Abstract
Human blinding disorders are often initiated by hereditary mutations that insult rod and/or cone photoreceptors and cause subsequent cellular death. Generally, the disease phenotype can be predicted from the specific mutation as many photoreceptor genes are specific to rods or cones; however certain genes, such as Retinal Degeneration Slow (RDS), are expressed in both cell types and cause different forms of retinal disease affecting rods, cones, or both photoreceptors. RDS is a transmembrane glycoprotein critical for photoreceptor outer segment disc morphogenesis, structural maintenance, and renewal. Studies using animal models with Rds mutations provide valuable insight into Rds gene function and regulation; and a better understanding of the physiology, pathology, and underlying degenerative mechanisms of inherited retinal disease. Furthermore, these models are an excellent tool in the process of developing therapeutic interventions for the treatment of inherited retinal degenerations. In this paper, we review these topics with particular focus on the use of rds models in gene therapy.
70.1 Introduction
Inherited retinal degenerations can be caused by mutations in over 100 different genes, and in many cases, the structure, function, and regulation of these genes are well documented. Among them, the RDS (retinal degeneration slow, also known as peripherin/rds or prph2) gene is an important target of study because: (1) over 80 different disease causing mutations have been identified in RDS (http://www.retinainternational.com/sci-news/rdsmut.htm), (2) these mutations account for a substantial fraction of inherited retinal diseases, and (3) the variety in RDS-associated disease phenotypes provides critical insight into the biological function of rod and cone photoreceptors (Boon et al. 2008).
RDS is located in the rim region of rod and cone outer segment (OS) discs and is critical for OS disc formation, orientation, renewal and structural stability (Connell et al. 1991; Molday et al. 1987). Interestingly though, the protein has been shown to play different roles in rod vs. cone OS morphogenesis (Farjo et al. 2006b; Nour et al. 2004).
70.2 Diseases Associated with RDS Mutations
Disease causing mutations in the human RDS gene were first reported in 1991 and were associated with an autosomal dominant retinitis pigmentosa (adRP) phenotype (Farrar et al. 1991; Kajiwara et al. 1991). Further investigation found that different mutations in the RDS gene cause not just adRP, but a wide spectrum of both cone- and rod-dominant retinal diseases including a variety of macular dystrophies, cone and cone-rod dystrophy, central areolar chroidal dystrophy, retinitis punctata albescens, and autosomal recessive Stargardt disease (Boon et al. 2008). While the phenotypes of RDS-associated diseases vary in severity, age of onset, and clinical presentation, most lead to debilitating blindness, and all are currently uncurable.
70.3 Current Animal Models
Animal models carrying Rds mutations are of great value in the investigation of the physiology and pathology of RDS-associated disease and for the establishment of potential therapies. The initial Rds animal model to be characterized was the rds mouse which has been studied for over 30 years (Farjo and Naash 2006; van Nie et al. 1978). This mouse contains a spontaneous 9 kb insertion into exon II of the mouse Rds gene and produces two large and stable Rds messages that cannot be translated to protein. The heterozygous mouse (rds+/−) has a striking adRP-like haploinsufficiency phenotype characterized by malformed OSs, a deficit in ERG function, and a progressive, slow loss of rods and cones (Cheng et al. 1997; Nour et al. 2004; Stricker et al. 2005). The homozygous mouse (rds−/−) does not form OSs and has little or no detectable ERG function accompanied by retinal degeneration (Farjo and Naash 2006). So far, several other animal models carrying deletion or missense mutations in Rds on either a wild-type (WT) or rds−/− background have been studied including nmf193 (Nystuen et al. 2008), P216L (Kedzierski et al. 1997), C214S (Nour et al. 2008; Stricker et al. 2005) and R172W (Conley et al. 2007; Ding et al. 2004). All these mice exhibit photoreceptor degeneration, abnormalities in OS structure, and reduction in ERG amplitudes. The mutant mice frequently share similar phenotypes to their human counterparts; e.g. C214S-RDS mice show an adRP like phenotype (Stricker et al. 2005) while R172W-RDS mice display a macular dystrophy phenotype (Conley et al. 2007; Ding et al. 2004).
70.4 Gene Therapy in rds Models
Gene therapy for the treatment of the diseased eye has become a common strategy; however, there are many factors which can influence its efficacy. Depending on the disease, a therapeutically beneficial effect can be achieved by directly targeting the primary genetic defect using gene replacement therapy (for loss-of-function phenotypes) or by suppressing mutant transcripts by RNA-based ribozymes or RNAi (shRNA). Alternatively, the desired effect may be achieved indirectly by modulating a secondary effect associated with the disease. Examples of this type of gene therapy in the eye include delivery of neurotrophic factors and anti-apoptotic genes to protect and improve photoreceptor survival in the presence of a degenerative insult (Danos 2008; Farrar et al. 2002; Hauswirth and Lewin 2000). Based on the characteristics of the delivery vehicles, gene therapies are commonly classified into two broad categories: viral and non-viral.
70.5 Viral Gene Therapy Approaches
Viral methods have been utilized quite successfully in the eye. Retrovirus, lentivirus, adenovirus, and adeno-associated virus (AAV) have all been used to transfer therapeutic genes to the retina (Hauswirth and Lewin 2000), but AAV vectors have been the most effective thus far. They are small in size, can efficiently and stably transduce a variety of dividing and non-dividing cell types, and site-specifically integrate without pathogenicity or immune response. AAV vectors are associated with high transduction efficiency, long term gene expression, and after therapeutic delivery to the eye, improvement in retinal function and vision (Acland et al. 2005; Acland et al. 2001). The only limitation of AAV vectors is capacity; traditional AAV vectors usually hold no more than 4.5–4.7 kb. Recently, however, one study reported that rAAV2/5 can incorporate up to 8.9 kb DNA (Allocca et al. 2008). So far, ocular AAV-mediated gene therapy in animals and humans has a good safety record (Mueller and Flotte 2008). For example, in several recent clinical trials, one in which rAAV-PEDF was delivered to treat age-related macular degeneration (AMD) (Campochiaro et al. 2006), and three in which rAAV-RPE65 was used to treat Leber’s Congenital Amaurosis (LCA) (Bainbridge et al. 2008; Cideciyan et al. 2008; Maguire et al. 2008), patients reported no severe side effects and some improvement in visual function.
Several viral approaches have been adopted for the treatment of Rds-associated disease. Ali’s group used the gene replacement approach, delivering Rds cDNA to the retinas of neonatal and adult rds−/− mice. They reported restoration of retinal ultrastructure and function; however, transduction efficiency was about 10%, gene expression decreased over time, and the treatment did not significantly ameliorate cell death (Ali et al. 2000; Sarra et al. 2001; Schlichtenbrede et al. 2003).
The neuroprotection approach has also been applied to rds models (Buch et al. 2006; LaVail et al. 1998). Combination therapy with glial cell line-derived neurotrophic factor (AAV.CBA.GDNF) and Rds (AAV.Rho.Prph2) delivered to the rds−/− retina was significantly more effective than gene replacement therapy alone (AAV.Rho.Prph2), but the positive effects did not persist beyond 3 months (Buch et al. 2006). Neonatal or adult delivery of ciliary neurotrophic factor (rAAV-CNTF) to the subretinal or the intravitreal space of P216L-RDS mice in either the rds+/− or rds−/− background significantly reduced photoreceptor death, increased rhodopsin expression in surviving rods, improved expression of key phototransduction genes, and improved rod ERG responses (Bok et al. 2002; Cayouette et al. 1998; Liang et al. 2001; Rhee et al. 2007). However, there is some controversy regarding the benefits of treatment with CNTF. Subsequent studies reported a dose-dependent reduction in retinal function and an abnormal nuclear phenotype in P216L-RDS mice after CNTF delivery (Bok et al. 2002; Buch et al. 2006; Rhee et al. 2007; Schlichtenbrede et al. 2003, 2003). The mechanisms underlying this deleterious effect are not understood and may be species specific. However the ability to attenuate photoreceptor cell death is clearly beneficial and further study of the effects of neurotrophic factors on Rds-associated retinal disease should be undertaken.
A final viral gene therapy approach which may be quite useful in the future, but has not yet been applied to the treatment of Rds-associated diseases is gene knockdown therapy. This approach will likely be a critical one to the successful treatment of Rds-associated macular diseases in particular since they tend to be associated with toxic, gain-of-function mutations. Ribozymes can reduce the production of mutated proteins by selectively cleaving the mutant mRNA molecules with a high degree of specificity (Hauswirth and Lewin 2000). Ribozymes have been successfully used to treat rhodopsin-associated adRP: AAV-ribozyme delivered to rhodopsin rats carrying the P23H mutation significantly decreased P23H transgene mRNA level, significantly reduced photoreceptor loss, increased outer nuclear layer (ONL) thickness, and improved scotopic b-wave amplitudes (Gorbatyuk et al. 2007a; Hauswirth et al. 2000; LaVail et al. 2000). An alternative knockdown strategy is delivery of shRNAs. This technology can be used to selectively knock down mutant alleles or more broadly to knock down both mutant and WT alleles, and has been applied to several different rhodopsin adRP models (Allen et al. 2007; Gorbatyuk et al. 2007b). While selective knockdown of a mutant allele may appear to be the ideal option for this type of therapy, this approach would require development of separate therapies for each individual mutation. In the case of RDS, the sheer number of different dominant mutations would likely make this cost-prohibitive. As an alternative, RNAi knockdown of both mutant and WT message (knockdown based on a recognition sequence not associated with the mutation) with concurrent administration of supplementary RNAi-resistant WT protein has been successfully tested in P23H rhodopsin mutant rats and would likely be a good strategy for dominant RDS mutations (O’Reilly et al. 2007).
70.6 Non-viral Approaches
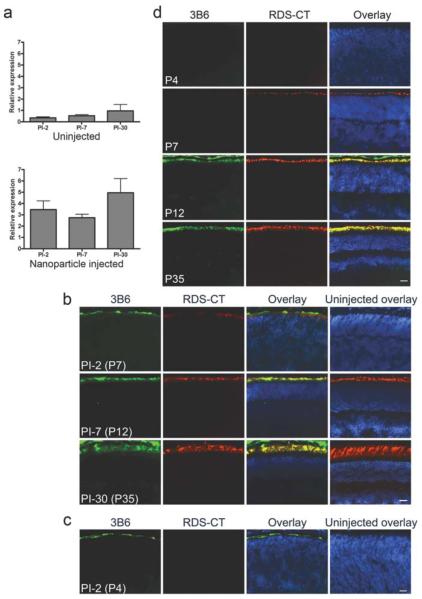
As an alternative to traditional viral-based gene delivery, non-viral vectors have recently become popular. The most common limitations encountered when using non-viral vectors is low transfection efficiency and transient gene expression. Many non-viral methods have been used in the eye including nanoparticles; liposomes; dendrimers; and plasmid DNA, with or without electroperation or ionotophoresis (Andrieu-Soler et al. 2006). In spite of the varying degrees of success associated with these strategies, so far, no non-viral vectors have been used clinically to treat ocular diseases. Recently, compacted-DNA nanoparticles (CK30PEG) were shown to be safe and effective in delivering the cystic fibrosis transmembrane receptor to the airways of cystic fibrosis patients in a type I/IIa clinical trial (Konstan et al. 2004). Given these positive results, we decided to investigate the utility of these nanoparticles in the eye. We subretinally injected CK30PEG nanoparticles containing a CMV-GFP vector into adult mice and demonstrated transfection of almost 100% of retinal cells beginning as early as two days post injection (PI) without any adverse effect on retinal structure or function. Since our ultimate goal is to use nanoparticles to treat Rds-associated adRP, we next generated nanoparticles containing the Rds cDNA with the P341Q modification at the C-terminus to enable specific recognition of the transferred protein with mAb 3B6. Rds cDNA expression was directed to rods and cones by the photoreceptor specific promoter IRBP (interphotoreceptor retinoid binding protein). We chose to deliver the treatment at postnatal day (P) 5, before the normal onset of RDS expression (P7) and OS formation (P8-10) (Cepko et al. 1996). As shown in Fig. 70.1a (qRT-PCR) and Fig. 70.1b (immunohistochemistry), after P5 injection into WT mice, the nanoparticles induced high levels of transgene expression as early as PI-2. In contrast to our results with the CMV-GFP nanoparticles (Farjo et al. 2006), IRBP nanoparticles drove sustained expression to PI-30 (the latest time point examined). At all timepoints, expression of the transferred protein (labeled with 3B6) was limited to the OS or developing OS layer, similar to the localization of the WT (RDS-CT) protein (Fig. 70.1b). To determine whether the nanoparticles could drive gene expression earlier than endogenous gene expression begins, a subset of animals was injected at P2. As shown in Fig. 70.1c, immunohistochemistry revealed that nanoparticle transferred protein (3B6) is detected at PI-2 after P2 injection, while endogenous protein is not apparent this early. Interestingly, when we used this same IRBP construct to generate stable transgenic mice, the transgenic RDS protein was not detected before the endogenous protein (Fig. 70.1d). Our data shown here and previously (Farjo et al. 2006) demonstrate that CK30PEG nanoparticles can efficiently transfect both mitotic and post-mitotic cells and induce rapid-onset and sustain gene expression. When the Rds gene is delivered, nanoparticles are capable of driving gene expression earlier than the native RDS without ectopic expression outside the photoreceptor OS layer. Our current studies are focused on determining whether these nanoparticles can rescue the rds+/− adRP disease phenotype, thus making them potential candidates for clinical use in the eye.
Fig. 70.
IRBP nanoparticles can drive persistent and elevated transgene expression in the WT Retina. WT mice were injected at P5 (a, b) or P2 (c) with IRBP nanoparticles. At various timepoints whole eyes were harvested and processed for qRT-PCR using Rds primers (a) or immunohistochemistry using mAB 3B6-green (specific for transferred/transgenic RDS) or RDS-CT polyclonal antibody-red (for endogenous RDS) with DAPI counterstain-blue (b–d). (a) Nanoparticle injection results in Rds message levels several fold higher than in uninjected contralateral control eyes. Levels remain elevated to PI-30. (b) Transferred RDS is detected at PI-2 in the nascent OS layer and co-localizes with the endogenous RDS at all timepoints examined. When nanoparticles are injected at P2, transferred protein is detected prior to the onset of endogenous RDS (c) although in stable transgenic mice generated using the same construct as the nanoparticle, transgenic RDS is not detectable until P12 (equivalent to PI-7) (d). Scale bar, 20 μm
References
- Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Ali RR, Sarra GM, Stephens C, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Allen D, Kenna PF, Palfi A, et al. Development of strategies for conditional RNA interference. J Gene Med. 2007;9:287–298. doi: 10.1002/jgm.1018. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Soler C, Bejjani RA, de Bizemont T, et al. Ocular gene therapy: a review of nonviral strategies. Mol Vis. 2006;12:1334–1347. [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Boon CJ, den Hollander AI, Hoyng CB, et al. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res. 2008;27:213–235. doi: 10.1016/j.preteyeres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Buch PK, MacLaren RE, Duran Y, et al. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006;14:700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Behn D, Sendtner M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, et al. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, Li S, et al. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley S, Nour M, Fliesler SJ, et al. Late-onset cone photoreceptor degeneration induced by R172W mutation in Rds and partial rescue by gene supplementation. Invest Ophthalmol Vis Sci. 2007;48:5397–5407. doi: 10.1167/iovs.07-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell G, Bascom R, Molday L, et al. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O. AAV vectors for RNA-based modulation of gene expression. Gene Ther. 2008;15:864–869. doi: 10.1038/gt.2008.69. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Nour M, Ritter LM, et al. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum Mol Genet. 2004;13:2075–2087. doi: 10.1093/hmg/ddh211. [DOI] [PubMed] [Google Scholar]
- Farjo R, Naash MI. The role of Rds in outer segment morphogenesis and human retinal disease. Ophthalmic Genet. 2006;27:117–122. doi: 10.1080/13816810600976806. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs JS, Nagel BA, et al. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol. 2006b;173:59–68. doi: 10.1083/jcb.200509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, et al. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006a;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Kenna PF, Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864. doi: 10.1093/emboj/21.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Kenna P, Jordan SA, et al. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991;354:478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, et al. Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp Eye Res. 2007a;84:44–52. doi: 10.1016/j.exer.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, et al. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vis Res. 2007b;47:1202–1208. doi: 10.1016/j.visres.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, LaVail MM, Flannery JG, et al. Ribozyme gene therapy for autosomal dominant retinal disease. Clin Chem Lab Med. 2000;38:147–153. doi: 10.1515/CCLM.2000.022. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Lewin AS. Ribozyme uses in retinal gene therapy. Prog Retin Eye Res. 2000;19:689–710. doi: 10.1016/s1350-9462(00)00007-0. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Hahn LB, Mukai S, et al. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991;354:480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Lloyd M, Birch DG, et al. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, et al. Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy. Proc Natl Acad Sci U S A. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Nour M, Ding XQ, Stricker H, et al. Modulating expression of peripherin/rds in transgenic mice: critical levels and the effect of overexpression. Invest Ophthalmol Vis Sci. 2004;45:2514–2521. doi: 10.1167/iovs.04-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Fliesler SJ, Naash MI. Genetic supplementation of RDS alleviates a loss-of-function phenotype in C214S model of retinitis pigmentosa. Adv Exp Med Biol. 2008;613:129–138. doi: 10.1007/978-0-387-74904-4_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystuen AM, Sachs AJ, Yuan Y, et al. A novel mutation in Prph2, a gene regulated by Nr2e3, causes retinal degeneration and outer-segment defects similar to Nr2e3 (rd7/rd7) retinas. Mamm Genome. 2008;9:623–633. doi: 10.1007/s00335-008-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M, Palfi A, Chadderton N, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Ruiz A, Duncan JL, et al. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007;48:1389–1400. doi: 10.1167/iovs.06-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, et al. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10:2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede FC, da Cruz L, Stephens C, et al. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J Gene Med. 2003;5:757–764. doi: 10.1002/jgm.401. [DOI] [PubMed] [Google Scholar]
- Stricker HM, Ding XQ, Quiambao A, et al. The Cys214–>Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice. Biochem J. 2005;388:605–613. doi: 10.1042/BJ20041960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R, Ivanyi D, Demant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978;12:106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]