Abstract
Introduction
Aromatase, a member of the cytochrome P450 family, converts androgens such as androstenedione and testosterone to estrone and estradiol respectively. Letrozole (1-[bis-(4-cyanophenyl)methyl]-1H-1,2,4-triazole, Femara®) is a high affinity aromatase inhibitor (Ki=11.5 nM) which has FDA approval for breast cancer treatment. Here we report the synthesis of carbon-11 labeled letrozole and its assessment as a radiotracer for brain aromatase in the baboon.
Methods
Letrozole and its precursor (4-[(4-bromophenyl)-1H-1,2,4-triazol-1-ylmethyl]benzonitrile, 3) were prepared in two-step syntheses from 4-cyanobenzyl bromide and 4-bromobenzyl bromide, respectively. The [11C]cyano group was introduced via the tetrakis(triphenylphosphine)palladium(0) catalyzed coupling of [11C]cyanide with the bromo-precursor (3). PET studies in the baboon brain were carried out to assess regional distribution and kinetics, reproducibility of repeated measures and saturability. The free fraction of letrozole in the plasma, log D, and the [11C-cyano]letrozole fraction in the arterial plasma were also measured.
Results
[11C-cyano]Letrozole was synthesized in 60 min with a radiochemical yield of 79–80%, with a radiochemical purity greater than 98% and a specific activity of 4.16±2.21 Ci/μmol at the end of bombardment (n=4). PET studies in the baboon revealed initial rapid and high uptake and initial rapid clearance followed by slow clearance of carbon-11 from the brain with no difference between brain regions. The brain kinetics was not affected by co-injection of unlabeled letrozole (0.1 mg/kg). The free fraction of letrozole in plasma was 48.9% and log D was 1.84.
Conclusion
[11C-cyano]Letrozole is readily synthesized via a palladium catalyzed coupling reaction with [11C]cyanide. Although it is unsuitable as a PET radiotracer for brain aromatase as revealed by the absence of regional specificity and saturability in brain regions, such as amygdala, which are known to contain aromatase, it may be useful in measuring letrozole distribution and pharmacokinetics in brain and peripheral organs.
Keywords: Letrozole (Femara®); PET; Carbon-11 (C-11,11C); Aromatase
Introduction
Aromatase, a member of the cytochrome P450 (CYP450) protein superfamily, is a unique gene product of the CYP19 gene [1]. Aromatase regulates the last step of estrogen biosynthesis, aromatizing the A ring of androgens such as androstenedione and testosterone to estrone and estradiol, respectively. It is expressed in gonadal tissues, such as ovaries [2], but it also occurs in brain and peripheral organs [3]. Brain aromatase is found mainly in the preoptic nucleus and ventromedial nucleus in hypothalamus, medial amygdala, and the bed nucleus of the stria terminalis [4]. Aromatase, along with other estrogen-synthesizing enzymes and receptors, has been implicated in cellular proliferation, sexual behavior, cognition function and neuroprotection [5].
Because of the pivotal role of aromatase in estrogen synthesis, a number of aromatase inhibitors (AI) have been developed as scientific tools for studies of aromatase and estrogen synthesis and as treatment for breast cancer. AIs are also used by body builders who self-administer these drugs to counteract the feminizing effects of excess testosterone [6]. The AI’s fall into two categories: (1) steroidal AI’s such as formestane and exemestane which bind irreversibly to the catalytic site of the enzyme [7] and (2) non-steroidal AI’s such as aminoglutethimide, fadrozole, anastrozole, letrozole, and vorozole whose binding mode is competitive and reversible [7]. Among these, anastrozole, letrozole, and exemestane are considered to be a new generation of FDA-approved AI’s for treating breast cancer because of their potency and selectivity.
Among the AI’s, only vorozole ((S)-6-[(4-chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-1-methyl-1H-benzotriazole, Ki=0.7 nM, [8]) has been labeled with carbon-11 using [11C]methyl iodide and evaluated as a radiotracer for imaging aromatase in brain and peripheral organs in rhesus monkeys [9, 10]. Brain scans revealed an uptake in the amygdala and hypothalamus both of which are rich in aromatase, though signal to noise was low [10]. More recently, carbon-11 labeled sulfonamide derivatives with moderate to excellent aromatase inhibitory potency have been synthesized, though no in vivo studies have been reported [11].
Letrozole (1-[Bis-(4-cyanophenyl)methyl]-1H-1,2,4-triazole, Femara®) is another high affinity inhibitor of aromatase (Ki=11.5 nM, [12]). It has been used extensively as a tool for studies of aromatase and is also approved for breast cancer treatment [13]. Here we report the labeling of letrozole with carbon-11 using tetrakis(triphenylphosphine)palladium(0) catalyzed cyanide coupling with no-carrier-added [11C]hydrogen cyanide, and the evaluation of [11C-cyano]letrozole as a PET radiotracer for imaging brain aromatase in vivo in a female baboon.
1. Material and Methods
1.1. General
All chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO), and used directly without further purification. 1H-NMR and 13C-NMR were obtained in chloroform-d by Bruker Avance 400 MHz NMR spectrometer (400 MHz for 1H and 100 MHz for 13C) (Bruker Instruments Inc., Billerica, MA), and their chemical shifts were measured in part per million downfield from tetramethylsilane. Melting points were measured using Fisher-Jones melting point apparatus (Fisher Scientific Co., Pittsburgh, PA). Thin Layer Chromatography (TLC) with UV detection (254 nm) was used to monitor all organic reactions. Palladium catalyst mediated cyanide labeling reaction was monitored by high performance liquid chromatography (HPLC) with a Knauer HPLC system (Sonntek Inc., Woodcliff Lake, NJ) equipped with model K-500 pump, a model 87 variable wavelength monitor (UV 254 nm), NaI radioactivity detector, and a Hewlett-Packard 3390A integrator. TLC radioactivity was scanned by a Bioscan System 200 Imaging Scanner (Bioscan Inc., Washington DC).
1.2. Chemistry
1.2.1. 1-(4-cyanobenzyl)-1H-1,2,4-triazole (1) and 1-(4-bromobenzyl)-1H-1,2,4-triazole (2)
Compounds 1 and 2 were prepared by an adaptation of the previous method [14]. A mixture solution of 4-cyanobenzyl bromide (1.00 mmol, 0.196 g) or 4-bromobenzyl bromide (1.00 mmol, 0.250 g), potassium carbonate (1.50 mmol, 0.207 g), potassium iodide (0.059 mmol, 0.0098 g), and 1,2,4-triazole (1.50 mmol, 0.104 g) in acetone (12 ml) was refluxed overnight. Saturated sodium carbonate solution was added to the reaction mixture followed by extraction with ethyl acetate. The organic layers were dried by anhydrous magnesium sulfate, filtered, and evaporated. Column chromatography eluting with ethyl acetate afforded 0.146 g and 0.187 g of Compounds 1 and 2 respectively. (80%, 79%) mp 108–110°C (lit: 77–79°C [15], 1), 83°C (lit: 77–79°C [16], 2). 1H NMR (CDCl3, 1): δ5.46 (s, 2H), 7.35–7.37 (d, J=8.4 Hz, 2H), 7.64–7.67 (d, J=8.3 Hz, 2H), 8.01 (s, 1H), 8.26 (s, 1H), 13C NMR (1): δ 152.37, 143.51, 140.01, 132.61, 128.24, 118.16, 112.20, 52.50. 1H NMR (CDCl3, 2): δ 5.30 (s, 2H), 7.12–7.15 (m, 2H), 7.48–7.51 (m, 2H), 7.97 (s, 1H), 8.08 (s, 1H). 13C NMR (2): δ 152.12, 143.05, 133.62, 131.97, 129.46, 122.49, 52.57.
1.2.2. 4-[(4-bromophenyl)-1H-1,2,4-triazol-1-ylmethyl]benzonitrile (3)
Compound 3 was prepared by an adaptation of the previous method [16]. To a slurry of potassium t-butoxide (30.24 mmol, 3.39 g) in 7 ml of anhydrous DMF at −55°C was added 1-(4-bromobenzyl)-1H-[1,2,4]triazole (2, 5.04 mmol, 1.20 g) solution in 7 ml of anhydrous DMF drop by drop. A solution of 4-fluorobenzonitrile (7.56 mmol, 0.92 g) in 7 ml of DMF was added to the reaction mixture at −55°C dropwise, and the reaction was stirred for one hour. The reaction mixture was slowly warmed to −30°C and stirred for one hour. The reaction mixture was quenched by 6 N HCl solution, and basified by saturated sodium bicarbonate solution. The aqueous solution was extracted with ethyl acetate. The organic solution was dried by anhydrous magnesium sulfate, filtered and evaporated. The residual DMF was further removed by high vacuum. The remaining residue was subjected to column chromatography (ethyl acetate:hexane=1:10, 1:5, 1:2, and then 2:1 co-solvent) to give 0.54 g of Compound 3. (32% yield) mp 98–99°C. 1H NMR (CDCl3): δ 6.90 (s, 1H), 7.12–7.14 (d, J=8.44 Hz, 2H), 7.29–7.31 (d, J=8.24 Hz, 2H), 7.50–7.52 (d, J=8.52 Hz, 2H), 7.64–7.66 (d, J=8.40 Hz, 2H), 8.03 (s, 1H), 8.21 (s, 1H). 13C NMR: δ 153.06, 143.80, 142.97, 135.76, 132.99, 132.74, 130.26, 128.78, 123.89, 118.25, 113.06, 66.75.
1.2.3. 1-[Bis-(4-cyanophenyl)methyl]-1H-1,2,4-triazole (Letrozole)
Letrozole was prepared by an adaptation of a previous method [14]. To a slurry solution of potassium t-butoxide (18 mmol, 2.0 g) in 30 ml of anhydrous DMF at room temperature was added Compound 1 (2.0 mmol, 0.37 g) slowly in small portions. The mixture was stirred for 30 minutes at room temperature. To the resultant solution was added 4-fluorobenzonitrile (3.0 mmol, 0.37 g) slowly followed by stirring for one hour. The reaction mixture was diluted into methylene chloride/water (50 ml/50 ml), cooled to 0°C, and neutralized with 6 N HCl. After separation of layers, the aqueous layer was extracted with methylene chloride (50 ml×2). The combined organic phases were washed with brine (50 ml), dried with anhydrous magnesium sulfate, and evaporated. The remaining crude mixture was purified by column chromatography eluting with hexane, ethyl acetate:hexane=1:10, 1:5, 1:2, and then 2:1 co-solvent to afford crude product. The crude product was further purified by recrystallization from ethyl acetate-hexane co-solvent to give 0.27 g of desired product after dryness in vacuo (47% yield) mp 185–186°C (lit: 182–185°C [14]). 1H NMR (CDCl3): δ 6.81 (s, 1H), 7.28–7.30 (d, J=8.24 Hz, 4H), 7.70–7.72 (d, J=8.36 Hz, 4H), 8.07 (s, 1H), 8.09 (s, 1H). 13C NMR: δ 153.06, 143.71, 141.75, 132.98, 128.94, 117.86, 113.37, 66.44.
1.3. Radiolabeling
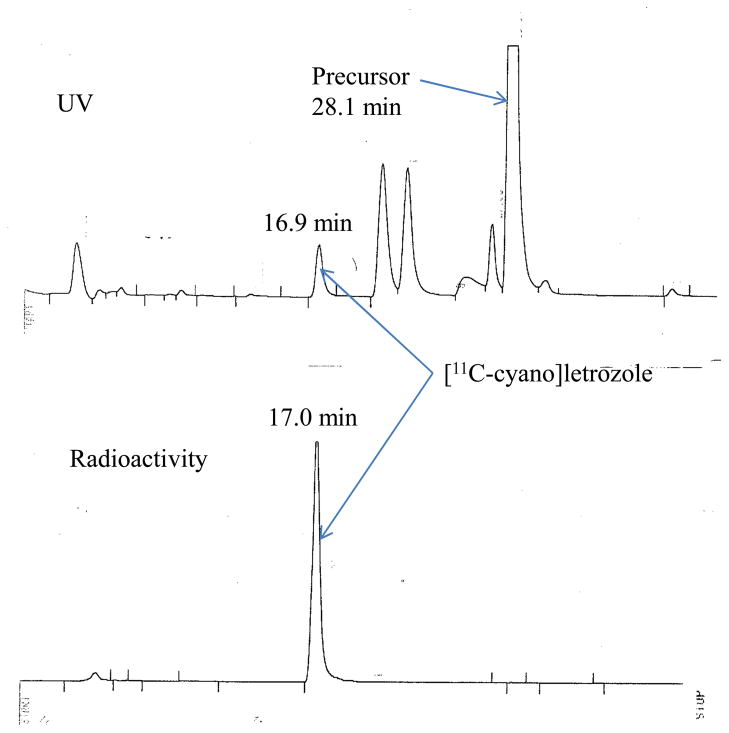
Carbon-11 was generated by proton bombardment of nitrogen gas with a trace of oxygen, and delivered to a home-made [11C]hydrogen cyanide production system as [11C]carbon dioxide. [11C]Carbon dioxide was reduced to [11C]methane by hydrogen with a nickel catalyst at 390°C for 5 minutes. [11C]Methane was passed together with ammonia through a platinum furnace at 920°C to convert into [11C]hydrogen cyanide according to the original procedure [17]. [11C]Hydrogen cyanide was transferred into long neck V-shaped vessel containing 1.0 mg of Compound 3 and 0.68 mg of tetrakis(triphenylphosphine)palladium(0) in 0.30 ml of DMSO. When carbon-11 radioactivity peaked in the reaction vessel as determined by NaI detector, the reaction vessel was sealed, and heated at 110°C for 5 minutes in an oil bath. The reaction mixture was diluted with 1 ml of water, and transferred to a C18 Sep-Pak® (Waters Corp., Milford, MA) which had been pretreated with methanol and water. The product was eluted with 5 ml of ether which was removed by argon flow in a warm water bath for 3 minutes. The residue was diluted with 1 ml of HPLC solvent before injection into the HPLC system. The reaction mixture was eluted with 37% acetonitrile:63% 0.1 M ammonium formate co-solvent at a flow rate of 4.0 ml/min on a Phenomenex Luna C18 (2) semi-preparative column (250 mm×10 mm, 5μm) with UV (254 nm) and radioactivity detection. [11C-cyano]Letrozole was collected around 17.0–17.5 min. After [11C-cyano]letrozole elution, the HPLC column was washed with 60% acetonitrile:40% 0.1 M ammonium formate to remove all side products with long retention times including the bromo-precursor (3). The collected product fraction was transferred to a rotary evaporator, and evaporated with acetonitrile until dryness. The residue was dissolved in 4 ml of 5% ethyl alcohol solution in saline and transferred to a sterile vial after passing through a 0.22μm Millipore® filter (Millipore Corp., Billerica, MA) for PET study. The synthesis time was 60 minutes. The radiochemical yield was 79–80% based on total [11C]hydrogen cyanide activity as determined by analytical HPLC comparing the amount of carbon-11 in the product peak relative to the amount of carbon-11 in the crude reaction mixture that was applied to the C18 Sep-Pak®. The specific activity was 4.16±2.21 Ci/μmol (n=4) at the end of bombardment and was determined as the ratio of the total carbon-11 eluted with the product peak to the mass of letrozole determined by the area under the HPLC peak from a standard curve. HPLC profiles are shown in Figure 2.
Figure 2.
HPLC profile of [11C-cyano]letrozole synthesis showing UV and radioactivity traces.
1.4. Quality control
Both analytical HPLC and TLC were used to determine the radiochemical purity of purified [11C-cyano]letrozole. Quality control was conducted according to a method described previously [18]. An aliquot of [11C]product (10μl) was injected into HPLC system equipped with UV and radioactivity detectors eluting with acetonitrile:0.1 M ammonium formate=1:1 co-solvent at a flow rate of 1.0 ml/min. The retention time was 10.82±1.5 min, and the radiochemical purity was >98%. When the product aliquot (20μl) was co-injected with unlabeled letrozole standard solution (5 μl), the UV and radioactivity profiles were congruent taking into account the time delay between the UV and radioactivity detectors. The radiochemical purity was also determined by TLC co-spotted with unlabeled letrozole standard solution eluting with ethyl acetate as a developing solvent on Macherey-Nagel Polygram Sil G/UV254 plastic back TLC plate with UV detection. Radiochemical purity determined by TLC was also over 98% (n=4, Rf=0.42).
1.5. Determination of log D of [11C-cyano]letrozole
The log D of [11C-cyano]letrozole was measured as described previously [18]. An aliquot (50 μl) of [11C]letrozole was added to a test tube containing 2.5 ml of octanol and 2.5 ml of pH 7.4 phosphate buffer solution. The test tube was vortexed for 2 minutes, and centrifuged for 2 minutes at highest speed. A sample (0.1 ml) was taken from the octanol layer, the other (1.0 ml) sample was taken from the phosphate buffer layer, and they were saved for radioactivity measurement. An aliquot solution (2.0 ml) from the octanol layer was transferred to next test tube containing 0.5 ml of octanol and 2.5 ml of pH 7.4 phosphate buffer solution, and the previous procedure (from vortex to transfer to next test tube) was repeated until six sets of aliquot samples were prepared. The radioactivity of each sample was measured in a well counter (Picker, Cleveland, OH). The log D of each set of sample was derived by the following equation: log D=log (Decay corrected radioactivity in octanol layer×10 / decay corrected radioactivity in phosphate buffer layer)
1.6. PET studies of [11C-cyano]letrozole in baboon
The baboon study was approved by the Brookhaven National Laboratory Institutional Animal Care and Use Committee. A female baboon (Papio anubis) was prepared and anesthetized as previously described [19]. Ketamine hydrochloride (10 mg/kg) was injected intramuscularly into the baboon followed by intubation. The animal was transferred to the PET laboratory in a temperature-maintained transportation cage. The baboon was anesthetized by nitrous oxide (1500 ml/min), oxygen (800 ml/min) and isoflurane (1–4%). Two catheters were inserted, one in the femoral artery for blood sampling and the other in the antecubital vein for radiotracer injection. Vital signs such as heart rate, respiration rate, arterial partial pressure of oxygen, and body temperature were monitored throughout the studies. Two PET studies were performed one month apart with the same baboon to assess the reproducibility and the effect of aromatase inhibition by letrozole. Before the [11C-cyano]letrozole injection, a transmission scan was conducted with a 68Ge rotating rod source. [11C-cyano]Letrozole was administered twice intravenously two hours apart during each study. In the first study, there was no intervention and in the second PET study, letrozole (2 mg, 0.1 mg/kg) dissolved in 8% ethanol in water (10 ml) was co-injected with [11C-cyano]letrozole. Injected doses of [11C-cyano]letrozole ranged from 2.6 mCi to 4.2 mCi (n=4), and specific activity at the time of injection was 0.52±0.28 Ci/μmol (n=4). All studies were performed for 90 minutes with a high-resolution PET (Siemens HR+; 63 slices; 4.5×4.5×4.5 mm at the center of the field of view) in 3D mode with the following time frames (1×10 sec, 12×5 sec, 1×20 sec, 1×30 sec, 8×60 sec, 4×300 sec, 8×600 sec). Arterial blood samples were collected every 2.5 second (OleDich blood sampling machine; Hvidore, Denmark) for 2.5 minutes after [11C-cyano]letrozole injection, and then collected at 5, 10, 20, 30, 60 minutes, and at the end of scan. All blood samples were centrifuged to obtain plasma and counted in a well counter. The plasma samples at 5, 10, 30, 60, and 90 minutes were further analyzed to determine the portion of parent compound.
1.7. HPLC determination of [11C-cyano]letrozole fraction in blood plasma
The fraction of parent compound in plasma was determined according to the procedure described previously [18]. Plasma samples obtained at 5, 10, 30, 60, and 90 minutes were added to each test tube containing acetonitrile (0.3 ml) and letrozole standard solution (20 μl, 1 mg/ml). The sample was counted, homogenized for 10 seconds and centrifuged for 5 minutes. After the supernatant and precipitant were separated, and counted, the sample from the supernatant was injected into HPLC system on a Phenomenex® Spherex C18 analytical column eluting with 35% acetonitrile:65% 50 mM ammonium formate co-solvent at a flow rate of 1.0 ml/min with UV detection. The retention time of [11C-cyano]letrozole was 11 minute. The percent of [11C-cyano]letrozole was obtained as the ratio of [11C-cyano]letrozole isolated from HPLC to the carbon-11 in the supernatant.
1.8. Plasma protein binding (PPB) of [11C-cyano]letrozole
The free fraction of [11C-cyano]letrozole in plasma was obtained by the procedure described previously [18]. An aliquot of [11C]product (10 μl) was added to an 0.8 ml baboon plasma sample. The mixture was incubated for 10 minutes at room temperature. After 20 μl of sample was taken from the mixture for measurement of total activity (AT, AT=Abound+Aunbound), 0.2 ml of sample was placed into the upper part of the Centrifree® tube (Amicon Inc., Beverly, MA), and centrifuged for 10 minutes. After the upper part of Centrifree® tube was discarded, the aliquot (20 μl) from bottom part of the tube was taken for the measurement of radioactivity of unbound sample (Aunbound). PPB was derived by the following equation: % unbound=Aunbound×100/AT.
1.9. Image and data analysis
All time frames were summed over 90 minutes of scanning time and planes were summed in groups of two to select regions of interest (ROI) in the brain. ROIs (cerebellum, striatum, thalamus, and amygdala) were chosen on the summed image, and then projected on to the dynamic frames to obtain time-activity curves (TAC’s). The radioactivity (nCi/cc) was converted to percent injected dose per cc (%dose/cc) by dividing by the injected dose and multiplying by 100. Baseline time activity curves were compared to the repeated baseline and to the letrozole pretreatment scans for the two studies.
2. Results and discussion
2.1. Chemistry and Carbon-11 Labeling
Letrozole and its bromo-precursor (3) were prepared by the scheme shown in Figure 1. Those two compounds were synthesized by two steps: alkylation of triazole followed by nucleophilic aromatic substitution after carbanion generation. This was a modification of the literature and patent procedures [14, 16].
Figure 1.
Synthetic scheme of letrozole and bromo-precursor (3) and for [11C-cyano]letrozole via palladium(0)-mediated carbon-11 cyanide coupling
Though this procedure worked well for the synthesis of letrozole, the carbanion of Compound 2 generated by treatment of potassium tert-butoxide was unstable at temperatures greater than 0°C. After optimization of base amount and temperature, we could generate the corresponding carbanion of Compound 2 by treatment with 6 eq. of potassium tert-butoxide at −55°C, carrying out the reaction at −55°C and −30°C for one hour after the addition of 4-fluorobenzonitrile.
Palladium catalyzed cyanide coupling was first applied to carbon-11 synthesis with [11C]cyanide by Andersson and Långström using tetrakis(triphenylphosphine)palladium(0), aryl tricarbonylchromium complex, or both catalysts [20]. Since then, there have been several examples of the use of this method for the introduction of the [11C]cyano group into organic compounds [21] using a number of different catalysts [22]. For example, it has been accomplished using a copper catalyst using the Rosenmund-von Brown reaction [22]. Sandell et. al. prepared [11C]NAD-299 by labeling with [11C]cyanide using Tris(dibenzylideneacetone) dipalladium(0) ([Pd2(dba)3]), 1,1′-bis(diphenylphosphino)ferrocene (dppf), and N-methyl-2-pyrrolidinone (NMP) [23]. We used tetrakis(triphenylphosphine)palladium(0) because this method had been shown to provide the best results with bromo- and iodo-substrates [20].
As reported previously [20], [11C]cyanide could be trapped in substrate and tetrakis(triphenylphospine)palladium(0) mixture solution at room temperature without any base. Ammonia, hydrogen and other carrier gas did not interfere with the reaction. We used 0.25 eq. of palladium catalyst relative to the bromo-precursor (3) with no-carrier-added [11C]cyanide and observed a rapid coupling reaction in DMSO at 110°C. DMSO proved to be the best solvent giving 79–80% radiochemical yield compared to THF, diglyme, and DMF which gave yields 29%, 43%, and 58% respectively.
After the radiolabeling reaction, the crude reaction mixture was passed through a C18 Sep-Pak® solid phase extraction column to remove palladium catalyst prior to HPLC injection. The total synthesis time was 60 minutes from end of bombardment. The HPLC profile is shown in Figure 2. Most of the carbon-11 eluting from the HPLC was [11C-cyano]letrozole (Rt=17.04 min). The UV peak from letrozole (Rt=16.90 min) was also observed. The difference in retention times occurs because the eluent passes through the UV detector before the radio-detector. The specific activity was 4.16±2.21 Ci/μmol (n=4) at the end of cyclotron bombardment. Log D derived from partitioning of [11C-cyano]letrozole between octanol and pH 7.4 phosphate buffer solution was 1.84.
2.2. Evaluation of [11C-cyano]letrozole with PET in the baboon brain
[11C-cyano]Letrozole was evaluated for reproducibility of repeated studies on the same day and for specificity in the anesthetized female baboon. In the reproducibility study, two consecutive baseline scans were conducted two hours apart after injection of [11C-cyano]letrozole. In the specificity/saturability study, co-administration with [11C-cyano]letrozole and unlabeled letrozole (0.1 mg/kg, dose was determined according to [24, 25]) was performed after the baseline scan was finished. In both sets of scans, time-activity curves (TAC’s) for [11C-cyano]letrozole in the arterial plasma were measured.
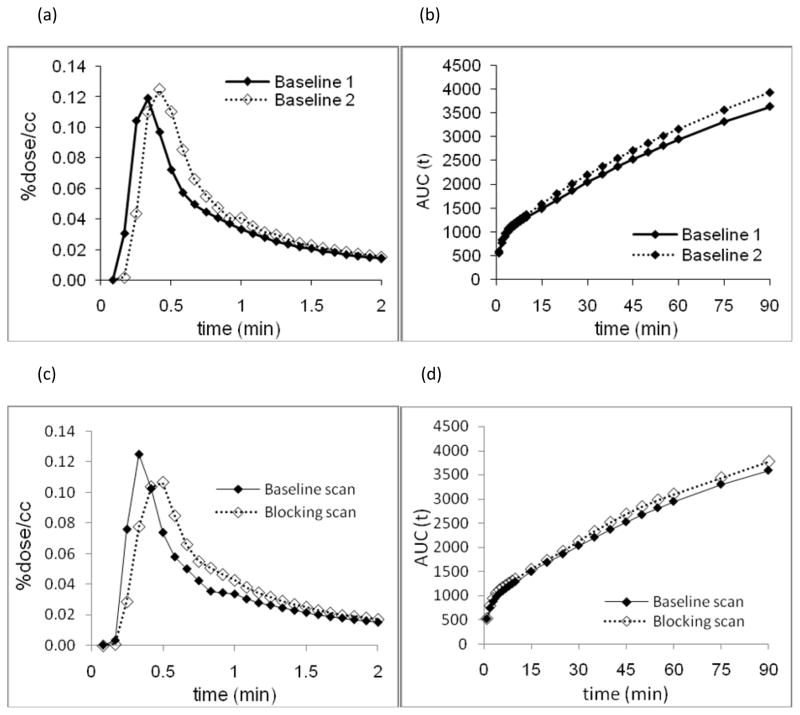
The TAC’s of plasma from the test-retest studies and the baseline-blocking studies were compared (Figure 3(a) and 3(c)). In both cases, the peak time in the plasma for the second study (i.e., the retest study and the blocking study) was slightly delayed and the integral over the time course of the second study was slightly elevated. The effect was small; however, it may be related to a different physiological state of the anaesthetized animal or slight differences in injection rate. The unbound fraction of [11C-cyano]letrozole in plasma was 48.9% and the fraction of carbon-11 in the plasma in the chemical form of [11C-cyano]letrozole was very high (99.2±0.3%, 99.0±0.3%, 98.2±0.3%. 96.9±1.1%, and 95.6±2.6% at 5, 10, 30, 60, and 90 minutes (n=4), respectively).
Figure 3.
(a) TAC’s of plasma from two baseline studies for the first two minutes. (b) Area under the curve (AUC) of [11C-cyano]letrozole concentration in plasma over a 90 minute experiment for the two repeated baseline scans. (c) TAC’s of plasma from baseline study and blocking study (0.1 mg/kg of unlabeled letrozole co-administration) for the first two minutes (d) AUC of [11C-cyano]letrozole concentration in plasma over a 90 minute experiment at baseline and after letrozole treatment.
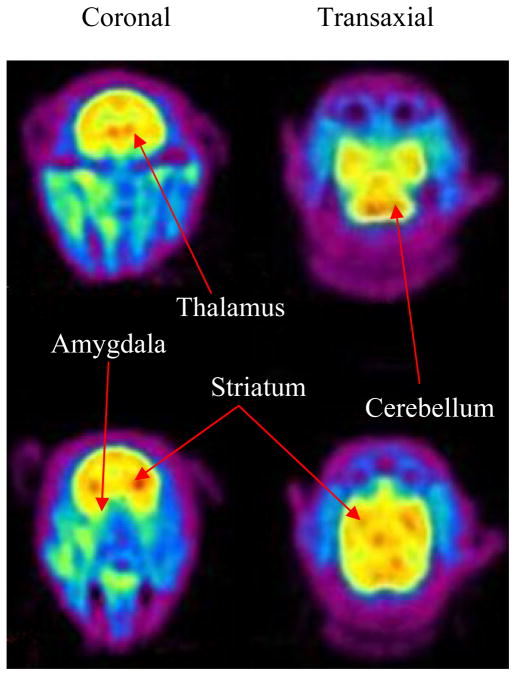
Representative PET images from the baseline scan are shown in Figure 4. The summed image revealed high carbon-11 uptake in all brain regions such as thalamus, striatum, and cerebellum. It is likely that the carbon-11 in the brain is in the form of [11C-cyano]letrozole based on the absence of labeled metabolites in the plasma.
Figure 4.
Summed frames PET images of baboon brain (0–90 min) from dynamic baseline scan. Carbon-11 uptake was observed in thalamus, striatum, and cerebellum.
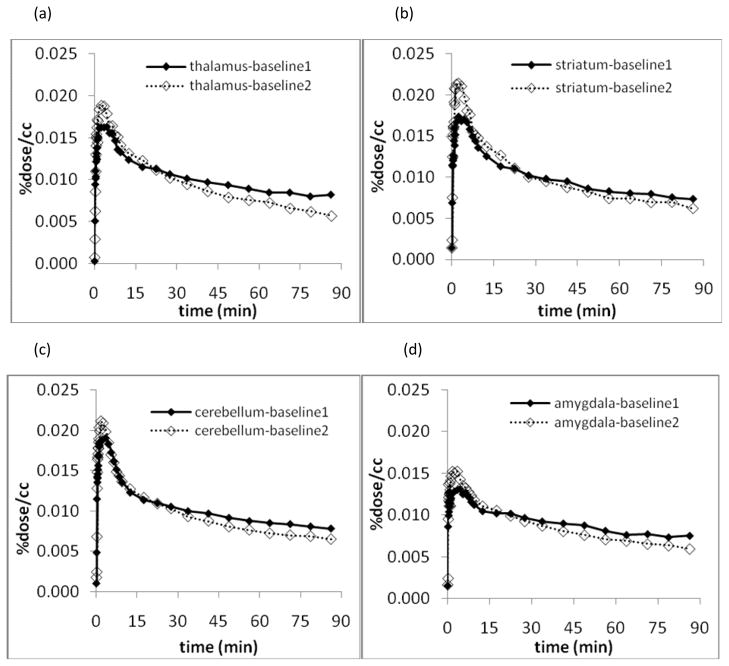
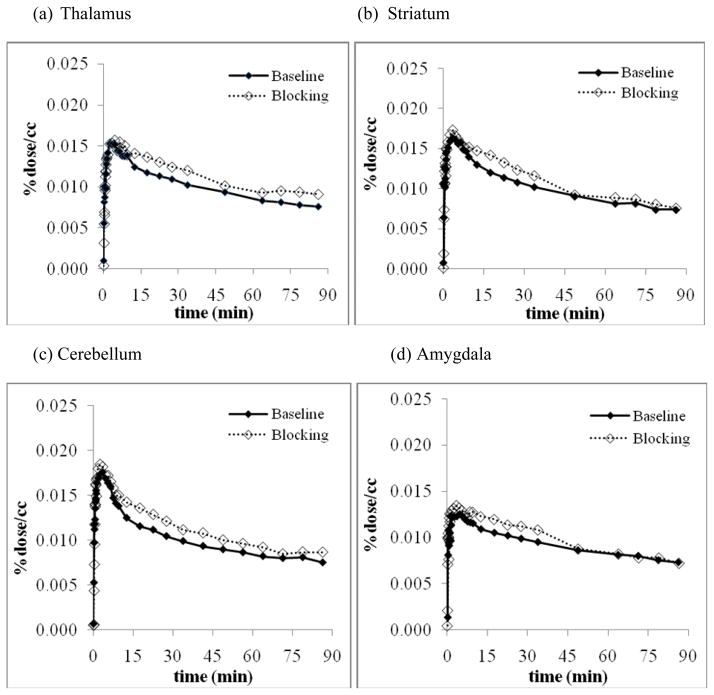
TAC’s for different brain regions were similar to each other with rapid initial uptake peaking at ~5 minutes followed by an initial rapid clearance and a very slow clearance from 15–90 minutes. (Figure 5) There was no enhanced accumulation in the amygdala. In the studies of reproducibility, there were some initial differences during the first few minutes but the TAC’s for different brain regions were generally similar with slight increase in clearance rate in the second scan on each day which may be due to a difference in physiological state of the animal after prolonged anesthesia. (Figure 5)
Figure 5.
Comparison of first and second dynamic scan for reproducibility in thalamus (a), striatum (b), cerebellum (c), and amygdala (d)
Co-administration of unlabeled letrozole produced global elevation in the TAC’s for different brain regions. Although there are some differences in peak uptake, these TAC’s displayed similar profiles throughout the scan times with no distinctive changes in the brain regions. (Figure 6) Lack of accumulation of [11C-cyano]letrozole in brain regions rich in aromatase and absence of regionally specific changes with co-administration of letrozole indicate that [11C-cyano]letrozole will not be a useful tracer for brain aromatase. This contrasts with previous studies of [11C]vorozole which report that amygdala and to a lesser extent hypothalamus were visualized and blocked with aromatase inhibition [10]. This may reflect the higher affinity of vorozole as compared to letrozole (0.7 nM vs 11.5 nM for human placental tissue, respectively [8, 12]).
Figure 6.
TAC’s from baseline study and blocking studies for thalamus (a), striatum (b), cerebellum (c), and amygdala (d). There was some movement of the head during the period 35–60 min necessitating deletion of two time frames for the small ROI’s (striatum, thalamus, and amygdala).
3. Conclusion
We synthesized [11C-cyano]letrozole using [11C]hydrogen cyanide by palladium-mediated cyanide coupling reaction. The radiochemical yield was critically dependant on solvent used. Our PET studies clearly showed that letrozole penetrated the blood brain barrier; however, there was no elevated accumulation of [11C-cyano]letrozole in brain regions rich in aromatase and no change with co-administration of letrozole. This limits its utility for imaging and quantification of brain aromatase. However, since letrozole is an approved drug for use for breast cancer treatment [13], labeled drugs such as [11C-cyano]letrozole may be useful for planning treatment where the drug distribution and pharmacokinetics can be measured.
Acknowledgments
This work was performed at the Brookhaven National Laboratory under contract DE-AC02-98CH10886 with Department of Energy and supported by its Office of Biological and Environmental Research and by the NIH K05DA020001, and also in part by Deutscher Akademischer Austausch Dienst (DAAD), Bonn, for a student fellowship for Andre Fischer. The authors thank to Donald Warner for PET operations, and Pauline Carter and Payton King for performance of baboon studies. The authors also thank to Colleen Shea, Youwen Xu, and Lisa Muench for performance of in-vitro studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danielson PB. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002;3:561–97. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 2.Kragie L. Aromatase in primate pregnancy: a review. Endocr Res. 2002;28:121–8. doi: 10.1081/erc-120015041. [DOI] [PubMed] [Google Scholar]
- 3.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase-A brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 4.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–23. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 5.Roselli CE. Brain aromatase: Roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106:143–50. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–54. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 7.Budzar A, Howell A. Advances in aromatase inhibition: Clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. 2001;7:2620–35. [PubMed] [Google Scholar]
- 8.Vanden Bossche H, Willemsens G, Roels I, Bellens D, Moereels H, Coene M-C, Le Jeune L, Lauwers W, Janssen PAJ. R76713 and enantiomers: selective nonsteroidal inhibitors of the cytochrome P450-dependent oestrogen synthesis. Biochem Pharmacol. 1990;40:1707–18. doi: 10.1016/0006-2952(90)90346-m. [DOI] [PubMed] [Google Scholar]
- 9.Lidström P, Bonasera TA, Kirilovas D, Lindblom B, Lu L, Bergström E, Bergström M, Westlin J-E, Långström B. Synthesis, in vivo rheus monkey biodistribution and in vitro evaluation of a 11C-labelled potent aromatase inhibitor: [N-methyl-11C]vorozole. Nucl Med Biol. 1998;25:497–501. doi: 10.1016/s0969-8051(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Bergström M, Frändberg P, Vesström E-L, Watanabe Y, Långström B. Imaging of aromatase distribution in rat and rhesus monkey brains with [11C]vorozole. Nucl Med Biol. 2006;33:599–605. doi: 10.1016/j.nucmedbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Lacy G, Gao M, Miller KD, Sledge GW, Zheng Q-H. Synthesis of carbon-11 labeled sulfoanilide analogues as new potential PET agents for imaging of aromatase in breast cancer. Bioorg Med Chem Lett. 2007;17:332–6. doi: 10.1016/j.bmcl.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Bhatnagar AS, Häusler A, Schieweck K, Lang M, Bowman R. highly selective inhibition of estrogen biosynthesis by CGS 20267, a new non-steroidal aromatase inhibitor. J Steroid Biochem Mol Biol. 1990;37:1021–7. doi: 10.1016/0960-0760(90)90460-3. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, Johnson JR, Li N, Chen G, Pazdur R. Approval summary: Letrozole in the treatment of postmenopausal women with advanced breast cancer. Clin Cancer Res. 2002;8:665–9. [PubMed] [Google Scholar]
- 14.Wood PM, Woo LWL, Humphreys A, Chander SK, Purohit A, Reed MJ, Potter BVL. A letrozole-based dual aromatase-sulphatase inhibitor with in vivo activity. J Steroid Biochem Mol Biol. 2005;94:123–30. doi: 10.1016/j.jsbmb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Bowman RM, Steele RE, Browne L. US 4,978,672 Alpha-heterocyclic substituted tolunitriles. 1990
- 16.Lang M, Differding E, Stanek J. US 5,227,393 α-fluoro-α-tetrazolyl-phenylmethyl derivatives useful as aromatase inhibitors. 1993
- 17.Christman DR, Finn RD, Karlstrom KI, Wolf AP. The production of ultra high activity 11C-labeled hydrogen cyanide, carbon dioxide, and methane via the 14N(p,α)11C reaction (XV) Int J Appl Radiat Isot. 1975;26:435–42. [Google Scholar]
- 18.Kil K-E, Ding Y-S, Lin K-S, Alexoff D, Kim SW, Shea C, Xu Y, Muench L, Fowler JS. Synthesis and positron emission tomography studies of carbon-11-labeled imatinib (Gleevec®) Nucl Med Biol. 2007;34:153–63. doi: 10.1016/j.nucmedbio.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Y-S, Fowler JS, Volkow ND, Logan J, Gatley SJ, Sugano Y. Carbon-11-D-threo-methylphenidate binding to dopamine transporter in baboon brain. J Nucl Med. 1995;36:2298–305. [PubMed] [Google Scholar]
- 20.Andersson Y, Långström B. Transition metal-mediated reactions using [11C]cyanide in synthesis of 11C-labelled aromatic compounds. J Chem Soc Perkin Trans I. 1994:1395–400. [Google Scholar]
- 21.Balatoni JA, Adam MJ, Hall LD. Synthesis of 11C-labeled aromatics using aryl chromium tricarbonyl intermediates. J Labelled Compd Radiopharm. 1999;26:159–64. [Google Scholar]
- 22.Ponchant M, Hinnen F, Demphel S, Crouzel C. [11C]Copper(I) cyanide: a new radioactive precursor for 11C-cyanization and functionalization of haloarenes. Appl Radiat Isot. 1997;48:755–62. [Google Scholar]
- 23.Sandell J, Halldin C, Hall A, Thorberg S-O, Werner T, Sohn D, Sedvall G, Farde L. Radiosynthesis and autoradiographic evaluation of [11C]NAD-299, a radioligand for visualization of 5-HT1A receptor. Nucl Med Biol. 1999;26:159–64. doi: 10.1016/s0969-8051(98)00091-2. [DOI] [PubMed] [Google Scholar]
- 24.Sioufi A, Sandrenan N, Godbillon J, Trunet P, Czendlik C, Howald H, Pfister CH, Ezzet F. Comparative bioavailability of letrozole under fed and fasting conditions in 12 healthy subjects after a 2. 5 mg single oral administration. Biopharm Drug Dispos. 1997;18:489–97. doi: 10.1002/(sici)1099-081x(199708)18:6<489::aid-bdd36>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Sioufi A, Gauducheau N, Pineau V, Marfil F, Jaouen A, Cardot JM, Godbillon J, Czendlik C, Howald H, Pfister CH, Vreeland F. Absolute bioavailability of letrozole in healthy postmenopausal women. Biopharm Drug Dispos. 1997;18:779–89. doi: 10.1002/(sici)1099-081x(199712)18:9<779::aid-bdd64>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]