Propofol is an intravenous (IV) sedative hypnotic which is commonly used as a bolus for the induction of general anesthesia. It is also used as an infusion for continuous sedation and as an adjunct for general anesthesia. The main advantage of propofol, as compared with other IV sedatives, is that it has an ultra-short half-life. This allows for a relatively rapid awakening.[1,2] Furthermore, propofol is devoid of the side effects of both nausea and vomiting. In addition, continuous low-dose infusions have been successfully utilized to treat recalcitrant nausea.[3]
Fospropofol is a water-soluble prodrug of propofol.[4,5] These two features give fospropofol clinical advantages, as well as disadvantages, with respect to propofol. Specifically, its water solubility allows it to be manufactured without egg lecithin, soy bean extract, or glycerol. Note that these are used as diluents with propofol. Furthermore, these additional ingredients may support microbial growth despite the presence of antimicrobial preservatives.[6–8] Moreover, administration of propofol to patients allergic to any of these additives is contraindicated. Hypertriglyceridemia, presumably from these diluents, has also been reported.[9,10] Of note, a lipid-free propofol formulation is currently being developed and investigated.[11]
Additionally, the current preparation of propofol produces pain on injection. This frequently requires the concomitant use of such medications as lidocaine, or ketamine, as analgesics.[12–15] It should be noted that ketamine has both local and general anesthetic properties.
In addition, propofol is lipophilic and is prepared as an emulsion. Thus, prolonged storage can lead to separation or “cracking” of the emulsion.[16,17] Propofol is also difficult to manufacture and numerous recalls, as well as shortages, of this drug have occurred.[18]
As shown in Figure 1, propofol is based upon phenol, with the addition of two isopropyl side chains, which are located at positions 2 and 6.
Figure 1.
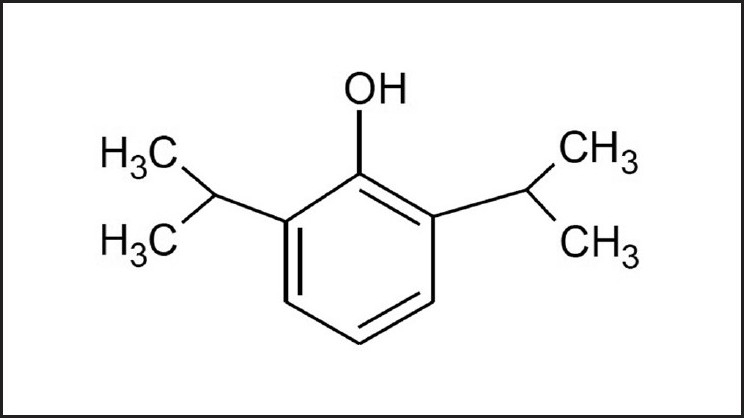
The lipophilic chemical structure of propofol
However, fospropofol, as shown in Figure 2, is a water-soluble salt which utilizes phosphate and sodium ions.
Figure 2.
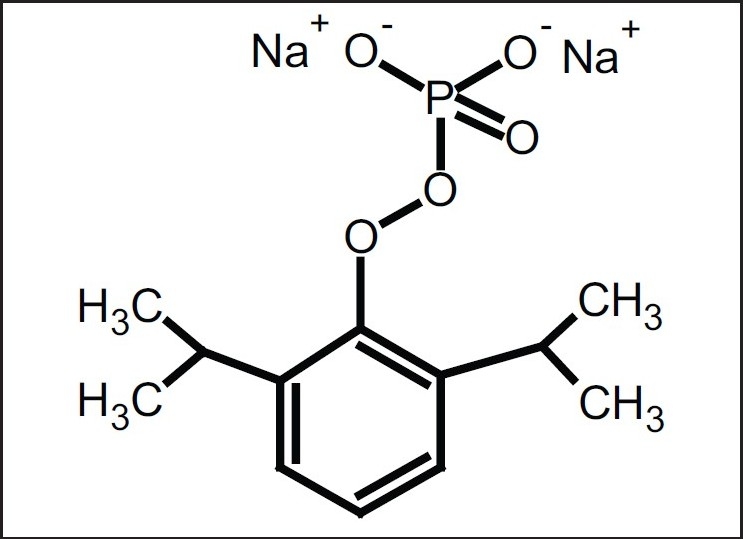
Fospropofol is a disodium-phosphate water-soluble salt which is enzymatically degraded to propofol by alkaline phosphatases
Since fospropofol is a prodrug, a delay in peak onset occurs following the administration of a single dose. Using a 10 mg/ kg IV bolus, the median time to sedation was reported as approximately 7 minutes with a range of 1 to 15 minutes.[4] This is in contradistinction to propofol which has a near-immediate effect.[2] Because of this, propofol is frequently used for rapid-sequence induction of general anesthesia, whereas this would not be possible with fospropofol. Furthermore, the time to awakening with fospropofol was reported within a range of 21 to 45 minutes; although still short, this is significantly longer than propofol.[4] Somewhat similar dose-response and pharmacodynamic properties have been reported in other clinical studies.[19,20] In addition, the major side effects of fospropofol are pruritus as well as paresthesias. These are transient.[4,18–21]
Alkaline phosphatases enzymatically degrade fospropofol to propofol, with formaldehyde, formate, and phosphate produced as metabolic byproducts.[4] These are illustrated in Figure 3. Of note, formate is the anion of formic acid which is responsible for the itching produced from insect venom. This may explain fospropofol's side effect of pruritus. However, the resultant blood formaldehyde levels are not considered clinically significant.
Figure 3.
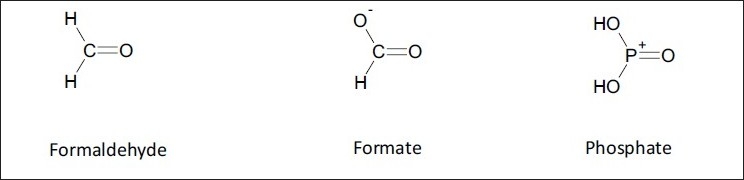
Formaldehyde, formate, and phosphate are also produced as fospropofol is metabolized
Currently, fospropofol is only administered in intermittent IV boluses. However, a continuous infusion of this drug would result in a more stable level of sedation. Furthermore, the potential for excessive propofol levels from an IV bolus may be reduced with a carefully administered continuous infusion. Lastly, the use of syringe pumps, or other similar technology, would be more convenient than repetitive bolus administration.
To accomplish this, a “target dose” of propofol from the administration of fospropofol would need to be determined. This would result from a “propofol equivalent” regimen of fospropofol dosing. An understanding of the gram-molar relationship between fospropofol and propofol is therefore necessary. Explicitly, one mole of fospropofol produces one mole of propofol.[4] This is in contradistinction to an “approximate milligram equivalence” which is typically used when comparing similar-acting medications. Furthermore, equimolar dosing is frequently utilized when establishing bioequivalence.[22]
Thus, the number of moles for a given amount of drug is a direct representation of its associated number of molecules. To further illustrate the concept of equimolar dosing, the number of moles of propofol produced from an infusion of 100 mg/(kg·min), is:[23]
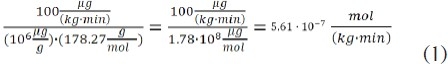
Note that the molecular weight of propofol is 178.27 g/ mole. However, the molecular weight of fospropofol is 332.24 g/ mole. Based upon the molar dose from equation (1), the dose of fospropofol to produce an equimolar amount of propofol is as follows:
![]()
Thus, in terms of producing an equivalent number of moles of propofol:
![]()
Furthermore, the above ratio in equation (3) remains valid for other “target dosages” of propofol which would be produced from an infusion of fospropofol:

It should also be noted that fospropofol is manufactured in a more concentrated form than propofol. Specifically, fospropofol has a concentration of 35 mg/ml, whereas the concentration of propofol is 10 mg/ml. Thus, the net flow rate for a fospropofol infusion is significantly less than that of an equimolar propofol infusion. This occurs despite the greater molecular weight of fospropofol.
As an example, for a propofol dose of 100 mg/(kg·min), the net flow rate, per kg body weight, is:
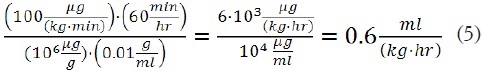
However, the flow rate per kg body weight for an equimolar dose of fospropofol is:
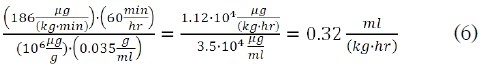
Further research is necessary to clinically evaluate the benefits and limitations of a “propofol equivalent” dosing regimen of a continuous infusion of fospropofol. This would include blood levels of propofol, formaldehyde, phosphate, and formate which are produced from the metabolism of fospropofol.
Appendix: Numerical Examples
Example 1. It is desired to administer fospropofol based upon a molar equivalent of 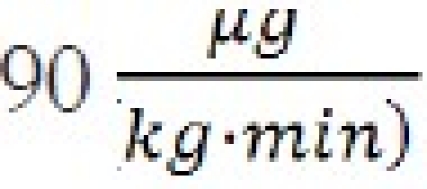 of propofol. Using Equation (4):
of propofol. Using Equation (4):
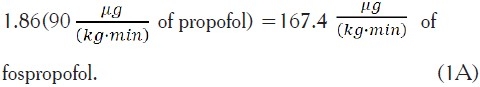
Based upon Equation (6), the associated flow rate per kg would be:
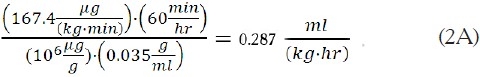
For a 70 kg patient, this would correspond to a flow rate of:
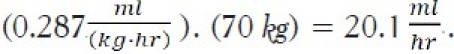
Example 2. It is desired to administer fospropofol using the molar equivalent of a propofol dose of 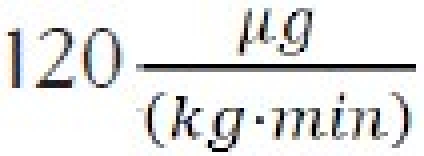 120. This would correspond to:
120. This would correspond to:

Based upon Equation (6), the associated flow rate per kg would be:
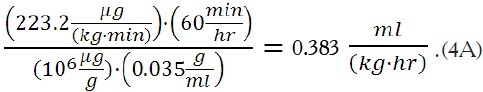
For a 70 kg patient, this would correspond to a flow rate of:
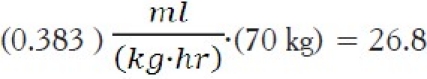
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82. doi: 10.1097/00000542-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: A multicenter study. Anesthesiology. 2000;92:727–38. doi: 10.1097/00000542-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Kim SI, Han TH, Kil HY, Lee JS, Kim SC. Prevention of postoperative nausea and vomiting by continuous infusion of subhypnotic propofol in female patients receiving intravenous patient-controlled analgesia. Br J Anaesth. 2000;85:898–900. doi: 10.1093/bja/85.6.898. [DOI] [PubMed] [Google Scholar]
- 4.Woodcliff Lake, NJ, USA: Eisai corp; 2009. Lusedra© (fospropofol disodium) package insert.Fospropofol prescribing information. [Google Scholar]
- 5.Bengalorkar GM, Bhuvana K, Sarala N, Kumar TN. Fospropofol: Clinical pharmacology. J Anaesth Clin Pharmacol. 2011;27:79–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehnert MJ, Webb RM, Jochimsen EM, Hancock GA, Arduino MJ, Hand S, et al. Staphylococcus aureus bloodstream infections among patients undergoing electroconvulsive therapy traced to breaks in infection control and possible extrinsic contamination by propofol. Anesth Analg. 1997;85:420–5. doi: 10.1097/00000539-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Wachowski I, Jolly DT, Hrazdil J, Galbraith JC, Greacen M, Clanachan AS. The growth of microorganisms in propofol and mixtures of propofol and lidocaine. Anesth Analg. 1999;88:209–12. doi: 10.1097/00000539-199901000-00039. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Hirose M, Mikami S, Takamura K, Goi T, Yamaguchi A, et al. Effect of anaesthesia maintained with sevoflurane and propofol on surgical site infection after elective open gastrointestinal surgery. J Hosp Infect. 2010;74:129–36. doi: 10.1016/j.jhin.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Knibbe CA, Naber H, Aarts LP, Kuks PF, Danhof M. Long-term sedation with propofol 60 mg/ml vs.propofol 10 mg/ml in critically ill, mechanically ventilated patients. Acta Anaesthesiol Scand. 2004;48:302–7. doi: 10.1111/j.1399-6576.2004.0339.x. [DOI] [PubMed] [Google Scholar]
- 10.Coetzee A, Blaine EM, Labadarios D, Schall R, Haus M. Effect of 1% and 2% propofol on blood lipids during long-term sedation. S Afr Med J. 2002;92:911–6. [PubMed] [Google Scholar]
- 11.Mohite SN, Kudalkar AG, Dalvi NP. A comparative study of premixed lipid-free propofol and lignocaine vs lipid emulsion of propofol in conscious sedation. J Anaesth Clin Pharmacol. 2010;26:303–6. [Google Scholar]
- 12.Lee P, Russell WJ. Preventing pain on injection of propofol: A comparison between lignocaine pre-treatment and lignocaine added to propofol. Anaesth Intensive Care. 2004;32:482–4. doi: 10.1177/0310057X0403200432. [DOI] [PubMed] [Google Scholar]
- 13.Tan CH, Onsiong MK, Kua SW. The effect of ketamine pretreatment on propofol injection pain in 100 women. Anaesthesia. 1998;53:302–5. doi: 10.1046/j.1365-2044.1998.00287.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner LE, 2nd, Gingrich KJ, Kulli JC, Yang J. Ketamine blockade of voltage-gated sodium channels: Evidence for a shared receptor site with local anesthetics. Anesthesiology. 2001;95:1406–13. doi: 10.1097/00000542-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Tverskoy M, Oren M, Vaskovich M, Dashkovsky I, Kissin I. Ketamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: A study in postoperative patients. Neurosci Lett. 1996;215:5–8. doi: 10.1016/s0304-3940(96)12922-0. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Davis SS, Washington C. Physical properties and stability of two emulsion formulations of propofol. Int J Pharm. 2001;215:207–20. doi: 10.1016/s0378-5173(00)00692-x. [DOI] [PubMed] [Google Scholar]
- 17.Chernin EL, Smiler B. Any propofol compatibility study must include an emulsion stability analysis. Anesth Analg. 2000;91:1307–8. doi: 10.1097/00000539-200011000-00052. [DOI] [PubMed] [Google Scholar]
- 18.Jensen V, Rappaport BA. The reality of drug shortages — the case of the injectable agent propofol. N Engl J Med. 2010;363:806–7. doi: 10.1056/NEJMp1005849. [DOI] [PubMed] [Google Scholar]
- 19.Gan TJ, Berry BD, Ekman EF, Muckerman RC, 2nd, Shore N, Hardi R. Safety evaluation of fospropofol for sedation during minor surgical procedures. J Clin Anesth. 2010;22:260–7. doi: 10.1016/j.jclinane.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Cohen LB. Clinical trial: A dose-response study of fospropofol disodium for moderate sedation during colonoscopy. Aliment Pharmacol Ther. 2008;27:597–608. doi: 10.1111/j.1365-2036.2008.03598.x. [DOI] [PubMed] [Google Scholar]
- 21.Garnock-Jones KP, Scott LJ. Fospropofol. Drugs. 2010;70:464–77. doi: 10.2165/11204450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Birkett DJ. Generics-equal or not? Aust Prescr. 2003;26:85–7. [Google Scholar]
- 23.Amiji MM, Sandmann BJ, editors. New York: McGraw-Hill; 2003. Applied physical pharmacy. [Google Scholar]


