Abstract
Dopamine has long been implicated in impulsivity, but the precise mechanisms linking human variability in dopamine signaling to differences in impulsive traits remain largely unknown. Using a dual PET scan approach in healthy human volunteers with amphetamine and the D2/D3 ligand 18F-fallypride, we found that higher levels of trait impulsivity were predicted by diminished midbrain D2/D3 autoreceptor binding and greater amphetamine-induced DA release in the striatum, which was in turn associated with stimulant craving. Path analysis confirmed that the impact of decreased midbrain D2/D3 autoreceptor availability on trait impulsivity is mediated in part through its effect on stimulated striatal dopamine release.
The ability to deliberate on the consequences of one’s actions is a critical component of adaptive human behavior. However, individuals differ strongly in their capacity for such deliberation, and a sizeable contingent of the population persistently makes rash, unpremeditated, and often destructive decisions. Such trait differences in impulsivity robustly predict liability for a range of externalizing disorders, including attention deficit/hyperactivity disorder (ADHD), antisocial personality disorder, and substance abuse(1–3).
Dopamine (DA) has been theorized to play a key role in impulsivity(4, 5), but the precise systems-level mechanisms linking variation in DA signaling to trait differences in impulsivity and its behavioral and psychopathological correlates remain unclear, particularly in humans. Extrapolating from preclinical and human findings of impulsivity-linked alterations in DA functioning, we have developed a neurobiological model of individual differences in human impulsivity. According to this model, highly impulsive individuals are characterized by diminished midbrain D2 autoreceptor availability, which leads to enhanced DA cell firing and potentiated DA release in terminal fields following exposure to novel, salient, or rewarding stimuli.
We scanned 32 physically and psychiatrically healthy volunteers using positron emission tomography with [18F]-fallypride – a D2/D3-selective ligand that labels striatal and extrastriatal receptors – at placebo and following the oral administration of .43 mg/kg d-amphetamine(6). Each subject completed the Barratt Impulsiveness Scale (BIS-11)(3). For each subject, we calculated voxelwise statistical parametric maps (SPMs) of D2/D3 receptor availability and amphetamine-induced DA release (percent change in [18F]-fallypride binding from placebo; Fig. S1), and correlated these SPMs with participants’ BIS-11 total scores(6).
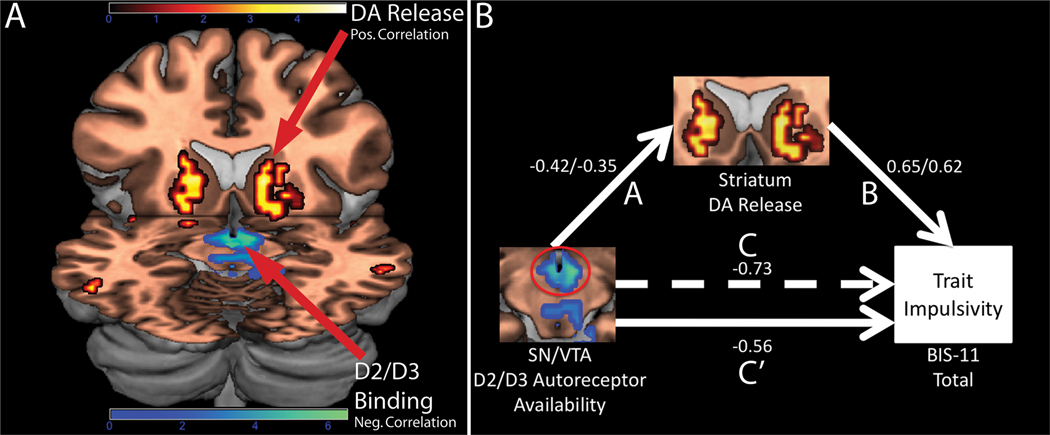
Trait impulsivity was inversely correlated with D2/D3 autoreceptor availability in the substantia nigra/ventral tegmental area (SN/VTA; Fig. 1A), and positively correlated with the magnitude of amphetamine-induced DA release in the striatum (Fig. 1A). Consistent with an inhibitory influence of midbrain autoreceptors on DA release in terminal fields (7), SN/VTA D2/D3 binding potential was inversely correlated with bilateral striatal DA release following amphetamine (r = −.42/−.35, left/right striatum, both p’s < 0.05). Using path modeling (mediation analysis), we tested our mechanistic hypothesis that lower SN/VTA D2/D3 availability leads to impulsive traits by enhancing stimulated DA release in the striatum. This analysis confirmed that the ability of SN/VTA D2/D3 binding to predict impulsive temperament is at least in part mediated through the impact of SN/VTA D2/D3 availability on amphetamine-induced DA release in the striatum (Fig. 1B).
Fig. 1.
(A) Rendering of two separate SPMs depicting negative correlations between BIS-11 scores and D2/D3 binding and positive correlations between BIS-11 scores and amphetamine-induced DA release (corrected for multiple comparisons at the cluster-level, pcorrected < 0.05; clusters defined using a height threshold of t > 3). For the D2/D3 binding, inverse associations emerged in the DA midbrain [anteriorly in the VTA, centered at 6, −3, −11 (x, y, z; MNI space) and posteriorly in the retrorubral fields)] as well as more anteriorly in the mammillary bodies and hypothalamus (not shown). Peak coordinates for the striatal AMPH-induced DA release correlations = −16, 15, 5 (left) and 20, 23, −3 (right); cluster sizes = 167 voxels (left) and 216 voxels (right). Colorbar represents Fisher-transformed z-statistic values for the correlations (d.f. = 31). SPMs rendered on a T1-weighted MRI template brain, with cuts at z = −11 and y = 16.
(B) Path analysis demonstrating that the influence of midbrain D2/D3 availability on trait impulsivity is mediated through an impact on striatal amphetamine-induced DA release. Path “A” shows path coefficients for the effect of midbrain D2/D3 binding (cluster highlighted with red circle) on striatal DA release (left/right striatum). Path “B” shows the path coefficient for the effect of striatal DA release on trait impulsivity (left/right striatum). Paths “C” and “C’” show coefficients for the total (dashed line) and direct (solid line) effects of midbrain D2/D3 binding on trait impulsivity. All coefficients standardized. Sobel test for mediation: Z = −1.94, p = 0.05, with left and right striatum modeled simultaneously.
To examine the relevance of enhanced striatal release to risk for psychopathology, we examined the relationship between amphetamine-induced striatal DA release and subjective responses to amphetamine. Increased striatal DA release predicted stronger subjective desire for more drug following amphetamine treatment (drug “Wanting” from the Drug Effects Questionnaire; left/right striatum: r = .48/.47, both p’s < 0.01). Given that heightened subjective “wanting” responses to initial psychostimulant exposure is a significant risk factor for future drug dependence(8), and that BIS-11 scores have been found to predict drug craving in substance dependent individuals (9), the present data suggest a neurobiological link between human impulsiveness and drug abuse vulnerability.
These results show that individual differences in midbrain D2/D3 availability are associated with the expression of impulsivity in humans, an effect that appears to be mediated, in part, through diminished inhibitory autoreceptor control over stimulated striatal DA release. Our findings suggest that dysregulation within ascending dopaminergic projection pathways subserving reward and motivation may produce deficits in impulse control, a critical feature of the psychopathological architecture underpinning substance abuse. Further, they provide a specific, plausible mechanism that links individual variability in DA network functioning to differences in human impulsivity.
Supplementary Material
References and Notes
- 1.Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. J Int Neuropsychol Soc. 2007 Jul;13:693. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- 2.Verdejo-Garcia A, Lawrence AJ, Clark L. Neurosci Biobehav Rev. 2008;32:777. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Psychiatry Res. 1999 May 31;86:163. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Cools R, Barker RA, Sahakian BJ, Robbins TW. Neuropsychologia. 2003;41:1431. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 5.Dalley JW, et al. Science. 2007 Mar 2;315:1267. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(Materials and methods are available as supporting material on Science Online)
- 7.Santiago M, Westerink BH. Eur J Pharmacol. 1991 Oct 29;204:79. doi: 10.1016/0014-2999(91)90838-h. [DOI] [PubMed] [Google Scholar]
- 8.Lambert NM, McLeod M, Schenk S. Addiction. 2006 May;101:713. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 9.Zilberman ML, Tavares H, el-Guebaly N. BMC Psychiatry. 2003 Jan 13;3:1. doi: 10.1186/1471-244X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.