion. N.I. was responsible for the maintenance of the animals. Yut.S. and D.J.D. wrote, reviewed, and edited the manuscript. Y.O. contributed to discussion. J.N. wrote, reviewed, and edited the manuscript.
The authors thank Michiko Yamada, Keiko Shimamoto, Naoko Furukawa, Chikako Nagase, and Mayumi Katagiri, from the Department of Endocrinology and Diabetes, Nagoya University Graduate School of Medicine, for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1462/-/DC1.
REFERENCES
- 1.Toth C, Brussee V, Cheng C, Zochodne DW. Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol 2004;63:561–573 [DOI] [PubMed] [Google Scholar]
- 2.Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve 2007;36:144–166 [DOI] [PubMed] [Google Scholar]
- 3.Kles KA, Vinik AI. Pathophysiology and treatment of diabetic peripheral neuropathy: the case for diabetic neurovascular function as an essential component. Curr Diabetes Rev 2006;2:131–145 [DOI] [PubMed] [Google Scholar]
- 4.Genuth S. Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocr Pract 2006;12(Suppl. 1):34–41 [DOI] [PubMed] [Google Scholar]
- 5.Nakae M, Kamiya H, Naruse K, et al. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes 2006;55:1470–1477 [DOI] [PubMed] [Google Scholar]
- 6.Naruse K, Hamada Y, Nakashima E, et al. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005;54:1823–1828 [DOI] [PubMed] [Google Scholar]
- 7.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008;57:3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 9.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269 [DOI] [PubMed] [Google Scholar]
- 10.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316–1322 [DOI] [PubMed] [Google Scholar]
- 11.Dupre J, Behme MT, Hramiak IM, et al. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 1995;44:626–630 [DOI] [PubMed] [Google Scholar]
- 12.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72 [DOI] [PubMed] [Google Scholar]
- 13.Hansotia T, Maida A, Flock G, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 2007;117:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 2003;72:603–612 [DOI] [PubMed] [Google Scholar]
- 15.During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 2003;9:1173–1179 [DOI] [PubMed] [Google Scholar]
- 16.Bertilsson G, Patrone C, Zachrisson O, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 2008;86:326–338 [DOI] [PubMed] [Google Scholar]
- 17.Belsham DD, Fick LJ, Dalvi PS, et al. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J 2009;23:4256–4265 [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA 2009;106:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry T, Lahiri DK, Chen D, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 2002;300:958–966 [DOI] [PubMed] [Google Scholar]
- 20.Perry T, Holloway HW, Weerasuriya A, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol 2007;203:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watabe K, Fukuda T, Tanaka J, Honda H, Toyohara K, Sakai O. Spontaneously immortalized adult mouse Schwann cells secrete autocrine and paracrine growth-promoting activities. J Neurosci Res 1995;41:279–290 [DOI] [PubMed] [Google Scholar]
- 22.Tosaki T, Kamiya H, Yasuda Y, et al. Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp Neurol 2008;213:381–387 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura J, Kato K, Hamada Y, et al. A protein kinase C-β–selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes 1999;48:2090–2095 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem 2008;110:351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010;59:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem 2008;56:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 29.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 1992;89:8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montrose-Rafizadeh C, Avdonin P, Garant MJ, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 1999;140:1132–1140 [DOI] [PubMed] [Google Scholar]
- 31.Kimura R, Okouchi M, Fujioka H, et al. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience 2009;162:1212–1219 [DOI] [PubMed] [Google Scholar]
- 32.Cabou C, Campistron G, Marsollier N, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes 2008;57:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res 2009;40:536–544 [DOI] [PubMed] [Google Scholar]
- 34.Mentlein R. Dipeptidyl-peptidase IV (CD26): role in the inactivation of regulatory peptides. Regul Pept 1999;85:9–24 [DOI] [PubMed] [Google Scholar]
- 35.De Meester I, Durinx C, Bal G, et al. Natural substrates of dipeptidyl peptidase IV. Adv Exp Med Biol 2000;477:67–87 [DOI] [PubMed] [Google Scholar]
- 36.Allen BJ, Rogers SD, Ghilardi JR, et al. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci 1997;17:5921–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci 1994;14:6423–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA 2007;104:20151–20158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katims JJ, Naviasky EH, Ng LK, Rendell M, Bleecker ML. New screening device for assessment of peripheral neuropathy. J Occup Med 1986;28:1219–1221 [PubMed] [Google Scholar]
- 40.Masson EA, Veves A, Fernando D, Boulton AJ. Current perception thresholds: a new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia 1989;32:724–728 [DOI] [PubMed] [Google Scholar]
- 41.Veves A, Young MJ, Manes C, Boulton AJ. Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy: a clinical study. Diabetes Care 1994;17:1200–1202 [DOI] [PubMed] [Google Scholar]
- 42.Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res 2005;33:442–453 [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002;110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barragán JM, Eng J, Rodríguez R, Blázquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol 1999;277:E784–E791 [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003;278:471–478 [DOI] [PubMed] [Google Scholar]
- 46.Maida A, Hansotia T, Longuet C, Seino Y, Drucker DJ. Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology 2009;137:2146–2157 [DOI] [PubMed] [Google Scholar]
- 47.Miki T, Liss B, Minami K, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 2001;4:507–512 [DOI] [PubMed] [Google Scholar]
- 48.Burcelin R, Knauf C, Cani PD. Pancreatic alpha-cell dysfunction in diabetes. Diabetes Metab 2008;34(Suppl. 2):S49–S55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.