Abstract
Pemphigus foliaceus (PF) is an autoimmune skin blistering disease mediated by pathogenic autoantibodies against the desmosomal core glycoprotein desmoglein 1 (Dsg1). This study demonstrated that the O-glycan-specific plant lectin jacalin binds Dsg1 and inhibits the interaction of Dsg1/PF IgG. N-glycosylation is not involved in the interaction of Dsg1/jacalin or Dsg1/PF IgG. Subcutaneous injection of jacalin into neonatal mice drastically reduced PF IgG deposition at the epidermal cell surface and blocked PF IgG-induced skin blisters, both clinically and histologically. Interestingly, another plant lectin peanut agglutinin (PNA), which shares the same carbohydrate specificity toward the O-linked carbohydrate structure known as Thomsen-Friedenreich antigen (TF antigen, Galβ1-3GalNAcα-O-Ser/Thr), also bound Dsg1 and blocked the skin blistering. In contrast, the plant lectin vicia villosa-B4 (VVL-B4), which shares the carbohydrate specificity toward the O-linked monosaccharide known as Thomsennouveau antigen (Tn antigen, GalNAc-α1-O-Ser/Thr) did not bind Dsg1 and did not show a protective effect against the disease induced by the autoantibodies. Collectively, these results suggest that the binding of jacalin to O-linked TF carbohydrate motifs on Dsg1 impairs the Dsg1/PF autoantibody interactions and abrogates its pathogenicity in vivo. TF-specific binding ligands may have a potential therapeutic value for PF.
Keywords: skin, autoantibody, desmoglein, pemphigus, lectin
Introduction
The endemic and nonendemic forms of pemphigus foliaceus (PF) are autoimmune blistering skin diseases characterized by superficial blisters, epidermal cell detachment (known as acantholysis) at the level of the granular layer of the epidermis, and IgG autoantibodies bound to the surface of detached keratinocytes (Beutner et al., 1968; Lever et al., 1953). The nonendemic form of PF occurs sporadically in many regions of the world and the endemic form, also known as Fogo Selvagem (FS), is common in certain rural areas of Brazil (Aoki et al., 2004). (Aoki et al., 2004).
The anti-epidermal autoantibodies in both clinical forms of PF are IgG4 subclass predominant and pathogenic as demonstrated by passive transfer of the IgG fraction of these sera into mice (Futei et al., 2001; Rock et al., 1989). Neonatal mice that are injected with PF IgG, IgG4, or Fab’ monovalent fragment develop skin blisters and subcorneal vesicles, which recapitulate the clinical and histological features of the human disease in these animals (Espana et al., 1997; Rock et al., 1990; Rock et al., 1989; Roscoe et al., 1985). The antigen targeted by pathogenic PF autoantibodies is desmoglein-1 (Dsg1), a desmosomal core glycoprotein expressed predominantly in the upper layers of the epidermis (Koulu et al., 1984; Rappersberger et al., 1992). Affinity-purified anti-Dsg1 autoantibodies from PF sera are able to induce epidermal blisters and subcorneal acantholysis in mice passively transferred with these fractions (Amagai et al., 1995a; Arteaga et al., 2002) .
In spite of the remarkable advances in understanding the molecular mechanisms of autoantibody-induced acantholysis in pemphigus (Culton et al., 2008; Kitajima and Aoyama, 2007; Rubenstein and Diaz, 2006; Sharma et al., 2007; Waschke, 2008), these knowledge has not been translated into newer therapies of patients. Current therapy of PF patients mainly relies on the use of corticosteroids and immunosuppressive agents. Although these therapies significantly reduce the mortality of the disease, they result in significant morbidity due to side effects.
Identifying means of interfering with the autoantibody-antigen interaction may represent an alternative approach to treat autoantibody-mediated autoimmune diseases. While investigating a possible case of IgA pemphigus, we noticed that jacalin-agarose, which was used to precipitate IgA, also precipitated the baculovirus expressed recombinant Dsg1 ectodomain. Further studies demonstrate that binding of jacalin interferes with PF autoantibody binding and protects mice against PF IgG-induced skin blisters.
Results
Jacalin binds Dsg1
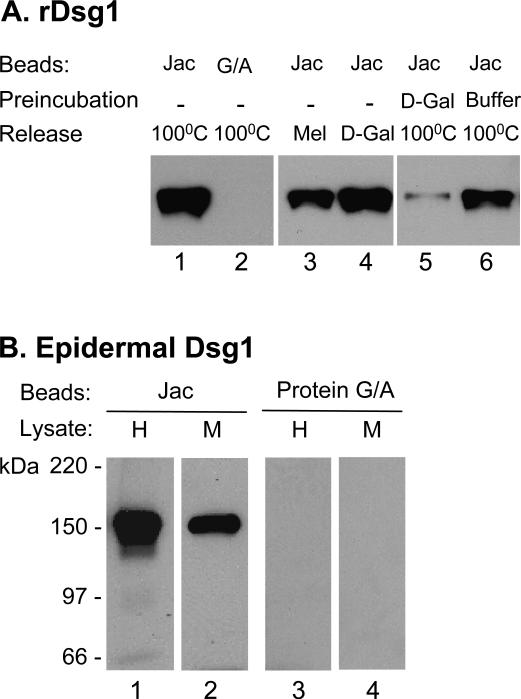
Aliquots of conditioned culture medium containing baculovirus-expressed human recombinant Dsg1 ectodomain (rDsg1) were incubated with agarose beads that were conjugated with jacalin or protein G/A (negative control). Following extensive wash (see Methods); bound proteins were released by boiling in SDS sample buffer and subjected to IB. As shown in Figure 1A, jacalin-agarose (lane 1), but not protein G/A-agarose (lane 2), was able to pull-down Dsg1. To demonstrate the carbohydrate-dependency of the binding, we tested the ability of jacalin-specific sugars to elute the bound Dsg1. We found that both melibiose (0.1 M) and D-galactose (0.8 M) were able to elute the bound Dsg1 (lanes 3 and 4). Moreover, preincubation of jacalin-agarose with D-galactose diminished its binding to Dsg1 (lane 5) as compared to those preincubated with TBS-Ca2+ buffer alone (lane 6). These results demonstrate that jacalin is able to bind the recombinant Dsg1 ectodomain and that the binding is galactose-dependent.
Figure 1. Jacalin binds Dsg1.
(A) Binding to rDsg1. rDsg1 was incubated with jacalin-agarose (lanes 1, 3-6) or protein G/A-agarose beads (lane 2). Bound-Dsg1 was released by boiling in SDS sample buffer (lanes 1-2 and 5-6) or eluted with jacalin-inhibiting sugars (lane 3: 0.1 M melibiose; lane 4: 0.8 M D-galactose). In one set of experiment, jacalin beads were preincubated with 0.8 M D-galactose (lane 5) or buffer (lane 6) before adding the PF serum to further show the sugar-dependency binding. Released Dsg1 was analyzed by IB using anti-His antibodies. (B) Binding to epidermal Dsg1. Tissue lysates from human (H) or mouse (M) epidermis were incubated with jacalin-agarose (lanes 1 and 2) or protein G/A-agarose beads (lanes 3 and 4) followed by IB using anti-Dsg1 antibodies.
Considering possible differences in glycosylation of proteins expressed by insect cells and mammalian epidermal keratinocytes, we examined the interaction of jacalin with Dsg1 extracted from the skin. Tissue lysates from human epidermis and mouse skin were incubated with jacalin-agarose or protein G/A agarose beads. Proteins bound by the beads were released and analyzed by IB using antibodies to Dsg1. As shown in Figure 1B, the 160-kDa Dsg1 from mouse or human epidermal extracts was precipitated by jacalin-beads (lanes 1 and 2), but not by protein G/A-beads (lanes 3 and 4).
Jacalin blocks the binding of PF autoantibodies to Dsg1
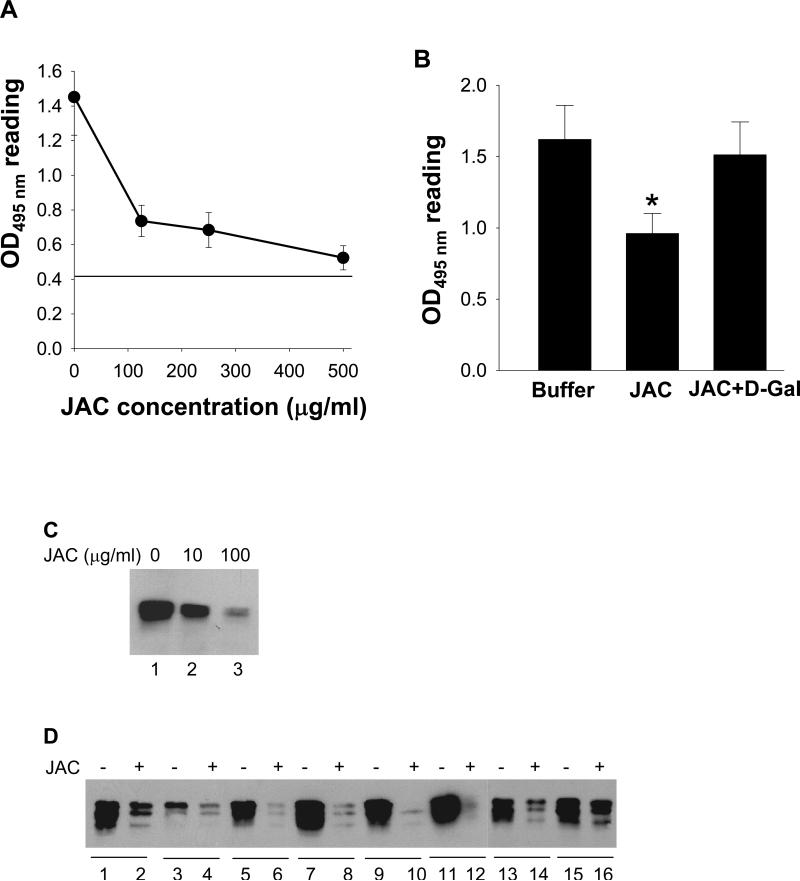
Finding that jacalin interacts with the ectodomain of Dsg1 prompted us to investigate whether such interaction could interfere with the binding of PF autoantibodies to Dsg1. We tested this possibility by ELISA and IP assays using a test FS serum (PF-1). ELISA results showed a concentration-dependent inhibition of jacalin on the binding of FS autoantibodies (Figure 2A), and that the inhibition was reversible by D-galactose (Figure 2B). A similar dose-dependent blocking effect on the binding of Dsg1 to FS autoantibodies was also observed by IP analysis (Figure 2C). To assess whether the jacalin inhibition on FS autoantibody binding is applicable to other FS/PF sera, we tested additional samples. Representative IP data is shown in Figure 2D. Among 20 PF/FS sera tested, jacalin significantly reduced Dsg1 binding in 18 samples (90%), and in 2 sera the reduction was modest (one of such sera, PF-2, is shown in lanes 15 and 16).
Figure 2. Jacalin inhibits PF autoantibody binding to Dsg1.
(A) ELISA assay showing dose-dependent inhibitory effect of jacalin. Dsg1-coated microplates were preincubated with jacalin (100, 250, and 500 μg/ml) followed by incubating with PF-1 serum. Cut-off value: 0.421. (B) Reverse effects of D-galactose on jacalin inhibition. Dsg1-microplates were preincubated with TBS-Ca2+ buffer (left column), jacalin (100 μg/ml) (center), or jacalin (100 μg/ml) plus D-galactose (0.8 M) (right) before adding PF-1 serum. [n=3, *p<0.05 (Student's t-test)]. (C) IP results showing dose-dependent inhibitory effect of jacalin. rDsg1 was preincubated with jacalin (0, 10 and 100 μg/ml) before IP. (D) Representative IP showing that 100 μg/ml of jacalin (even-numbered lanes) inhibits the interaction of rDsg1 with 8 PF/FS sera compared with the controls that were preincubated with buffer (odd-numbered lanes).
Interaction of Dsg1/jacalin and Dsg1/PF autoantibodies is N-glycosylation-independent
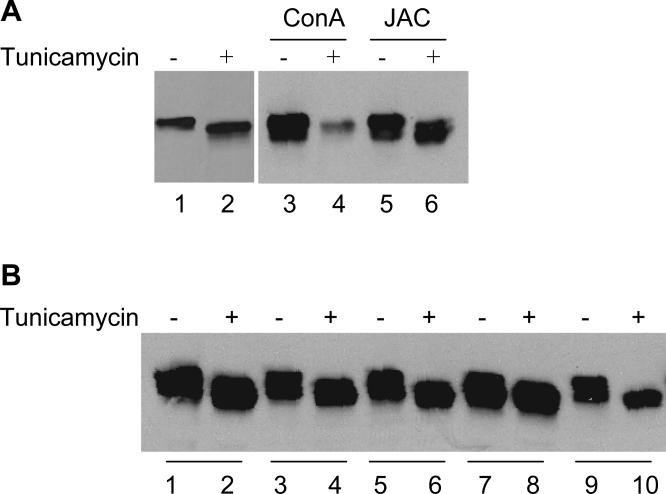
Although jacalin is a well-known O-glycan-specific plant lectin (Hortin, 1990; Tachibana et al., 2006), a few studies have suggested that jacalin may also have affinity for N-glycan (Bourne et al., 2002; Do and Lee, 1998). To evaluate whether jacalin binds to N-glycans on Dsg1, we produced the Dsg1 in High-Five cells in the presence of tunicamycin to inhibit N-glycosylations. As expected, Dsg1 expressed in the presence of tunicamycin showed a reduced molecular weight on SDS-PAGE (Figure 3A, lane 1 vs. lane 2), an indication of N-glycosylation inhibition. We then used the same amount of tunicamycin-treated and nontreated-Dsg1 for lectin pull-down assay. As expected, immobilized concanavalin A (ConA) reacted poorly with tunicamycin-treated-Dsg1 (Figure 3A, lanes 3 vs. 4), a further indication of the N-glycosylation inhibition. In contrast, immobilized jacalin was able to precipitate both the tunicamycin-treated and nontreated-Dsg1 (Figure 3A, lanes 5 vs. 6). This result demonstrates that N-glycans on Dsg1 are not involved in jacalin binding.
Figure 3. N-glycosylation is not involved in Dsg1/jacalin and Dsg1/PF IgG binding.
Baculovirus expressed Dsg1 ectodomain produced in the presence or absence of tunicamycin (0.5 mg/ml) was used for the experiments. (A) IB shows that tunicamycin treatment reduced the molecular weight of Dsg1 (untreated in lane 1 vs treated in lane 2). ConA-beads precipitated poorly with Dsg1 produced in the presence of tunicamycin (lanes 3) compared with nontreated-Dsg1 (lane 4). In contrast, jacalin-agarose precipitated both the tunicamycin-treated (lane 6) and nontreated-Dsg1 (lane 5). (B) Representative IP shows that 5 PF/FS sera reacted with tunicamycin-treated Dsg1 (even-numbered lanes) as well as nontreated-Dsg1 (odd-numbered lanes).
We also examined the binding ability of tunicamycin-treated Dsg1 with PF autoantibodies since the role of N-glycosylation of Dsg1 in PF autoantibody binding has been controversial (Amagai et al., 1995b; Olague-Alcala M., 1993; Ortiz-Urda et al., 2003). We tested 12 PF/FS sera (6 PF and 6 FS) by IP, and all reacted well with both tunicamycin-treated and nontreated-Dsg1. Representative IP data are shown in Figure 3B.
Jacalin binds the epidermis and inhibits PF autoantibodies binding
We next examined the binding of jacalin to cryostat sections of human and murine skin by IF techniques. Sections of human or mouse skin incubated with FITC-conjugated jacalin produced a “pemphigus-like” staining pattern (Figure S1-A). Preincubation of the human or mouse skin sections with jacalin significantly inhibited PF IgG binding to the epidermis (Figure S1-B, panels b and d) as compared to those preincubated with TBS-Ca2+ buffer alone (panels a and c).
Jacalin abrogates the pathogenicity of PF autoantibodies in mice
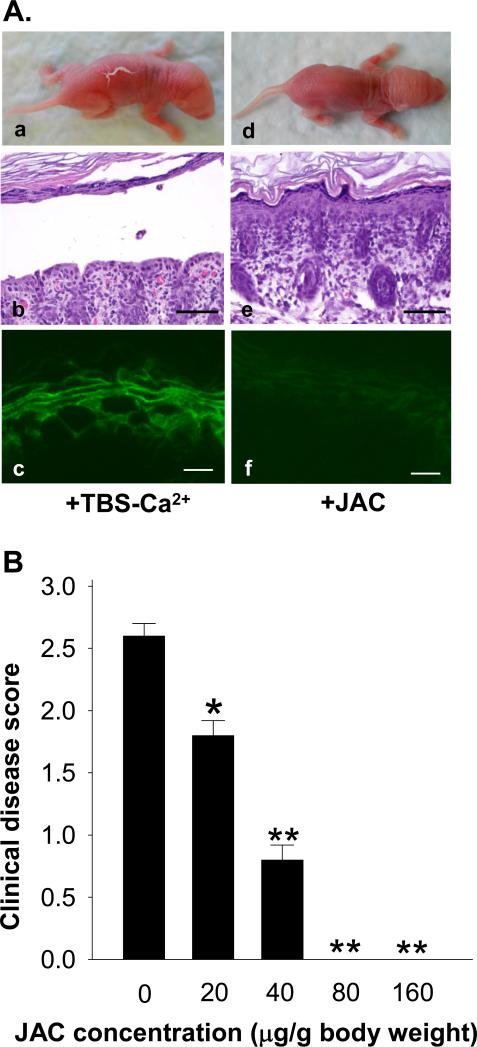
We further investigated the in vivo effect of jacalin in the PF mouse model using pathogenic IgG isolated from PF-1, a serum that was significantly inhibited by jacalin (see Figures 2A-C). Control mice (n=4) pretreated with vehicle alone developed blisters, clinically and histologically (Figure 4A, panels a and b) 20 hrs post PF IgG injections. In contrast, mice pretreated with jacalin (80 μg/g b.w., n=4; 160 μg/g b.w., n=4) did not develop skin lesions (panels d and e). Direct IF staining revealed a significant reduction of IgG binding to the epiderms in jacalin-treated mice (panel f) compared to those of control mice (panel c). The inhibition of jacalin on the induction of skin lesions in mice was dose-dependent (Figure 4B). These results suggest that jacalin affects the in vivo binding of PF autoantibodies to the epidermis and thus inhibits disease.
Figure 4. Jacalin protects mice from developing PF.
(A) Representative results showing that mice preinjected with control buffer (n=4) developed clinical and histological blisters upon injection with IgG from PF-1 (a, b). In contrast, mice pretreated with jacalin (80 ug/g b. w., n=4; 160 ug/g b. w., n=4) did not develop skin blisters clinically and histologically (d, e). Direct IF staining showed a significant reduction of IgG binding to the epidermis in mice treated with jacalin (f) compared to buffer-treated controls (c). Scales bars = 100 μm (b, e) and 10 μm (c, f), respectively. (B) Jacalin inhibits PF blistering in a dose-dependent fashion. Animals were pretreated (s.c) with different doses of jacalin and then injected (s.c.) with the same dose of pathogenic PF IgG. Clinical disease was scored for each group of experimental mice. [n=4; *p<0.05, **p<0.01 (Student's t-test)].
Study of other plant lectins
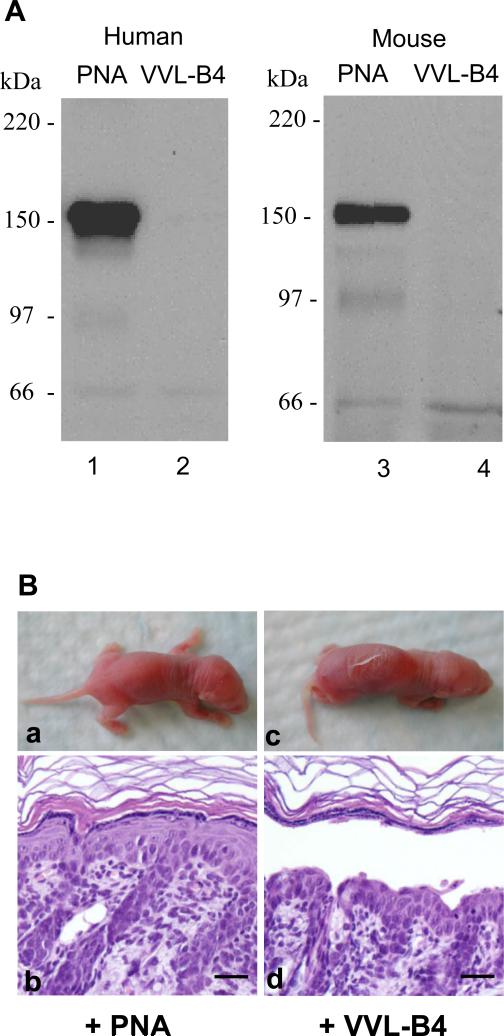
Jacalin is known to have binding specificities for the O-linked disaccharide Thomsen-Friedenreich antigen (TF antigen, Galβ1-3GalNAc-α1-OSer/Thr) and the monosaccharide Thomsen-nouveau antigen (Tn antigen, GalNAc-α1-OSer/Thr) in either the asialylated or sialylated form. It is known that other lectins have overlapping and distinct binding specificities as jacalin. For example, the peanut agglutinin (PNA) has binding affinity for TF antigen in its asialylated form only, whereas vicia villosa-B4 (VVL-B4) binds specifically to Tn antigen. Therefore, we tested the reactivity of these two lectins with Dsg1 by the lectin pull-down assay. The results showed that PNA, but not VVL-B4, interacted with the epidermal Dsg1 extracted from human or mouse skin (Figure 5A), suggesting that Dsg1 carries the asialylated form of TF antigen but not the Tn antigen. Moreover, preinjection of PNA (80 μg/g b.w., n=9) abrogated the pathogenicity of PF IgG, while preinjection the same dose of VVL-B4 (n=3) did not show any blocking effect (Figure 5B). Like jacalin, subcutaneous injection of PNA or VVL-B4 alone did not cause any abnormal appearance of the mouse skin. Table 1 summarizes the binding specificity of the lectins tested and their effect on PF IgG-induced skin blisters in mice.
Figure 5. PNA, but not VVL-B4, binds to Dsg1 and abolishes the pathogenicity of PF autoantibodies.
(A) Lectin pull-down assay. Tissue lysates from human epidermis (lanes 1 and 2) or mouse skin (lanes 3 and 4) were incubated with PNA-agarose (lanes 1 and 3) or VVL-B4-agarose beads (lanes 2 and 4). Dsg1 was detected by IB using anti-Dsg1 antibodies. PNA but not VVL-B4 pull-down the epidermal Dsg1. (B) IgG passive transfer. Neonatal mice pretreated with PNA (160 μg/g b.w. n=9) or VVL-B4 (160 μg/g b.w., n=3), followed by s.c. injection of pathogenic IgG from PF-1. Injected mice were examined 20 h post IgG injection. PNA (panels a and b), but not VVL-B4 (panels c and d), inhibits PF IgG-induced skin blisters in mice. Scale bar = 50 μm
Table 1.
In vivo effect of plant lectins in IgG passive transfer mouse model of PF
| IgG | Treatment | Oligosaccharide specificity | # mice tested | # mice with skin blister | # mice without skin blister |
|---|---|---|---|---|---|
| PF-1 | Vehicle | 15 | 15 | 0 | |
| Jacalin | Galβ1-3GalNAc-α (asialo or sialo) | 8 | 0 | 8 | |
| PNA | GalNAc Galβ1-3GalNAc-α or -β (asialo) | 9 | 1* | 8 | |
| VVL-B4 | GalNAc | 3 | 3 | 0 | |
| PF-2 | Vehicle | 6 | 6 | 0 | |
| Jacalin | 6 | 5** | 1 |
Neonatal mice were preinjected (s.c.) with vehicle (TBS-Ca2+) or lectins for 2 h followed by pathogenic PF IgG injection (s.c.). Pathogenic IgG from two patients’ sera was used. PF-1 serum showed a remarkable inhibition in Dsgl binding by jacalin, whereas PF-2 showed a marginal reduction in Dsgl binding by jacalin. Animals were evaluated 20 h post PF IgG injection. Jacalin: 80 to 160 μg/g b.w.; PNA and VVL-B4: 160 μg/g b.w. Abbreviations: Gal, galactose; GalNAc, N-acetylglactosamine; Glc, glucose; PNA, peanut agglutinin; VVL-B4, vicia villosa-B4 lectin.
Minimum disease score (0.5+).
Overall less disease score (1.25 + 0.36) compared to those preinjected with vehicle (2.58 + 0.20) (p<0.01, Students’ t-test).
Effect of jacalin on the pathogenicity of an additional PF serum that showed a modest reduction in Dsg1 binding by jacalin
As described above, two patients’ sera, including PF-2, showed a modest reduction on the binding of Dsg1 by IP when preincubated with jacalin. We expected that jacalin would have a lesser inhibitory effect on the pathogenicity of these PF autoantibodies. We tested this hypothesis in mice using pathogenic IgG prepared from PF-2 serum. Out of six animals preinjected with jacalin, five developed skin blisters, but the disease score was significantly reduced (p < 0.01, Student's t-test) compared to those pretreated with vehicle (Table 1).
Discussion
Jacalin is a plant lectin that is highly specific for the α-O-glycoside of the terminal disaccharide Thomsen-Friedenreich (TF) carbohydrate motif (asialylated or sialylated) and its monosaccharide precursor Tn antigen (Kabir, 1998; Mahanta et al., 1990; Tachibana et al., 2006). Immobilized jacalin is often used for isolation of IgA1 from human serum and purifying other O-glycosylated proteins (Hortin, 1990; Roque-Barreira and Campos-Neto, 1985). In this study, we found that jacalin-agarose beads precipitated the recombinant Dsg1 ectodomain (Figure 1A) as well as the native Dsg1 from human and mouse epidermal lysates (Figure 1B). We demonstrated that the binding between jacalin and Dsg1 is galactose-dependent ((Figure 1A), but N-glycosylation-independent (Figure 3A), supporting the notion that jacalin has no or extremely low affinity for N-glycans (Arockia Jeyaprakash et al., 2005; Hortin and Trimpe, 1990). While Dsg1 is known to be N-glycosylated (Amagai et al., 1995b; Koch et al., 1991; Olague-Alcala M., 1993; Ortiz-Urda et al., 2003), its possible modification by O-linked oligosaccharides is less defined. The observation that Dsg1 interacts with jacalin and PNA but not VVL-B4 (Figure 5A) indicates that Dsg1 carries O-linked disaccharide TF antigen (asialylated) but not the monosaccharide Tn antigen.
By competition ELISA and IP, we showed that jacalin was able to inhibit the binding of PF autoantibodies to Dsg1 in concentration- and galactose-dependent manners (Figure 2A-C). A significant inhibitory effect was observed in the majority of patients’ sera tested (90%, n=20, Figure 2D) including PF-1, which was used throughout the study. Jacalin also markedly inhibited the binding of PF autoantibodies to human and mouse epidermis (Figure S1). Furthermore, preinjection (s.c.) of jacalin into mice diminished the in vivo binding of PF-1 autoantibody to the epidermis and blocked skin disease (Figure 4). Interestingly, injection (s.c.) of jacalin alone did not cause any abnormal effect on mouse skin (data not shown), suggesting that binding of jacalin does not impair the adhesive function of Dsg1. Another lectin PNA, which shares the same carbohydrate specificity for the O-linked asialylated TF antigen but not the sialylated form, interacted with Dsg1 and blocked PF-1 autoantibody-induced skin blisters in mice (Figure 5). In contrast, lectin VVL-B4, which shares the carbohydrate specificity for the O-linked Tn antigen, did not interact with Dsg1 and did not show any beneficial effect on the disease induced by PF-1 IgG (Figure 5). These data suggest that binding of the O-linked TF carbohydrate motif of Dsg1 by jacalin and PNA can abrogate the pathogenicity of PF autoantibodies. However, there are a few PF sera (2 out of 20) where the Dsg1/PF autoantibody interaction was modestly inhibited by jacalin, and the in vivo protective effect of jacalin on disease was less effective as demonstrated with PF-2 serum (Table 1).
The molecular mechanism involved in the inhibitory effects of jacalin on PF autoantibody binding to Dsg1 is not clear. It is known that pathogenic PF autoantibodies bind calcium-dependent conformational epitopes located in the NH2-terminal region (EC1-2 or 1-161 residues) of Dsg1 (Eyre and Stanley, 1987; Kowalczyk et al., 1995; Li et al., 2003; Sekiguchi et al., 2001). These epitopes are unlikely to be composed of the carbohydrate moieties since bacterial expressed Dsg1 ectodomain, which had no glycosylation modifications, was still recognized by PF sera (Dmochowski et al., 1994). It is possible that jacalin binds the TF carbohydrate motifs of Dsg1 and spatially masks the major epitopes recognized by PF autoantibodies, thereby inhibiting the subsequent binding of PF autoantibodies. Alternatively, the binding of jacalin to Dsg1 may induce conformational changes of Dsg1, which impairs its interaction with PF autoantibodies. Identification of the position of the O-glycosylation sites on Dsg1 may help to understand the mechanism of action of jacalin. Potential O-glycosylation sites are predicted to be located in the membrane-proximal EC5 domain and possibly in other domains (EC1-4), depending on which predicting programs/methods are used (http://turing.cs.iastate.edu/EnsembleGly; http://www.cbs.dtu.dk/services/netoglyc; http://comp.chem.nottingham.ac.uk/glyco). Further experimental determination of the O-glycosylation sites on Dsg1 may shed light into the inhibitory mechanism.
Two putative N-glycosylation sites (residues 61 and 131) are identified within the NH2-terminal region of Dsg1 where PF autoantibodies bind to. The influence of the N-lined glycosylation on PF anutantibody binding has been examined with conflicting results reported. While earlier studies showed that the binding of PF autoantibodies to Dsg1 is N-glycosylation-independent (Amagai et al., 1995b; Olague-Alcala and Diaz, 1993), a recent study by Ortiz-Urda et al. (Ortiz-Urda et al., 2003) suggested that most PF epitopes are determined by N-linked carbohydrates. Ortiz-Urda et al. further showed that topically applied lectin wheat germ agglutinin (WGA), which binds the N-linked carbohydrates, was able to block most PF autoantibody binding to the epidermis and abrogated their pathogenicity in mice (Ortiz-Urda et al., 2003).
In contrast to the results by Ortiz-Urda et al. (Ortiz-Urda et al., 2003), our IP data show that inhibition of N-glycosylation of Dsg1 by tunicamycin does not attenuate their binding to PF/FS sera (n=12) (Figure 3B). Our results support the notion that binding of PF IgG to Dsg1 is N-glycosylation-independent (Amagai et al., 1995b; Olague-Alcala and Diaz, 1993). It should be noted that the conclusion of N-glycosylation-dependent PF autoantibody binding was mainly based on the observation that, by immunoblotting (IB), the majority of PF sera (7 out of 12 sera tested) failed to react with human epidermal Dsg1 that has been digested with PNGase F (Ortiz-Urda et al., 2003). However, it is known that IB analysis is not an optimal technique to reveal the binding of PF autoantibodies to Dsg1 because more than half of PF sera failed to react with Dsg1 by this technique, presumably due to the loss of the conformational epitopes under the denatured conditions inherent to IB (Amagai et al., 1995a; Eyre and Stanley, 1987; Hashimoto et al., 1990; Kowalczyk et al., 1995). It is probable that the failure of recognizing Dsg1 by most of the PF sera in the study of Ortiz-Urda et al. (Ortiz-Urda et al., 2003) was due to the limited sensitivity of the IB analysis rather than the removal of N-linked carbohydrates. Furthermore, we could not confirm that WGA was capable of blocking PF IgG-induced skin blistering in mice. Instead of a beneficial effect, we found that WGA itself induces intraepidermal cell detachment (acantholysis-like) and skin blisters upon injection into mice (unpublished observation). The reason for this conflicting observation is not clear and requires further investigation.
The TF disaccharide (alpha-anomer, asialylated), recognized both by jacalin and PNA, is a tumor-associated carbohydrate structure. This glycotope occurs on the cell surface of a few normal epithelial cells; but is overexpressed on many types of carcinomas as a result of incomplete O-glycosylation of cell membrane proteins (Cao et al., 1996; Springer, 1984). Its cryptic form (sialylated TF), which interacts with jacalin but not PNA, is expressed on many normal tissues/organs including blood cells (Cao et al., 1996; Kabir, 1998). The beta-anomer of TF (Galβ1-3GalNAc-β), found in the termini of certain glycolipids such as GM1 ganglioside and asialo-GM1 ganglioside (Rittenhouse-Olson, 2007) may interact with PNA but not jacalin. Although jacalin and PNA are dietary lectins that can be eaten raw, and local administration of the two lectins into neonatal mice did not induce any obvious toxicity within 24 hour duration, it is possible that systemic administration of these lectins, especially jacalin, may be toxic/harmful due to its agglutinating properties of erythrocytes and its mitogenicity for human CD4+ T cells (Cao et al., 1996; Kabir, 1998). Our findings, however, may lead to a new therapeutic strategy for PF, i.e, targeting the TF glycotope with other more specific TF-binding ligands, such as anti-TF antibodies (Heimburg et al., 2006) or peptides (Landon et al., 2004). These agents react only with the asialylated alpha-anomer TF disaccharide, but not with its more common cryptic form (sialylated TF) that are expressed on cell surface of many normal cells including erythrocytes. Further studies are required to test the potential therapeutic value of the TF-specific agents for PF,
Materials and Methods
Patient sera
Sera from 20 PF/FS patients (11 PF sera from the U.S.A. and 9 FS sera from Brazil) were included in this study. A well-characterized FS serum (PF-1), which was available in a relatively large quantity, was used throughout this study. A second PF serum (PF-2) that showed a modest reduction in Dsg1 binding by jacalin was also used for IgG passive transfer experiments (Table 1). The diagnosis of PF/FS had previously been confirmed using established clinical, histological, and serological criteria. This study was approved by the Institutional Review Board at UNC.
Plant lectins
Jacalin (Artocarpus integrifolia agglutinin), peanut agglutinin (PNA), vicia villosa isolectin B4 (VVL-B4), concanavalin A (ConA), PNA-agarose, and VVL-B4-agarose were purchased from Sigma (St. Louis, MO). Agarose-bound jacalin was from ICN Pharmaceuticals (Irvine, CA). ConA-sepharose was from Amersham Phamacia Biotech. Fluorescein-isothiocyanate (FITC)-conjugated jacalin was purchased from Vector Laboratories Inc.
Production of recombinant Dsg1
The ectodomain of human Dsg1 with a carboxyl-terminal histidine (His) tag was produced in a baculovirus expression system as described previously (Li et al., 2003). To produce Dsg1 without N-glycosylation, we expressed Dsg1 in High-Five cells in the presence of tunicamycin (0.5 μg/ml) as described by Amagai et al. (Amagai et al., 1995b). For ELISA, Dsg1 was purified by nickel affinity chromatography as described previously (Ding et al., 1999).
Lectin pull-down assay
Conditioned culture medium containing rDsg1 or skin/epidermal lysates were incubated with immobilized lectins for 1.5 hrs at 4 °C. Negative control experiments were carried out using agarose beads conjugated with protein G/A. After centrifugation, the beads were washed three times for 10 min, each with either of the following buffers: a) 0.1% triton X-100 in TBS-Ca2+ (10 mM TrisHCl, pH 7.4, 140 mM NaCl, and 5 mM CaCl2), b) RIPA buffer (1% NP40, 0.25% sodium deoxycholate, 0.1% SDS, 50 mM TrisHCl, pH 7.4, and 150 mM NaCl), or c) high ionic buffer (100 mM TrisHCl, pH 7.4 and 500 mM NaCl). Proteins bound to the beads were released by boiling in 2x SDS sample buffer or eluted with 0.8 M D-galactose or 0.1 M melibiose. The eluates were fractioned by SDS-PAGE and then subjected to immunoblotting (IB) using anti-His or anti-Dsg1 antibodies (Emery et al., 1995).
Enzyme-linked immunosorbent assay (ELISA)
Dsg1-ELISA was performed as previously reported (Warren et al., 2000) with the following modifications. Various amounts of jacalin in TBS-Ca2+ buffer containing 0.2%BSA and 0.05% Tween 20 were added in duplicate to the wells. In some experiments, jacalin was preincubated with 0.8 M D-galactose prior to adding to the plates. Following a 1-hour incubation at R.T, the plate was washed five times with TBS-Ca2+ buffer (pH 7.2) containing 0.05% Tween 20. The plate was then subjected to ELISA assay using PF serum samples (1:200).
Immunoprecipitation (IP)
Cold IP was performed as previously described (Li et al., 2003). In brief, aliquots of conditioned culture supernatant containing the His-tagged rDsg1 were preincubated with buffer or jacalin and then incubated with PF serum (1:200). The mixture was then subjected to IP, followed by IB using HRP-conjugated anti-His antibodies.
Induction of experimental PF and testing the effect of plant lectins
Neonatal BALB/c mice (1-2 days old) were used for experimental PF induction (Anhalt et al., 1982; Rock et al., 1989). Animal care/experiments were approved by the Animal Care Committee at the institute and were in accordance with NIH guidelines. To test the effect of lectins, animals were subcutaneously (s.c.) injected with lectins or vehicle (TBS-Ca2+ buffer); two hours later animals were injected (s.c.) with pathogenic PF IgG (0.4 mg/g b.w.). Twenty hours post IgG injection, the extent of skin disease was evaluated and scored on a scale of 0 to 3+ as described previously (Li et al., 2005).
Statistical analysis
Data were presented as mean ± standard deviation (SD). The Student's t test (unpaired, two-tailed) was used to evaluate for statistical significance. P < 0.05 was considered significant.
Acknowledgements
This work was supported in part by US Public Health Service National Institutes of Health (NIH) Grants AR053313, AR052109 awarded to N. Li., AI40768 and AI61430 to Z. Liu, and AR30281, AR32599 awarded to L.A. Diaz,
Abbreviations
- ConA
concanavalin A
- Dsg
desmoglein
- Jac
jacalin
- PF
pemphigus foliaceus
- PNA
peanut agglutinin
- s.c.
subcutaneous
- TF antigen
Thomsen-Friedenreich antigen (Galβ1-3GalNAcα-O-Ser/Thr)
- Tn antigen
Thomsen-nouveau antigen (GalNAc-α1-O-Ser/Thr)
- VVL-B4
vicia villosa-B4 lectin
Footnotes
Conflict of Interest: The authors state no conflict of interest.
Reference
- Amagai M, Hashimoto T, Green KJ, Shimizu N, Nishikawa T. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995a;104:895–901. doi: 10.1111/1523-1747.ep12606168. [DOI] [PubMed] [Google Scholar]
- Amagai M, Ishii K, Hashimoto T, Gamou S, Shimizu N, Nishikawa T. Conformational epitopes of pemphigus antigens (Dsg1 and Dsg3) are calcium dependent and glycosylation independent. J Invest Dermatol. 1995b;105:243–247. doi: 10.1111/1523-1747.ep12317587. [DOI] [PubMed] [Google Scholar]
- Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren SJ, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem). J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- Arockia Jeyaprakash A, Jayashree G, Mahanta SK, Swaminathan CP, Sekar K, Surolia A, et al. Structural basis for the energetics of jacalin-sugar interactions: promiscuity versus specificity. J Mol Biol. 2005;347:181–188. doi: 10.1016/j.jmb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Arteaga LA, Prisayanh PS, Warren SJ, Liu Z, Diaz LA, Lin MS. A subset of pemphigus foliaceus patients exhibits pathogenic autoantibodies against both desmoglein-1 and desmoglein-3. J Invest Dermatol. 2002;118:806–811. doi: 10.1046/j.1523-1747.2002.01743.x. [DOI] [PubMed] [Google Scholar]
- Beutner EH, Prigenzi LS, Hale W, Leme Cde A, Bier OG. Immunofluorescent studies of autoantibodies to intercellular areas of epithelia in Brazilian pemphigus foliaceus. Proc Soc Exp Biol Med. 1968;127:81–86. doi: 10.3181/00379727-127-32626. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Astoul CH, Zamboni V, Peumans WJ, Menu-Bouaouiche L, Van Damme EJ, et al. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem J. 2002;364:173–180. doi: 10.1042/bj3640173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- Culton DA, Qian Y, Li N, Rubenstein D, Aoki V, Filhio GH, et al. Advances in pemphigus and its endemic pemphigus foliaceus (Fogo Selvagem) phenotype: a paradigm of human autoimmunity. J Autoimmun. 2008;31:311–324. doi: 10.1016/j.jaut.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Dmochowski M, Hashimoto T, Amagai M, Kudoh J, Shimizu N, Koch PJ, et al. The extracellular aminoterminal domain of bovine desmoglein 1 (Dsg1) is recognized only by certain pemphigus foliaceus sera, whereas its intracellular domain is recognized by both pemphigus vulgaris and pemphigus foliaceus sera. J Invest Dermatol. 1994;103:173–177. doi: 10.1111/1523-1747.ep12392664. [DOI] [PubMed] [Google Scholar]
- Do SI, Lee KY. Jacalin interacts with Asn-linked glycopeptides containing multi-antennary oligosaccharide structure with terminal alpha-linked galactose. FEBS Lett. 1998;421:169–173. doi: 10.1016/s0014-5793(97)01539-1. [DOI] [PubMed] [Google Scholar]
- Emery DJ, Diaz LA, Fairley JA, Lopez A, Taylor AF, Giudice GJ. Pemphigus foliaceus and pemphigus vulgaris autoantibodies react with the extracellular domain of desmoglein-1. J Invest Dermatol. 1995;104:323–328. doi: 10.1111/1523-1747.ep12665364. [DOI] [PubMed] [Google Scholar]
- Espana A, Diaz LA, Mascaro JM, Jr., Giudice GJ, Fairley JA, Till GO, et al. Mechanisms of acantholysis in pemphigus foliaceus. Clin Immunol Immunopathol. 1997;85:83–89. doi: 10.1006/clin.1997.4407. [DOI] [PubMed] [Google Scholar]
- Eyre RW, Stanley JR. Human autoantibodies against a desmosomal protein complex with a calcium-sensitive epitope are characteristic of pemphigus foliaceus patients. J Exp Med. 1987;165:1719–1724. doi: 10.1084/jem.165.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Ogawa MM, Konohana A, Nishikawa T. Detection of pemphigus vulgaris and pemphigus foliaceus antigens by immunoblot analysis using different antigen sources. J Invest Dermatol. 1990;94:327–331. doi: 10.1111/1523-1747.ep12874456. [DOI] [PubMed] [Google Scholar]
- Heimburg J, Yan J, Morey S, Glinskii OV, Huxley VH, Wild L, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8:939–948. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin GL. Isolation of glycopeptides containing O-linked oligosaccharides by lectin affinity chromatography on jacalin-agarose. Anal Biochem. 1990;191:262–267. doi: 10.1016/0003-2697(90)90217-w. [DOI] [PubMed] [Google Scholar]
- Hortin GL, Trimpe BL. Lectin affinity chromatography of proteins bearing O-linked oligosaccharides: application of jacalin-agarose. Anal Biochem. 1990;188:271–277. doi: 10.1016/0003-2697(90)90605-9. [DOI] [PubMed] [Google Scholar]
- Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J Immunol Methods. 1998;212:193–211. doi: 10.1016/s0022-1759(98)00021-0. [DOI] [PubMed] [Google Scholar]
- Kitajima Y, Aoyama Y. A perspective of pemphigus from bedside and laboratory-bench. Clin Rev Allergy Immunol. 2007;33:57–66. doi: 10.1007/s12016-007-0036-5. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Walsh MJ, Zimbelmann R, Franke WW. Complete amino acid sequence of the epidermal desmoglein precursor polypeptide and identification of a second type of desmoglein gene. Eur J Cell Biol. 1991;55:200–208. [PubMed] [Google Scholar]
- Koulu L, Kusumi A, Steinberg MS, Klaus-Kovtun V, Stanley JR. Human autoantibodies against a desmosomal core protein in pemphigus foliaceus. J Exp Med. 1984;160:1509–1518. doi: 10.1084/jem.160.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Anderson JE, Borgwardt JE, Hashimoto T, Stanley JR, Green KJ. Pemphigus sera recognize conformationally sensitive epitopes in the amino-terminal region of desmoglein-1. J Invest Dermatol. 1995;105:147–152. doi: 10.1111/1523-1747.ep12316680. [DOI] [PubMed] [Google Scholar]
- Landon LA, Zou J, Deutscher SL. Effective combinatorial strategy to increase affinity of carbohydrate binding by peptides. Mol Divers. 2004;8:35–50. doi: 10.1023/b:modi.0000006897.40575.41. [DOI] [PubMed] [Google Scholar]
- Lever WF, Smith PA, Hurley NA. Effects of intravenous heparin on the plasma lipoproteins in primary hypercholesteremic xanthomatosis and idiopathic hyperlipemia. Science. 1953;118:653–654. doi: 10.1126/science.118.3074.653. [DOI] [PubMed] [Google Scholar]
- Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem). J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanta SK, Sastry MV, Surolia A. Topography of the combining region of a Thomsen-Friedenreich-antigen-specific lectin jacalin (Artocarpus integrifolia agglutinin). A thermodynamic and circular-dichroism spectroscopic study. Biochem J. 1990;265:831–840. doi: 10.1042/bj2650831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olague-Alcala M, Diaz LA. The epitopes on bovine pemphigus foliaceus antigen are calcium-dependent and located on the peptide backbone of this glycoprotein. Chron Dermatol. 1993;2:189–209. [Google Scholar]
- Olague-Alcala M, DL The epitopes on bovine pemphigus foliaceus antigen are calcium-dependent and located on the peptide backbone of this glycoprotein. Chron Dermatol. 1993;2:189–209. [Google Scholar]
- Ortiz-Urda S, Elbe-Burger A, Smolle J, Marquart Y, Chudnovsky Y, Ridky TW, et al. The plant lectin wheat germ agglutinin inhibits the binding of pemphigus foliaceus autoantibodies to desmoglein 1 in a majority of patients and prevents pathomechanisms of pemphigus foliaceus in vitro and in vivo. J Immunol. 2003;171:6244–6250. doi: 10.4049/jimmunol.171.11.6244. [DOI] [PubMed] [Google Scholar]
- Rappersberger K, Roos N, Stanley JR. Immunomorphologic and biochemical identification of the pemphigus foliaceous autoantigen within desmosomes. J Invest Dermatol. 1992;99:323–330. doi: 10.1111/1523-1747.ep12616659. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Olson K. Jaa-f11: extending the life of mice with breast cancer. Expert Opin Biol Ther. 2007;7:923–928. doi: 10.1517/14712598.7.7.923. [DOI] [PubMed] [Google Scholar]
- Rock B, Labib RS, Diaz LA. Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990;85:296–299. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem). N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- Roque-Barreira MC, Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985;134:1740–1743. [PubMed] [Google Scholar]
- Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, et al. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985;85:538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- Rubenstein DS, Diaz LA. Pemphigus antibody induced phosphorylation of keratinocyte proteins. Autoimmunity. 2006;39:577–586. doi: 10.1080/08916930600971885. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J Immunol. 2001;167:5439–5448. doi: 10.4049/jimmunol.167.9.5439. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci. 2007;48:1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakamura S, Wang H, Iwasaki H, Maebara K, Cheng L, et al. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, et al. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. N Engl J Med. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- Waschke J. The desmosome and pemphigus. Histochem Cell Biol. 2008;130:21–54. doi: 10.1007/s00418-008-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]