Abstract
The ribonuclease ranpirnase (Onconase) has been used empirically to treat malignant mesothelioma (MM) patients, and some of them had prolonged survivals. The aim of this study was to investigate the mechanisms of the therapeutic function of ranpirnase in MM cells. The effects of ranpirnase were studied in vivo and in vitro on 2 MM cell lines (epithelioid REN and sarcomatoid PPM-Mill). We found that ranpirnase was able to inhibit NF-κB nuclear translocation, evaluated by cell fractionation and immunoblotting as well as by immunofluorescence. Also, MMP9 secretion by MM cells was decreased by ranpirnase treatment, as assessed by the reduction of metalloproteinase activity, evaluated by zymography on culture-conditioned media. Ranpirnase induced apoptosis of MM cells in vitro and in vivo, causing a powerful inhibition of MM tumor growth in SCID xenografts, determined by In Vivo Imaging System (IVIS) of tumor cells engineered by lentiviral transduction of the luciferase gene. Finally, mice treated with ranpirnase showed a significantly prolonged survival. Our data provide a mechanistic rationale to explain the beneficial antitumor activity observed in some patients treated with ranpirnase and demonstrate that ranpirnase interferes with the NF-κB pathway, thus influencing MM tumor cell invasiveness and survival. It is hoped that this information will also facilitate the identification of those patients who are more likely to benefit from this drug and will also open a new frontier for the use of this drug in tumor types other than MM.
Keywords: mesothelioma, ranpirnase, NF-κB, MMP9, TNF-α
Introduction
Mesothelioma (MM) is a very aggressive tumor that arises from mesothelial cells of the lining of pleural, pericardial, and peritoneal cavities. Histologically, MM can present an epithelioid, sarcomatoid, or biphasic (both epithelioid and sarcomatoid) morphology. The relationship between asbestos exposure and MM is well established, with approximately 60% to 70% of patients with pleural MM in the United States having been previously exposed to asbestos.1 In the US, an estimated 2,500 people are diagnosed with MM each year,2 with nearly 100,000 new cases expected to occur over the next 40 years.3 The prognosis of MM remains poor, with the current standard of care resulting in a median survival of approximately 12 months from diagnosis. Sarcomatoid MM is refractory to therapy and is associated with a survival of 6 to 7 months.4,5 Recent multimodality studies utilizing neoadjuvant chemotherapy, followed by surgery and radiotherapy, have yielded slightly more favorable outcomes.6 Continued efforts to find more effective treatments are ongoing, and a number of therapeutic agents have shown promising results in a subset of patients. Among them is ranpirnase, a ribonuclease (RNase) isolated from early embryos of the Northern Leopard Frog.7 Ranpirnase acts by preferentially degrading tRNA, thus inhibiting protein synthesis.8 Because of its cytostatic function, causing minimal side effects, ranpirnase is considered an attractive drug.9,10 Ranpirnase treatment of Jurkat SN acute T-lymphocytic leukemia cells resulted in a decrease of NF-κB expression and of cell survival.11 The combination therapy of ranpirnase and cepharanthine showed synergistic effects in leukemia, lymphoma, myeloma, and prostate cancer–derived cell lines, promoting strong antitumor effects.12
The antitumor effect of ranpirnase has been studied in vitro and in vivo in various malignancies including MM.10,13 Ranpirnase has been used in phase I and II MM trials14 and in a confirmatory phase IIIb MM clinical trial. This trial was initiated as a result of an exploratory phase III study that showed a survival advantage after adjusting for a prognostic imbalance in the 2 treatment groups by using the Cancer and Leukemia Group B (CALGB) prognostic scoring system.5,9 The results demonstrate that the combination of ranpirnase and doxorubicin is a safe and feasible treatment in unresectable MM and showed a significant impact on the survival of pretreated patients compared to doxorubicin alone.15 Although it is known that ranpirnase is a RNAse that suppresses protein synthesis, its precise antitumor mechanisms in MM remain elusive.
Our previous studies have linked TNF-α secretion and NF-κB activity to the pathogenesis of MM.16,17 We found that asbestos causes programmed necrosis of mesothelial cells, thus causing the release of the cytokine high mobility group box 1 (HMGB1), which in turn induces TNF-α secretion by macrophages and mesothelial cells. TNF-α activates the nuclear translocation of the p65 subunit of NF-κB, a transcription factor that leads to the survival of mesothelial cells, thus favoring carcinogenesis.16,17
Here, we demonstrate that ranpirnase is a potent inhibitor of TNF-α–dependent NF-κB activity in MM cells. We demonstrated that by blocking NF-κB activity, ranpirnase inhibits MMP9 secretion and consequently inhibits MM cell invasiveness and causes apoptosis of MM tumor cells. Moreover, we show that ranpirnase is able to inhibit MM tumor growth in SCID mice xenografts, leading to significant prolonged survival.
Results
Ranpirnase inhibits NF-κB nuclear translocation induced by TNF-α
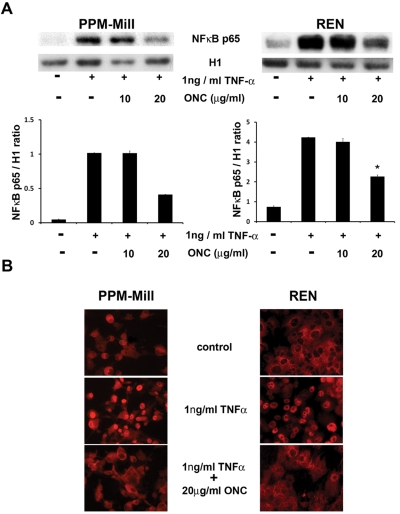
We previously showed that nuclear translocation of the transcription factor NF-κB occurs after TNF-α treatment of mesothelial cells, with a maximum effect between 30 minutes and 1 hour.16 To test if the antitumor effect of ranpirnase occurred by targeting this pathway, we incubated PPM-Mill and REN MM cells for 72 hours with 10 µg/mL and 20 µg/mL of ranpirnase, followed by a 30-minute treatment with 1 ng/mL of TNF-α. Nuclear extracts were analyzed by immunoblotting for the NF-κB p65 subunit. The immunoblotting densitometry analysis revealed that pretreatment with 20 µg/mL ranpirnase significantly reduced NF-κB nuclear translocation (P < 0.05) upon TNF-α exposure (Fig. 1A). Similar results were obtained by immunofluorescence with NF-κB p65 antibodies on the same cells. In PPM-Mill and REN cells pretreated with 20 µg/mL ranpirnase, NF-κB was instead detected in the cytoplasm (Fig. 1B), which demonstrated that ranpirnase impaired TNF-α–induced nuclear translocation of NF-κB. Therefore, we conclude that ranpirnase targets NF-κB activity by interfering with its nuclear translocation.
Figure 1.
Ranpirnase inhibits NF-κB nuclear translocation induced by TNF-α in mesothelioma (MM) cells. (A) Western blotting of nuclear extracts from PPM-Mill and REN cells, probed with NF-κB p65 and histone 1 antibodies. Cells were preincubated 72 hours with the indicated concentrations of ranpirnase (Onconase [ONC]) or with vehicle (-), followed by a treatment with 1 ng/mL TNF-α for 30 minutes (+). TNF-α induced NF-κB nuclear translocation in both cell types, but in cells pretreated with 20 µg/mL ranpirnase, nuclear translocation was significantly inhibited. The ratio between NF-κB p65 and histone 1 band intensities, measured by a densitometer, is shown in the lower bar graphs, generated by Image-J software. Asterisks (*) indicate P < 0.05 significance. (B) Immunofluorescence analysis with rabbit polyclonal NF-κB antibodies on PPM-Mill and REN cells. In cells treated with 1 ng/mL TNF-α, the nuclear localization of NF-κB was evident, while this was inhibited when cells were preincubated with 20 µg/mL ranpirnase (ONC). Secondary anti-rabbit antibodies were conjugated with Alexa Fluor 568 fluorochrome (red).
Ranpirnase inhibits cell invasiveness through inhibition of MMP9 secretion
Cell invasiveness is one of the hallmarks of cancer, and MM cells acquire the capability of matrix invasion upon transformation.18 It has been shown that this process is linked to the enhanced secretion of metalloproteinase (mainly MMP9), which is mediated by NF-κB activity.19 Because of the observed inhibitory effect of ranpirnase on TNF-α–induced NF-κB activity, we verified if MMP9 was released into the conditioned medium by MM and also detected whether its release was affected by ranpirnase.
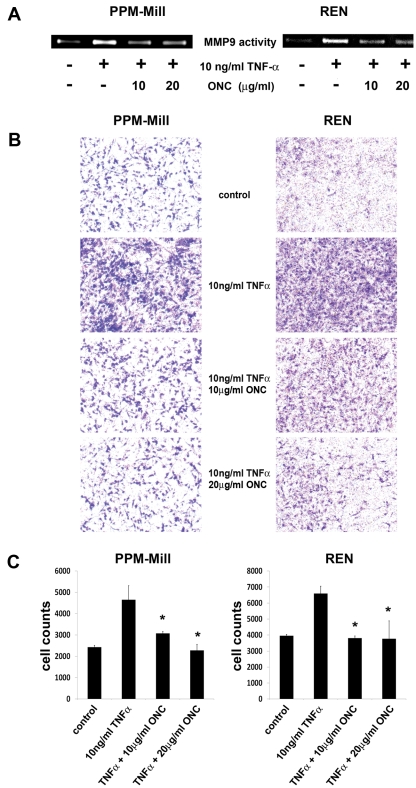
PPM-Mill and REN cells were incubated for 72 hours with 10 and 20 µg/mL ranpirnase. There was 10 ng/mL of TNF-α or vehicle solution added into the culture media in the last 24 hours. Dose-response curve experiments indicated that this timing and dose resulted in the maximum increase of MMP9 release and activity (data not shown). The cell-conditioned media were collected and assayed by zymography, as described in Materials and Methods. MMP9 activity was detected in the conditioned medium of cells treated with TNF-α, while a strong inhibition of MMP9 secretion was observed upon ranpirnase pretreatment, at both drug concentrations (Fig. 2A).
Figure 2.
Ranpirnase impairs MMP9 release and cell invasiveness induced by TNF-α in mesothelioma (MM) cells. (A) Gelatin zymography was performed, as described in Materials and Methods, on conditioned media from PPM-Mill and REN cells. Cells were preincubated for 72 hours with the indicated concentrations of ranpirnase (ONC) or with vehicle (-), followed by a treatment with TNF-α (10 ng/mL) for 30 minutes (+). A sharp reduction of MMP9 activity is evident at both ranpirnase concentrations. (B) Cell invasion was assessed by Matrigel-coated Transwell, as described in Materials and Methods. In both MM cell lines, TNF-α strongly induced cell invasiveness, which was inhibited by ranpirnase (ONC) in a dose-dependent manner. (C) The numbers of cells invading the coated membrane of the Transwell top chamber were quantified by Image-J software and are shown by the bar graph. Asterisks (*) indicate P < 0.05 significance.
We assessed the possible effects of ranpirnase on MM cell invasiveness by using the Transwell cell invasion assay (Corning, Barre, PA) in the same conditions used for the detection of MMP9 release. Upon TNF-α treatment, a high number of cells invaded into the lower compartment crossing the Matrigel-coated membrane (BD Biosciences, Bedford, MA), which provided further evidence supporting the role of TNF-α in MM cells’ invasiveness (Fig. 2B). In contrast, incubation of MM cells with both ranpirnase and TNF-α for 72 hours resulted in a dramatic reduction in cell invasiveness. The reduction was significant (P < 0.05) at 10 µg/mL ranpirnase and was even more pronounced when 20 µg/mL of the drug was used (Fig. 2C). These results indicate that ranpirnase has a strong inhibiting effect on cell invasion induced by TNF-α. We conclude that ranpirnase targets TNF-α–induced MMP9 secretion possibly through interfering with the NF-κB pathway19 and that this leads to a strong inhibition of cell invasiveness in MM cells.
Ranpirnase induces apoptosis in MM cells
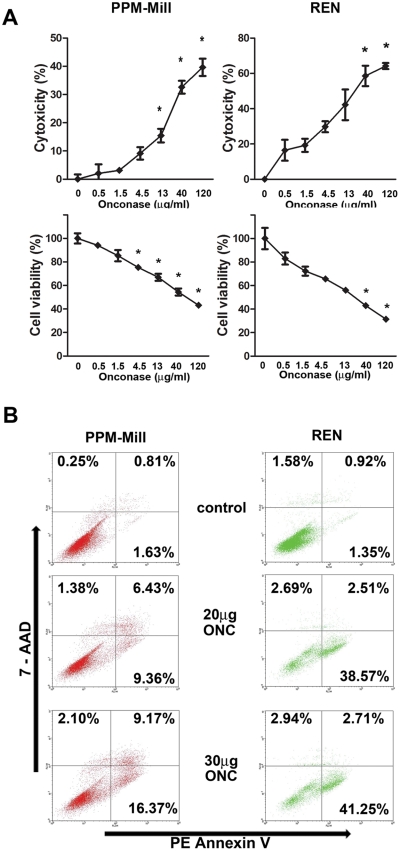
We tested the possible effects of ranpirnase in influencing MM cell death. The release of lactate dehydrogenase (LDH) from cultured cells was used to evaluate the cytotoxicity induced by 72 hours’ treatment with increasing concentrations of ranpirnase, ranging from 0.5 µg/mL to 120 µg/mL. Ranpirnase induced MM cell death in a dose-response manner. A maximum of 40% and 64% of cell death was observed for PPM-Mill and REN cells, respectively. These findings were verified by an MTT assay that measures cell metabolic activity (Fig. 3A).
Figure 3.
Ranpirnase has a powerful proapoptotic effect on mesothelioma (MM) cells. (A) LDH (cytotoxicity) and MTT (cell viability) assays were performed on PPM-Mill and REN cells in the presence of increasing concentrations of ranpirnase (ONC) as indicated. In both the LDH assay (upper graphs) and MTT assay (lower graphs), a dose-dependent increase of cytotoxicity and decrease of cell survival, respectively, are evident. Asterisks (*) indicate P < 0.05 significance. The experiments were conducted in triplicates. (B) PE annexin V/7-AAD flow cytometry assay was conducted on PPM-Mill and REN cells in the presence of the indicated concentrations of ranpirnase (ONC) to evaluate the induction of apoptosis. Cells were incubated 72 hours before fixation and flow cytometry. The percentages of cell subpopulations in early apoptosis (lower right), late apoptosis (upper right), and necrosis (upper left) are indicated.
Ranpirnase-induced apoptosis was evaluated by flow cytometry with the PE/annexin V apoptosis assay. PPM-Mill and REN cells were exposed to 20 µg/mL and 30 µg/mL of ranpirnase for 72 hours. Flow cytometry analysis revealed that ranpirnase induced both early and late apoptosis in drug-treated cells. Ranpirnase, at the dose of 20 µg/mL, caused apoptosis in 16% of PPM-Mill and in 41% of REN cells. Ranpirnase, at the dose of 30 µg/mL, caused apoptosis in 26% and 44% of PPM-Mill and REN cells, respectively (Fig. 3B). Ranpirnase did not appear to induce programmed cell necrosis. Notably, these results are consistent with those obtained by the cytotoxicity assay, with PPM-Mill cells being more resistant to ranpirnase-dependent cell death than REN cells.
Ranpirnase reduces tumor burden and prolongs survival in human MM xenografted SCID mice
To examine the effects of ranpirnase on tumor growth in vivo, we injected SCID mice with PPM-Mill or REN cells engineered to express the firefly luciferase (PPM-Mill/luc or REN/luc) as previously described.20 Tumor progression was monitored weekly by bioluminescence as described in Materials and Methods and as in Bertino et al. 20
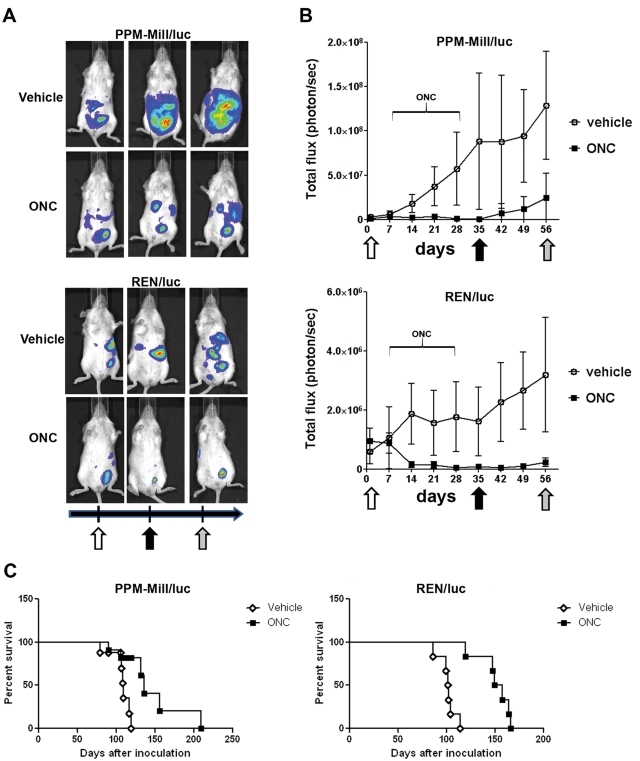
Tumor burden was markedly reduced in the mice bearing xenografts of MM cells and treated with ranpirnase compared to the respective control groups. Similar results were obtained in both MM cell lines. Notably, the tumors from treated mice resumed growth after discontinuation of drug treatment, although their size remained much smaller than the vehicle-treated group (Fig. 4A and 4B). These results indicate that ranpirnase has a strong and early antitumor efficacy and that these effects are long lasting.
Figure 4.
Ranpirnase suppresses mesothelioma (MM) growth in vivo and extends animal survival. (A) The growth of PPM-Mill and REN xenografts was monitored by IVIS weekly. Representative mice tumor imaging 1 week before treatment (white arrow) and 1 week after (black arrow) and 4 weeks (gray arrow) after the end of treatment is shown. (B) Quantitative analysis of bioluminescence photon counts as a measure of tumor growth during the entire experiment. Ranpirnase (ONC) treatment nearly suppressed tumor growth after 2 weeks in both groups, and the reduction in tumor size was evident even 4 weeks after discontinuation of ranpirnase treatment. Open symbol curve = vehicle control mice; closed symbol curve = ranpirnase (2.5 µg/g body weight). The horizontal line above the curves indicates the interval of ranpirnase (ONC) administration. (C) Kaplan-Meier survival curves of ranpirnase (ONC)–treated mice show median survival values of 136 days (PPM-Mill) and 153 days (REN) compared to the vehicle control mice median survival of 109 days (PPM-Mill) and 101.5 days (REN). Significance: P < 0.01.
Mice survival was evaluated up to 160 days after xenograft injection for REN/luc and about 200 days for PPM-Mill/luc. Kaplan-Meier curves revealed a significant (P < 0.01) increase in median survival of ranpirnase- treated mice compared to vehicle- treated animals: 136 days versus 109 days for PPM-Mill/luc and 153 days versus 101.5 days for REN/luc (Fig. 4C).
Tumors collected at necropsy were fixed and embedded in paraffin. Histological sections were stained with H&E and examined by 2 experienced pathologists (M.C. and D.S.), who confirmed the MM phenotype. Sections were processed for immunohistochemical analysis of apoptosis, evaluated by TUNEL. Tumor tissues from both ranpirnase-treated and vehicle-treated mice were stained positively for human cytokeratin, which confirmed that the tumors were originated from the injected human MM cell lines. When serial paraffin sections were analyzed, a significant (P < 0.005) fraction of cells stained positively for TUNEL in the ranpirnase-treated mice compared to that observed in vehicle-treated mice (Fig. 5).
Figure 5.
Ranpirnase induces massive apoptosis in human mesothelioma (MM) xenografts. Immunohistochemical analysis of tissues from tumors grown in the peritoneal cavity of SCID mice and collected at necropsy. Parallel tissue sections were immunostained for human cytokeratin, as a marker of mesothelial origin, by the TUNEL apoptosis assay. The total number of cells and the number of TUNEL-positive apoptotic cells were determined by Image-J software on 8 different fields from 2 different sections. The percentages of apoptotic cells (±SD) are reported on the pictures of representative sections from vehicle- and ranpirnase (ONC)–treated animals.
Discussion
We have previously shown that TNF-α inhibits asbestos-induced cytotoxicity via induction of NF-κB.16 Moreover, when we impaired NF-κB functions by using an inhibitor of IκB phosphorylation, we observed a remarkable increase in cytotoxicity.16 More recently, we demonstrated that asbestos induces programmed cell necrosis and the release of HMGB1 in the extracellular space, leading to TNF-α secretion by macrophages.17 In turn, TNF-α–induced NF-κB activity leads to cellular proliferation and survival, favoring cancer development.21 NF-κB is a transcription factor that is implicated in a multitude of critical cellular functions including inflammation, immunity, cell proliferation, and apoptosis.22,23 The inhibition of NF-κB activity in MM cells and MM mouse xenografts decreased tumorigenicity and increased animal survival.24
The RNase inhibitor ranpirnase has been investigated as a potential chemotherapeutic drug because of its cytostatic effect and as a potential radiosensitizer and chemosensitizer.25 We demonstrate here that ranpirnase has an inhibitory effect on NF-κB via suppression of its nuclear translocation, providing a mechanistic explanation to previous studies on the effects of this drug.11,26 Our results parallel and match with our recent results in vitro, showing the ranpirnase effect on 2 microRNAs, Hsa-miR-17* and Hsa-miR-30c, which specifically regulate NF-κB protein expression.27 We show that ranpirnase is effective in suppressing tumor growth in SCID mice xenografted with MM cells and in extending mice median survival up to 50 days compared to untreated controls. Immunohistochemical analysis of the MM tumors from ranpirnase-treated mice revealed a significantly higher percentage of TUNEL-positive cells compared to untreated controls. These in vivo data confirmed our in vitro data on the powerful proapoptotic effect of ranpirnase on MM in cell culture. A proapoptotic effect had been also observed in a leukemia cell line treated with ranpirnase.28
Previous studies have implicated metalloproteinases as important mediators in tumor metastasis.29-32 In addition, the metalloproteinase MMP9 has been associated also with tumor neoangiogenesis.31-33 Our results show that ranpirnase treatment of MM cells leads to a strong reduction of MMP9 activity, an effect that is specifically induced by TNF-α–mediated activation of NF-κB. In parallel to MMP9 activity inhibition, our results also demonstrate that ranpirnase blocks MM cell invasiveness in dose-dependent manner. These findings suggest that the metalloproteinase MMP9 may be a major target for the potential therapeutic efficacy of ranpirnase on MM tumor growth and possibly as an antimetastatic agent. Although clinical trials conducted with various MMP inhibitors did not give encouraging results,31 a better understanding of the role that MMPs play in tumor metastasis may lead to better outcomes.32
Chemoresistance to ranpirnase needs to be addressed to predict the efficacy of this drug. It has been reported that high levels of constitutive Akt activity were associated with increased resistance to ranpirnase by MM cells and that phosphoinositide 3-kinase (PI3K) inhibitors displayed a synergic effect in combination with ranpirnase.34 Our results indicate that some cells are more resistant to ranpirnase-induced cytotoxicity than others, having higher levels of Akt activity, which match previous findings34 (data not shown). This indicates that also Akt can be considered a valuable prognostic marker of responsiveness to ranpirnase treatment. Furthermore, a study conducted by microarray analysis revealed differential gene expression profiles upon ranpirnase treatment of several human MM cells and identified 155 genes regulated by ranpirnase. The functions of these ranpirnase-responsive genes were largely involved in transcriptional activity controlling apoptosis, inflammation, and immune response.35 These findings could be helpful to identify the subsets of MM patients who may be more responsive to ranpirnase than others, as suggested by the phase IIIb MM clinical trial.15
In conclusion, we showed that ranpirnase inhibits NF-κB pathway, cell proliferation, survival, and invasiveness, as well as tumor growth in vivo, leading to increased animal survival. Our data also further strengthen the important role played by NF-κB in MM onset and progression, indicating that blocking this signaling effector by ranpirnase leads to improved survival. Overall, our data provide mechanistic rationale and support the observed beneficial effect of ranpirnase in a subset of MM patients.15 It is hoped that our findings combined with those of Altomare and collaborators35 will facilitate the identification of those patients who are more likely to benefit from this drug.
Materials and Methods
Cell cultures
Two different MM cell lines were used in this study: REN, an epithelioid MM cell line from Dr. S.M. Albelda (University of Pennsylvania, Philadelphia, PA), and PPM-Mill, a sarcomatoid MM cell line from Dr. H. Pass (New York University, New York, NY). These MM cells were established in cell culture from MM tumor biopsies and cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM, Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (FBS).
Gelatin zymography assay
There was 40 µL of cell culture media loaded on SDS polyacrylamide gel with 0.1% gelatin. After running, the gel was washed for at least 1 hour in renaturing buffer (0.25% Triton X-100 in water) and incubated at 37°C overnight in developing buffer (50 mM Tris, pH8, 5 mM CaCl2, 200 mM NaCl, and 0.02% Brij-35 nonanionic detergent). The gel was stained with 0.5% Coomassie blue for at least 30 minutes and then destained (10% acetic acid, 30% ethanol).
Western blotting
Primary antibodies: rabbit polyclonal anti–NF-κB p65, mouse monoclonal anti–histone 1, and mouse monoclonal anti-MMP9 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase–conjugated secondary antibody and the chemiluminescence system Western Lightning Plus-ECL (PerkinElmer Life and Analytical Sciences, Shelton, CT) were used to detect the signal.
Immunofluorescence and immunohistochemistry
REN or PPM-Mill cells were seeded on 4-well chamber slides. Cells were incubated with ranpirnase (Onconase, Alfacell Corporation, Paramus, NJ) for 72 hours followed by treatment with TNF-α for 30 minutes. The cells were then fixed with 4% paraformaldehyde. NF-κB p65 rabbit polyclonal antibody (Santa Cruz Biotechnology) and Alexa Fluor 568–labeled goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) were used for this assay. Tumor tissues collected from SCID mice at necropsy were analyzed by immunohistochemistry on formalin-fixed, paraffin-embedded (FFPE) sections (5 µm) and incubated with human AE1/AE3 cytokeratin monoclonal antibodies (Invitrogen), followed by horseradish peroxide–conjugated anti-mouse immunoglobulin antibodies. After development with substrate (VIP Substrate kit, Vector Laboratories, Burlingame, CA), tissue sections were counterstained with Gill’s hematoxylin 1X (Fisher Scientific, Hampton, NH) for 1 minute at room temperature. The tissue sections were then mounted after dehydration.
Cell invasion assay
Transwell permeable supports (Corning) were coated with Matrigel (BD Biosciences) diluted (1:3) with DMEM and were used to monitor invasiveness of MM cells in the presence of 2% serum. The cells that migrated into the lower side of the permeable support were stained using the Hema 3 stain kit (Fisher, Middletown, VA).
Cell viability and cytotoxicity assays
The Cell Proliferation Kit I (MTT assay) and Cytotoxicity Detection Kit (LDH assay), both from Roche Applied Science (Indianapolis, IN), were used to verify the sensitivity of REN and PPM-Mill cells to ranpirnase-mediated cytotoxicity. In both assays, MM cells were seeded in 96-well plates (2 × 104 cells/well) and incubated with increasing concentrations of ranpirnase in 1% FBS. After 72 hours, the cells were processed following the manufacturer’s instructions, and the final absorbance was measured in a Bio-Rad microplate reader (Hercules, CA).
Apoptosis assays
PE/Annexin V Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA) was used to estimate the percentage of apoptotic cells upon ranpirnase treatment. The fluorescence intensity of annexin V and of 7-AAD of individual cells was measured by flow cytometry using the Becton Dickinson (BD, San Jose, CA) FACScan. Data were analyzed by the BD CellQuest Pro Software. TUNEL assay, according to the DeadEnd Colorimetric TUNEL system (Promega, Madison, WI), was used to evaluate the fraction of cells undergoing apoptosis in tumor tissues from xenografts.
Animal experiments
Six-week-old female NOD.CB17-Prkdcscid/J SCID mice (Jackson Laboratory, Bar Harbor, ME) were injected intraperitoneum (i.p.) with 1 × 106 MM cells, engineered to express the luciferase reporter gene (REN/luc, PPM-Mill/luc) and suspended in 400 uL of PBS, as previously described.20 Xenografts were visualized by luminescence, after luciferin injection, using the In Vivo Imaging System (IVIS, Xenogen Corp., Alameda, CA), with regions of interest quantified as total photon counts, processed by Living Image software (Xenogen Corp.). After the first IVIS measurement, mice that established active tumor growth were stratified into 2 groups of 6 mice each. In the first group, mice were injected with 2.5 µg/g ranpirnase, 100 to 200 µL i.p., twice a week. The second group was injected with PBS (200 µL) as control. Mice were euthanized when severe ascites was observed.
Acknowledgments
The authors thank Dr. Toshihiko Kawamori and Dr. Florian Sulzmaier for fruitful discussion and technical advice. Ranpirnase (Onconase) was provided by Alfacell Corporation (Paramus, NJ).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This work was supported by the National Cancer Institute (NCI) [grant numbers P01-CA114047, R01-CA106567, R01-CA092657 (to M.C.)]; the Mesothelioma Applied Research Foundation (MARF) and the Riviera United 4 a CURE (to H.Y.); and the Hawaii Community Foundation’s Fund (to G.G. and H.Y).
References
- 1. Sebbag G, Sugarbaker PH. Peritoneal mesothelioma proposal for a staging system. Eur J Surg Oncol. 2001; 27(3):223-4 [DOI] [PubMed] [Google Scholar]
- 2. Ismail-Khan R, Robinson LA, Williams CC, Jr., Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006; 13(4):255-63 [DOI] [PubMed] [Google Scholar]
- 3. Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009; 20(6):935-44 [DOI] [PubMed] [Google Scholar]
- 4. Carbone M, Albelda SM, Broaddus VC, et al. Eighth international mesothelioma interest group. Oncogene. 2007; 26(49):6959-67 [DOI] [PubMed] [Google Scholar]
- 5. Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008; 135(3):620-6 [DOI] [PubMed] [Google Scholar]
- 6. Kaufman AJ, Pass HI. Current concepts in malignant pleural mesothelioma. Expert Rev Anticancer Ther. 2008; 8(2):293-303 [DOI] [PubMed] [Google Scholar]
- 7. Ardelt W, Mikulski SM, Shogen K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos: homology to pancreatic ribonucleases. J Biol Chem. 1991; 266(1):245-51 [PubMed] [Google Scholar]
- 8. Porta C, Paglino C, Mutti L. Ranpirnase and its potential for the treatment of unresectable malignant mesothelioma. Biologics. 2008; 2(4):601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavlakis N, Vogelzang NJ. Ranpirnase–an antitumour ribonuclease: its potential role in malignant mesothelioma. Expert Opin Biol Ther. 2006; 6(4):391-9 [DOI] [PubMed] [Google Scholar]
- 10. Costanzi J, Sidransky D, Navon A, Goldsweig H. Ribonucleases as a novel pro-apoptotic anticancer strategy: review of the preclinical and clinical data for ranpirnase. Cancer Invest. 2005; 23(7):643-50 [DOI] [PubMed] [Google Scholar]
- 11. Tsai SY, Ardelt B, Hsieh TC, Darzynkiewicz Z, Shogen K, Wu JM. Treatment of Jurkat acute T-lymphocytic leukemia cells by onconase (ranpirnase) is accompanied by an altered nucleocytoplasmic distribution and reduced expression of transcription factor NF-kappaB. Int J Oncol. 2004; 25(6):1745-52 [PubMed] [Google Scholar]
- 12. Ita M, Halicka HD, Tanaka T, et al. Remarkable enhancement of cytotoxicity of onconase and cepharanthine when used in combination on various tumor cell lines. Cancer Biol Ther. 2008; 7(7):1104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck AK, Pass HI, Carbone M, Yang H. Ranpirnase as a potential antitumor ribonuclease treatment for mesothelioma and other malignancies. Future Oncol. 2008; 4(3):341-9 [DOI] [PubMed] [Google Scholar]
- 14. Mikulski SM, Costanzi JJ, Vogelzang NJ, et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol. 2002; 20(1):274-81 [DOI] [PubMed] [Google Scholar]
- 15. Reck M, Krzakowski M, Jassem J, et al. Randomized, multicenter phase III study of ranpirnase plus doxorubicin (DOX) versus DOX in patients with unresectable malignant mesothelioma (MM) [abstract]. J Clin Oncol. 2009;27:15s [Google Scholar]
- 16. Yang H, Bocchetta M, Kroczynska B, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006; 103(27):10397-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010; 107(28):12611-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroczynska B, Cutrone R, Bocchetta M, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci U S A. 2006; 103(38):14128-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li W, Li H, Bocking AD, Challis JR. Tumor necrosis factor stimulates matrix metalloproteinase 9 secretion from cultured human chorionic trophoblast cells through TNF receptor 1 signaling to IKBKB-NFKB and MAPK1/3 pathway. Biol Reprod. 2010; 83(3):481-7 [DOI] [PubMed] [Google Scholar]
- 20. Bertino P, Piccardi F, Porta C, et al. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res. 2008; 14(2):541-8 [DOI] [PubMed] [Google Scholar]
- 21. Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004; 6(3):297-305 [DOI] [PubMed] [Google Scholar]
- 22. Ea CK, Baltimore D. Regulation of NF-kappaB activity through lysine monomethylation of p65. Proc Natl Acad Sci U S A. 2009; 106(45):18972-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways: emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010; 14(1):45-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sartore-Bianchi A, Gasparri F, Galvani A, et al. Bortezomib inhibits nuclear factor-kappaB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007; 13(19):5942-51 [DOI] [PubMed] [Google Scholar]
- 25. Lee I. Ranpirnase (Onconase), a cytotoxic amphibian ribonuclease, manipulates tumour physiological parameters as a selective killer and a potential enhancer for chemotherapy and radiation in cancer therapy. Expert Opin Biol Ther. 2008; 8(6):813-27 [DOI] [PubMed] [Google Scholar]
- 26. Deptala A, Halicka HD, Ardelt B, et al. Potentiation of tumor necrosis factor induced apoptosis by onconase. Int J Oncol. 1998; 13(1):11-6 [DOI] [PubMed] [Google Scholar]
- 27. Goparaju CM, Blasberg JD, Volinia S, et al. Onconase mediated NFKbeta downregulation in malignant pleural mesothelioma. Oncogene. Epub 2011 Feb 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grabarek J, Ardelt B, Du L, Darzynkiewicz Z. Activation of caspases and serine proteases during apoptosis induced by Onconase (ranpirnase). Exp Cell Res. 2002; 278(1):61-71 [DOI] [PubMed] [Google Scholar]
- 29. Johansson N, Ahonen M, Kahari VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000; 57(1):5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13(5):534-40 [DOI] [PubMed] [Google Scholar]
- 31. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2(3):161-74 [DOI] [PubMed] [Google Scholar]
- 32. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25(1):9-34 [DOI] [PubMed] [Google Scholar]
- 33. Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007; 26(3-4):717-24 [DOI] [PubMed] [Google Scholar]
- 34. Ramos-Nino ME, Vianale G, Sabo-Attwood T, et al. Human mesothelioma cells exhibit tumor cell-specific differences in phosphatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther. 2005; 4(5):835-42 [DOI] [PubMed] [Google Scholar]
- 35. Altomare DA, Rybak SM, Pei J, et al. Onconase responsive genes in human mesothelioma cells: implications for an RNA damaging therapeutic agent. BMC Cancer. 2010;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]