Abstract
Nuclear-associated oscillations in calcium act as a secondary messenger in the symbiotic signaling pathway of legumes. These are decoded by a nuclear-localized calcium and calmodulin-dependent protein kinase, the activation of which is sufficient to drive downstream responses. This implies that the calcium oscillations within the nucleus are the predominant signals for legume symbiosis. However, the mechanisms that allow targeted release of calcium in the nuclear region have not been defined. Here we show that symbiosis-induced calcium changes occur in both the nucleoplasm and the perinuclear cytoplasm and seem to originate from the nuclear membranes. Reaction diffusion simulations suggest that spike generation within the nucleoplasm is not possible through transmission of a calcium wave from the cytoplasm alone and that calcium is likely to be released across the inner nuclear membrane to allow nuclear calcium changes. In agreement with this, we found that the cation channel DMI1, which is essential for symbiotic calcium oscillations, is preferentially located on the inner nuclear membrane, implying an essential function for the inner nuclear membrane in symbiotic calcium signaling. Furthermore, a sarco/endoplasmic reticulum calcium ATPase (SERCA) essential for symbiotic calcium oscillations is targeted to the inner nuclear membrane, as well as the outer nuclear membrane and endoplasmic reticulum (ER). We propose that release of calcium across the inner nuclear membrane allows targeted release of the ER calcium store, and efficient reloading of this calcium store necessitates the capture of calcium from the nucleoplasm and nuclear-associated cytoplasm.
Legumes form mutualistic symbiotic interactions with mycorrhizal fungi and with rhizobial bacteria that aid in the uptake of nutrients (1, 2). Establishment of both symbioses requires the common symbiosis (Sym) signaling pathway (1, 2) that involves calcium oscillations after perception of diffusible signals from the symbionts (3, 4): Nod factors from rhizobia and Myc factors from mycorrhizal fungi (4–7). The calcium oscillations are concentrated in the perinuclear region (3), and a nuclear-targeted calcium reporter showed that part of these oscillations occurs in the nucleoplasm (8). The decoder of the calcium oscillations, a calcium and calmodulin-dependent protein kinase (CCaMK), is localized to the nucleoplasm (9, 10), implying that intranuclear calcium changes are paramount. Furthermore, some of the components of the Sym pathway required for the induction of calcium oscillations are localized to the nuclear envelope: two cation channels and three components of the nuclear pore (11–14). All of this points to the nuclear membrane as playing a central role in symbiotic calcium oscillations.
The question of whether calcium changes can derive from the nucleus has been a contentious point for many years (15, 16). In animals, it is widely accepted that calcium events in and around the nucleus have significant effects on signaling pathways in the nucleus (15). Depending on the event studied, nuclear signals can be independent of or dependent on cytosolic calcium surges, and this is influenced by the mechanics of the nuclear pore complex (that can either permit or exclude ions from the nucleus), the distance of the cytosolic event to the nucleus, the viscosity and convection of the cytosol, and the local battery of calcium signaling proteins being expressed in a given cell (channels, buffers, pumps). Nuclear calcium signals in animal cells can have far-reaching effects on cellular function, in part through affecting transcription of specific genes (15, 16).
Although calcium changes within the nucleus have been reported in plant cells (3, 8, 17), the mechanisms of their induction and regulation are poorly understood. It has been suggested that the nucleus of plant cells can regulate calcium levels, independent of cytosolic calcium changes (17). Targeting calcium changes to the nuclear region requires either the targeting of calcium release channels to the nuclear membranes or the nuclear-targeted release of secondary messengers that activate existing nuclear calcium channels. As such, understanding the induction of nuclear calcium changes in plants requires an understanding of the calcium machinery present within the nuclear region. The outer nuclear membrane is contiguous with the endoplasmic reticulum (ER), but the inner nuclear membrane is a specialized membrane connected to the outer membrane through the nuclear pore. Integral membrane proteins targeted to this membrane require nuclear localization signals (NLS): it has been suggested that the nuclear pore is involved in translocating membrane proteins containing an NLS from the outer to the inner nuclear membrane (18).
Calcium channels regulate the movement of calcium, but the proteins that fulfill these roles in plants have only begun to be identified (19). Calcium pumps are responsible for the active reuptake of calcium into the internal store, using ATP or the electrochemical gradient to drive the movement of calcium against its concentration gradient. A calcium ATPase has been reported on the nuclear envelope of tomato, but the function of this protein has not been defined (17, 20). Here, we report the identification of a calcium ATPase, MCA8, localized to the nuclear envelope and ER, which is required for nuclear calcium signaling during symbiotic interactions. Also present on the nuclear envelope of Medicago truncatula is the cation channel DMI1, which genetic studies have shown to be essential for the induction of symbiotic calcium oscillations (21). We show that DMI1 preferentially localizes to the inner nuclear membrane. This, in conjunction with calcium imaging and mathematical modeling, supports the hypothesis that the symbiotic calcium changes occur across the inner nuclear membrane and this provides a mechanism for the targeted release of the ER calcium store in the nuclear region.
Results
Calcium Oscillations Occur in and Around the Root Hair Nucleus.
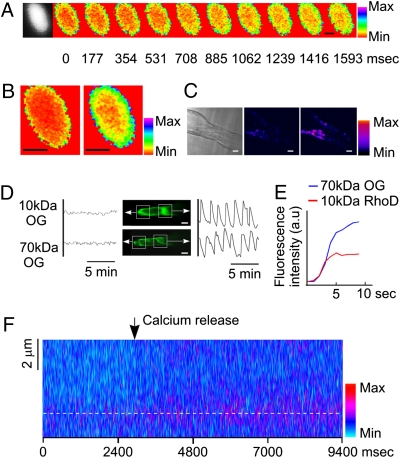
A nuclear-localized yellow cameleon (8) expressed in M. truncatula roots (Fig. S1A) revealed that Nod factor-induced nuclear calcium oscillations originate from the periphery of the nucleus (Fig. 1A and Fig. S2A; 17 cells analyzed on five plants), where the maximal changes of calcium occur (Fig. 1B), suggesting that calcium enters the nucleus from the inner nuclear envelope or diffuses into the nucleus from the cytosol. Cells expressing yellow cameleon present in the nucleus and cytoplasm (Fig. S1B) showed that the calcium oscillations in the cell are mostly restricted to the nuclear region (Fig. 1C), implying that either the nucleus itself or the nuclear-localized ER must be the source of calcium. However, these oscillations do occur in the nuclear-associated cytoplasm, because calcium oscillations could be measured in the nuclear region using the calcium dye Oregon Green fused to a 70-kDa dextran, which excludes it from the nucleoplasm (Fig. 1D; four cells analyzed on four plants). To assess for differences in the timing of the calcium response we injected M. truncatula root hair cells with Oregon Green fused to a 70-kDa dextran (excluded from the nucleus) in combination with a second calcium-responsive dye, RhoD, fused to a 10-kDa dextran (free to diffuse into the nucleus), thus allowing discrimination of nuclear and cytosolic regions. This assay revealed no difference in the timing of the calcium oscillation in the nucleus relative to the cytosol (Fig. 1E and Fig. S2C; three cells analyzed on three plants). Furthermore, using confocal imaging of cells expressing yellow cameleon revealed no evidence for temporal differentiation in the calcium oscillations either side of the nuclear envelope (Fig. 1F and Fig. S2B). This work demonstrates the importance of the nuclear periphery for regulating the calcium levels during calcium oscillations but does not discriminate between calcium released from the inner nuclear membrane or calcium oscillations occurring outside the nucleus and diffusing through the nuclear pore. Discriminating between these two scenarios is difficult using imaging alone, given the current spatial and temporal resolution limits and the dye characteristics.
Fig. 1.
Nod factor-induced nuclear calcium transients in M. truncatula root hair cells. (A) Imaging of a single Nod factor-induced calcium transient from a root hair cell expressing the nuclear-targeted cameleon (8) using time-lapse confocal scanning microscopy. The images show a single calcium transient from its initiation to its peak. Each frame is 177 ms apart. (B) Maximum intensity stack (Left) and average intensities (Right) of the images in A show a strong signal associated with the nuclear periphery. (C) Confocal imaging of a root hair cell expressing the YC2.1 cameleon lacking an NLS reveals calcium changes restricted to the nuclear region. Left: Cell before a calcium spike. Right: Same cell at the peak of the calcium spike. The timeframe between the two images is 1.32 s. (D) Calcium measurements within selected regions of the root hair cells treated with 1 nM Nod factor, after injection with the calcium-responsive dye Oregon Green (OG) fused to a 10-kDa dextran molecule (which diffuses into the nucleus) or fused to a 70-kDa dextran molecule (which is excluded from the nucleus). Boxes indicate the regions assessed for the calcium oscillations shown on either side of the image, with an arrow indicating the trace for each region; the tip of the root hair is shown to the left of each image and the nuclear region to the right. The vertical axis shows Oregon Green fluorescence intensity (arbitrary units). (E) A root hair cell of M. truncatula microinjected with Oregon Green fused to 70-kDa dextran (excluded from the nucleus) and RhoD fused to 10-kDa dextran (diffusible into the nucleus). Note that calcium changes measured using RhoD occur with a similar timing as those measured with Oregon Green. (F) Confocal imaging of a cell expressing YC2.1 lacking an NLS. Thin sections (2 μm) of a nuclear region were scanned at 73 ms, and each resulting image has been lined up together to show the calcium changes across the nuclear envelope (dotted line) over time. The images trace a single calcium transient from its inception to its peak. Note that calcium changes (magenta) occur either side of the nuclear envelope. The images in A, C, and E are pseudocolored to indicate calcium intensity that reflects ratio changes in the cameleon signal. (Scale bars, 5 μm in A and B, 10 μm in C and D.)
Perinuclear Reaction Diffusion Modeling.
To better discriminate between nuclear calcium oscillations deriving from the inner nuclear membrane or diffusing through the nuclear pore, we constructed a modified version of the popular fire-diffuse-fire (fdf) model. The modifications essentially account for the spheroid nature of the nucleus and the presence of nuclear pores. This model has previously proven successful in recreating the salutatory, continuous, and spiral wave patterns that have been observed in ventricular and cardiac myocytes (22, 23). The fdf model is based on calcium-induced calcium release mechanisms (CICR), such as would be found in systems dominated by inositol 1,4,5-trisphosphate (IP3) and/or ryanodine receptors. There is currently no evidence whether such mechanisms are important for symbiotic calcium oscillations; however, the presumption of CICR provides the most conservative model for this study, because the model gives us great flexibility in that we can control channel cluster release quantities, cluster arrangement and number, cluster refractory periods, calcium pump rates, and we can make any number of these traits stochastic to better approximate the current theory of channel behavior (24).
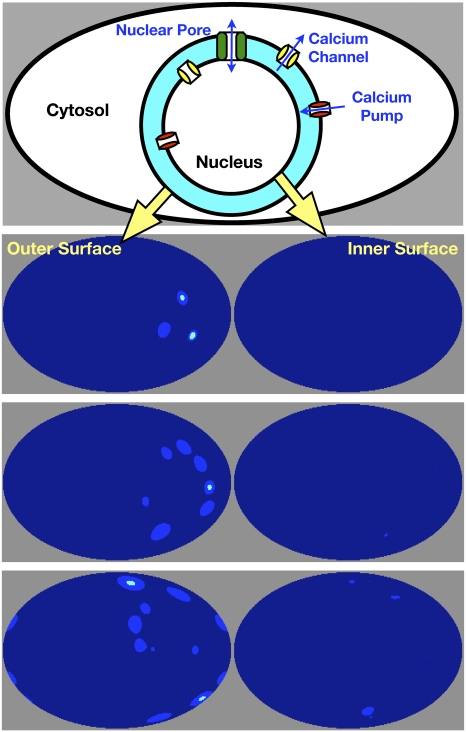
The nuclear pores were constructed according to published values for their diameters and cylindrical heights (25), placing up to 4,000 pores across the model nucleus. This number was chosen on the basis of the typical observed pore number in mammalian cells, which is between 3,000 and 4,000 (25). If we initiate CICR on the nuclear interior, then with careful parameter selection the model has the ability to reproduce calcium profiles across the surface of the nuclear envelope that accurately match the confocal observations (Fig. 2). However, without calcium release from the inner nuclear membrane we were not able to reproduce calcium oscillations in the nucleus using calcium diffusion from outside the nucleus, and vice versa (Fig. 2). In the unrealistic case in which the calcium pump rate has been removed and the cluster release quantity approaches concentrations of 38 μM, far higher than what is observed (26), we are able to achieve calcium release outside the nucleus of up to 1.8 μM within 2 s. However, even under these conditions the calcium that diffuses through the pores into the nucleus only reaches an average concentration of 25 nM within this time, well below the concentrations measured during symbiotic calcium oscillations (3), although these measurements are only an approximation. These low calcium diffusion rates for the nuclear pores are explained by the very low permeability of the nuclear pore complement: if we take an exceptionally conservative value of 150 μm2 (27) for the surface of the nucleus (whereas nuclei in M. truncatula seem to be more on the order of 600–800 μm2), then 4,000 pores at their typical resting diameter account for only 0.1% of the nuclear surface, and 4,000 fully dilated pores would only occupy 1.3%. These figures and the full simulations suggest that diffusion through the pores is not likely to account for the near-simultaneous interior/exterior calcium changes. We conclude that calcium is likely to be released either side of the nuclear envelope to create the observed calcium oscillations within the nucleus and within the nuclear-associated cytoplasm (Fig. 1).
Fig. 2.
Modeling reveals that nuclear calcium changes cannot be explained by diffusion from the cytosol. The top cartoon shows the essential components, channels, pumps, and pores used in the reaction diffusion model of calcium signal generation. The columns below show an atlas view of the calcium distribution on the nuclear surface. A spike was initiated on the outer side of the nuclear envelope (Left), and then we allow for free diffusion of calcium through the nuclear pores to the inner nuclear membrane (Right). Left: We depict channel clusters on the outer nuclear membrane firing and generating a spike over a time period of 0.6 s. The highest concentrations (cyan) correspond to 20 μM. Right: Calcium changes on the inner nuclear membrane, which fail to rise above a maximum of 15 nM and are negligible on average. In our models, diffusion of calcium through the nuclear pores was not sufficient to allow for oscillations to be transmitted from one side of the nucleus to other.
The Cation Channel DMI1 Is Preferentially Localized to the Inner Nuclear Membrane.
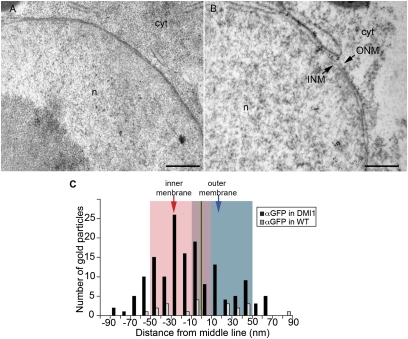
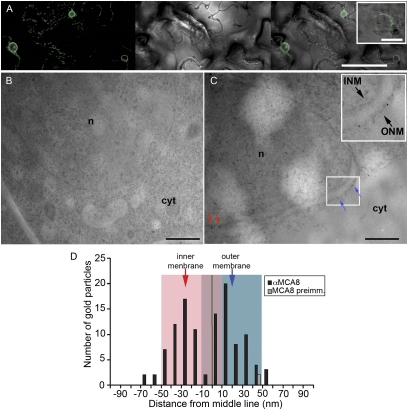
The modeling work implies there is calcium release machinery present on the inner nuclear membrane to allow calcium accumulation in the nucleus. Genetic dissection of Nod factor signaling in M. truncatula led to the identification of a cation channel, DMI1, that is required for the activation of symbiotic calcium oscillations (21, 28). The location of this essential cation channel should inform us of the membrane from which the calcium changes occur. Because of the poor specificity of native DMI1 antiserum, we used a monoclonal anti-GFP antibody on M. truncatula roots expressing a DMI1:GFP fusion protein that fully rescued dmi1 mutants (13). Transgenic roots were fixed with a high-pressure freezing, cryo-substitution technique that maintains the integrity of membranes and allowed us to discriminate between the inner and outer nuclear membranes. Immunogold labeling validated the previously shown (13) nuclear envelope location of DMI1 (Fig. 3). Quantification of 151 gold particles (16 nuclei) relative to the midline between the inner and outer nuclear membranes revealed a preference for DMI1 to locate on the inner nuclear membrane (Fig. 3 B and C). No such labeling was observed in roots lacking the DMI1:GFP construct (20 particles on 22 nuclei; Fig. 3 A and C). The preferential location of DMI1 on the inner nuclear membrane implies that modifications specific to the inner nuclear membrane are necessary for the activation of the symbiotic calcium oscillations.
Fig. 3.
M. truncatula DMI1 preferentially localizes to the inner nuclear membrane. (A) Immunogold labeling of nontransgenic M. truncatula root samples using anti-GFP antibody; few nonspecific signals were detected. (B) Immunogold labeling of DMI1::GFP-expressing transgenic hairy root cells using anti-GFP antibody; signals were detected on both inner and outer nuclear membranes. (C) Quantitative analysis of the relative abundance of DMI1 in the nuclear membranes. Arrows in B indicate the inner (INM) and outer (ONM) nuclear membranes. Black bars are DMI1-GFP transformed roots, grey bars are control roots. n, nucleus; cyt, cytoplasm. (Scale bar, 500 nm.)
SERCA-Type Calcium ATPase Involved in Nuclear Calcium Spiking of M. truncatula.
Considering the presence of calcium in the nuclear compartment and the poor diffusion of calcium from the nucleus, we would predict that efficient reuptake of the nuclear calcium would require the presence of a calcium pump on the inner nuclear membrane. Previous pharmacological approaches have implicated SERCA-type calcium ATPases in symbiosis signaling, because cyclopiazonic acid (CPA), which inhibits SERCA-type pumps, can reversibly inhibit Nod factor-induced calcium oscillations (29, 30) and gene expression (31). Indeed, both the inhibitor CPA and the activators of SERCA-type calcium ATPases, gingerol and butylated hydroxyanisole (32, 33), block Nod factor-induced calcium oscillations and resultant Early Nodulin (ENOD) 11 gene expression (Fig. S3). The fact that inhibitors as well as activators are able to block the response implies that a tight regulation of calcium pumps is necessary for maintenance of Nod factor-induced calcium oscillations. The M. truncatula calcium ATPase family (MCA) consists of at least 10 members that fall into the type IIA (SERCA) and type IIB subfamilies (Fig. S4A) (34). We looked for SERCA-type calcium ATPases that contained predicted NLSs. Only one SERCA-type calcium ATPase in M. truncatula, MCA8, was predicted to be nuclear-localized (Fig. S4B). The predicted NLS of this protein is conserved in the Lotus japonicus ortholog (Fig. S4B). MCA8 is predominantly expressed in the root, where these symbioses occur (Fig. S4C), and is not significantly up-regulated during nodulation (Fig. S4D), but is induced at late stages of the mycorrhizal interaction (Fig. S4E).
Nuclear Calcium Oscillations Depend on MCA8.
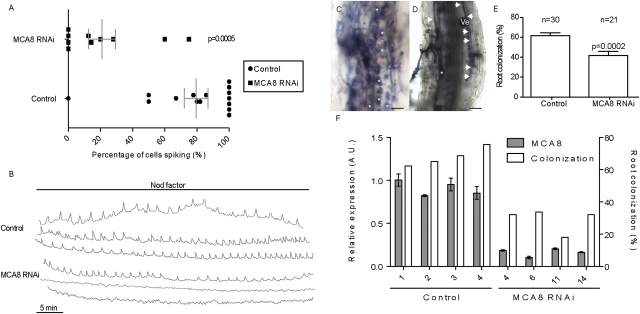
To ascertain whether this predicted nuclear-localized calcium ATPase functions in symbiotic calcium signaling, we silenced MCA8 in M. truncatula roots carrying the calcium reporter yellow cameleon YC2.1 (35). We used the 3′ UTR that has no homology with any other M. truncatula calcium ATPase gene. This construct strongly reduced transcript (Fig. 4F) and protein levels of MCA8 (Fig. S5). Plants transformed with the empty vector showed an average of 80% of root hair cells (on 90% of roots tested) with Nod factor-induced calcium spiking (Fig. 4 A and B). In contrast, silencing MCA8 had a significant effect, with only 20% of imaged root hair cells, on 70% of roots tested, initiating calcium spiking (Fig. 4 A–C). Combining the pharmacology and the effects of MCA8 silencing, we conclude that the SERCA-type calcium ATPase MCA8 is likely to be the predominant calcium ATPase functioning in symbiotic calcium signaling.
Fig. 4.
Silencing MCA8 blocks Nod factor-induced calcium oscillations and reduces mycorrhizal colonization. (A) Scatterplot comparing the percentage of spiking root hair cells in control (empty vector) and MCA8 silenced roots. Each dot represents one transformed root plotted to indicate the percentage of cells on that root that showed calcium spiking upon Nod factor treatment. Only 20% of cells spiked in the silenced roots, compared with 80% in control vector transformed lines. The difference was significant (P value indicated in the graph). Shown in gray is the median for each construct with the SEM. (B) Representative traces from the analysis in A observed for the different constructs used. Of the cells responding with calcium spiking, MCA8 silenced roots showed some cells with calcium oscillations equivalent to the empty vector control. (C–F) Mycorrhization phenotype of MCA8 RNAi roots. (C) Fungal colonization of a control root; asterisks indicate arbuscules. (D) In MCA8 RNAi roots aberrant colonization can be observed: septate hyphae (arrowheads) and reduced number or absence of arbuscules (asterisk) and vesicles (Ve). (E) Average mycorrhizal colonization per construct; error bars are SEM. (F) Quantification of RNA levels in representative silencing lines compared with control lines, demonstrating efficient knockdown of MCA8. Also plotted is the colonization percentage of the roots assessed for MCA8 expression levels. (Scale bars in C and D, 0.1 mm.) A.U., arbitrary units.
Considering this role, we tested whether MCA8 functions in the establishment of symbiotic interactions. We did not observe statistically significant differences in the levels of nodulation in MCA8 silenced lines (Fig. S6 A–C). This is not surprising, because studies of a number of mutants has revealed that only a few cells on a root need to respond to Nod factors to activate a nodule (28, 36). Considering that 20% of root hair cells are still capable of inducing calcium spiking in MCA8 silenced roots and that these responding cells appear normal (Fig. 4B), it is not surprising that nodules develop on these roots. Although we did not see major differences in the levels of nodules, we were able to see significant differences during mycorrhizal colonization. Compared with control roots, MCA8 RNAi roots showed reduced levels of colonization (Fig. 4F) that correlated with reduced levels of PT4 expression (Fig. S6D), a phosphate transporter activated in arbuscules (37). The reduced colonization of MCA8 silenced roots seems in part due to a defect in the initial penetration of fungal hyphopodia, the swollen fungal structure associated with infection. Whereas penetration of control roots occurred with a single hyphopodium (Fig. S6E), the silenced roots showed exaggerated hyphopodium formation and septate hyphae (Fig. 4D), hallmarks of incompatible interactions and multiple penetration attempts (Fig. S6F), indicating an impairment in fungal penetration. There was a strong correlation between colonization level and MCA8 RNA levels in silenced roots (Fig. 4F).
MCA8 Localizes to the Nuclear Envelope and ER.
MCA8 is predicted to contain an NLS. To assess the functionality of this predicted NLS we generated a GFP fusion protein containing the predicted NLS and three transmembrane domains of MCA8 and transiently expressed these in Nicotiana benthamiana. GFP fused to the C terminus of this truncated protein showed localization to the nuclear envelope (Fig. 5A). This work implies that the NLS of MCA8 can target proteins to the nuclear envelope.
Fig. 5.
Localization of MCA8 in root epidermal cells of M. truncatula. (A) Transient expression of a truncated version of MCA8 containing the predicted NLS and three transmembrane domains. The GFP signal is concentrated in the nuclear envelope, and some signal can be seen in the ER. Left: Fluorescent image; Center: light microscopic image; Right: merge. Inset: Detail of a nucleus with GFP signal restricted to the nuclear envelope. (B) Immunogold labeling of M. truncatula root cells using preimmune serum for MCA8; no significant signal was observed. (C) Immunogold labeling of M. truncatula root cells using an MCA8 antibody; signals were detected on the nuclear membranes. Inset: Nuclear envelope showing gold particles on the inner (INM) and outer (ONM) nuclear membranes. (D) Quantitative analysis of relative abundance of MCA8 in respective nuclear membranes. MCA8 is equally distributed over both inner and outer nuclear membranes in root epidermal cells of M. truncatula. Red arrow indicates gold particles on the inner nuclear membrane; blue arrow indicates gold particles on the outer nuclear membrane. n, nucleus; cyt, cytoplasm. (Scale bars, 100 μm in A, 15 μm in A Inset, 1 μm in B, 1 μm in C.)
To better define the location of MCA8 we generated a polyclonal antiserum raised against an MCA8 peptide, and this detected a single MCA8-specific band in M. truncatula (Fig. S5). Immunolocalization in M. truncatula root cells revealed that MCA8 location overlapped with DAPI staining (Fig. S7B), suggesting a nuclear localization, which was not seen in the preimmune controls (Fig. S7A). Isolated M. truncatula root nuclei also showed MCA8 localized around the periphery of the nucleus in discrete speckles (Fig. S7 C and D), suggesting a nuclear location of MCA8. However, the resolution and quality of fixation we were able to obtain with these techniques was limited and so we used the MCA8 antiserum in immunogold labeling on sections generated from M. truncatula roots using high-pressure freezing, cryo-substitution. Immunogold particles were predominantly located to the nuclear envelope, with some labeling on the ER (Fig. 5C and Fig. S8); this was not seen in the preimmune controls (10 randomly distributed particles in 16 nuclei; Fig. 5B). One hundred twelve gold particles present on the nuclear envelope (18 nuclei) were measured relative to the midline of the nuclear envelope, and this revealed that, unlike DMI1, MCA8 is equally present on both inner and outer nuclear membranes (Fig. 5D, compare with Fig. 2C). Interestingly, this result is in contrast to the nuclear calcium ATPases in animal systems that appear strictly localized to the outer nuclear membrane (38).
Discussion
Previous studies have shown that symbiotic calcium oscillations are associated with the nucleus and occur in the nucleoplasm (3, 8). This, in conjunction with the nuclear localization of symbiotic signaling components (11–14, 36), indicates an essential role for the nucleus in symbiotic calcium signaling. However, it cannot be concluded from this previous work whether calcium changes occur across the nuclear membranes vs. calcium released into the cytosol that then diffuses into the nucleus through the nuclear pores. We show that symbiotic calcium oscillations occur in both the nucleoplasm and the nuclear-associated cytoplasm and that the calcium changes seem to occur concurrently either side of the nuclear envelope. Reaction diffusion simulations indicate that even under highly favorable parameter settings the mechanism of diffusion is not sufficient to generate oscillations inside the nucleus from a cytosolic oscillation alone, and vice versa. This suggests that both the inner and outer nuclear membranes are likely to regulate calcium changes during symbiotic calcium signaling. This does not exclude calcium changes also occurring across the nuclear-associated ER.
The lumen of the nuclear envelope is contiguous with the ER lumen, and one mechanism for the nuclear-targeted release of the ER calcium store is the targeting of the channels responsible for their release to the nuclear membranes. There are already components of the symbiosis signaling pathway that are required for the induction of calcium oscillations that reside on or are associated with the nuclear envelope: three components of the nuclear pore (11–14, 36) and two cation channels (11, 13, 21, 39). The cation channel DMI1 is necessary for symbiotic calcium oscillations, and we show that this channel is preferentially located on the inner nuclear membrane, implying an essential function for this membrane in the activation of the calcium oscillations. The targeting of DMI1 to the inner nuclear membrane may explain the requirement for components of the nuclear pore in symbiosis signaling (11–14), because the nuclear pore seems to be involved in flipping proteins between the outer and inner nuclear membranes (18).
A specific requirement for the inner nuclear membrane in symbiotic calcium signaling provides a means for a targeted nuclear release of the ER calcium store. For efficient reuptake of nuclear calcium, we would predict the presence of a calcium ATPase on the inner nuclear membrane. Considering that the symbiotic calcium changes also occur in the nuclear-associated cytoplasm, we would anticipate the additional need for calcium reuptake from the outer nuclear membrane and nuclear-associated ER. The calcium pump(s) involved in symbiotic calcium signaling have not been revealed from forward genetic studies. In an attempt to identify these components, we used the presumed nuclear localization as a means to identify a calcium pump that is required for symbiotic calcium signaling. We show that MCA8, a nuclear-localized SERCA-type calcium ATPase, is involved in symbiotic calcium signaling. It seems this protein may have additional functions in plant development, because we have been unable to generate homozygous lines from two independent MCA8 knockout mutants. Importantly, MCA8 is present on the inner nuclear membrane, implying directed targeting of this calcium pump to this membrane and providing an efficient mechanism for the recapture of nuclear calcium during symbiotic signaling. Interestingly, MCA8 is also present on the outer nuclear membrane and the ER, suggesting that MCA8 may also function in the capture of calcium released into the nuclear-associated cytoplasm, thus efficiently reloading the ER/nuclear envelope calcium store. As far as we can tell MCA8 is the only nuclear-localized calcium ATPase in M. truncatula. This suggests that MCA8 plays an essential role in the recapture of calcium released into the nucleus. However, the reloading of the ER calcium store from cytoplasmic calcium is likely to be the function of MCA8 and possibly other calcium ATPases.
Taken together, the calcium imaging that implies calcium release concurrently either side of the nuclear envelope, the mathematical modeling showing that oscillations are unlikely to be generated on both sides of the nuclear envelope solely by diffusion through the nuclear pore, the preferential location of DMI1 on the inner nuclear membrane, and the presence of an essential calcium ATPase also on the inner nuclear membrane strongly suggest that calcium is released across the inner nuclear membrane during symbiotic calcium signaling. Such targeted release of calcium from the inner nuclear membrane would allow a nuclear-specific release from ER calcium stores. Our work does not exclude calcium release also from the outer nuclear membrane or nuclear-associated ER, and indeed the modeling strongly implies that the perinuclear cytosolic calcium release that we observed must be a function of calcium release outside the nucleus. However, the presence of the symbiotic calcium decoder CCaMK in the nucleus (9, 10) implies that the calcium changes across the inner nuclear membrane are likely to be the calcium changes that drive downstream symbiotic responses. Efficient reuptake of symbiotic calcium requires the capture of calcium from the nucleoplasm and nuclear-associated cytoplasm, and MCA8 is well positioned to fill this role. Much work remains to be done to define the exact mechanisms by which symbiotic calcium oscillations are induced and maintained. The nuclear location of this response has facilitated the identification of the calcium ATPase that is required, and using these criteria may allow identification of other components on the nuclear membrane necessary for this process.
Materials and Methods
For single-cell calcium imaging, root hair cells were injected with the calcium-sensitive dye Oregon Green and imaged after challenge with Nod factor. In other instances roots containing the calcium sensor yc2.1 was used. Composite plants carrying transgenic roots with a hairpin construct targeting MCA8 were analyzed for Nod factor-induced calcium spiking, nodulation, and mycorrhization. Expression levels were analyzed using quantitative RT-PCR to confirm specific knockdown of target genes or expression levels in wild-type roots. Fluorescent protein fusions were analyzed using transient expression assays in N. benthamiana leaves. Antibodies were raised against an MCA8-specific peptide and confirmed to be specific for MCA8. High-pressure frozen M. truncatula root tissue was stained with the anti-MCA8 antibody to analyze subcellular localization.
Supplementary Material
Acknowledgments
We thank Christine Faulkner for help with microscopy; Ali Pendle and Kim Findlay for help with immunohistochemistry; Ben Miller, Tatiana Vernie, Enrico Gobbato, and Ertao Wang for preparing RNA samples; and Marielle Vigouroux for help with phylogeny. This work was supported by the European Research Council, a grant-in-aid from the Biotechnology and Biological Science Research Council, the European Molecular Biology Organization (as a postdoctoral fellowship to W.C.), National Science Foundation Grants 0701846 (to J.-M.A.) and MCB-0843151 (to M.S.O.), and US Department of Agriculture Hatch WIS01163 (to J.-M.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. BK007879).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107912108/-/DCSupplemental.
References
- 1.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 2.Oldroyd GED, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 3.Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 4.Kosuta S, et al. Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc Natl Acad Sci USA. 2008;105:9823–9828. doi: 10.1073/pnas.0803499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabaud M, et al. Arbuscular mycorrhizal hyphopodia and germinated spore exudates trigger Ca2+ spiking in the legume and nonlegume root epidermis. New Phytol. 2011;189:347–355. doi: 10.1111/j.1469-8137.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 6.Lerouge P, et al. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- 7.Maillet F, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63. doi: 10.1038/nature09622. [DOI] [PubMed] [Google Scholar]
- 8.Sieberer BJ, et al. A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol. 2009;151:1197–1206. doi: 10.1104/pp.109.142851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaló P, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- 10.Smit P, et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 11.Charpentier M, et al. Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell. 2008;20:3467–3479. doi: 10.1105/tpc.108.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanamori N, et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA. 2006;103:359–364. doi: 10.1073/pnas.0508883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riely BK, Lougnon G, Ané JM, Cook DR. The symbiotic ion channel homolog DMI1 is localized in the nuclear membrane of Medicago truncatula roots. Plant J. 2007;49:208–216. doi: 10.1111/j.1365-313X.2006.02957.x. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell. 2007;19:610–624. doi: 10.1105/tpc.106.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci. 2009;122:2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 16.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci. 2004;117:3087–3094. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- 17.Pauly N, et al. Control of free calcium in plant cell nuclei. Nature. 2000;405:754–755. doi: 10.1038/35015671. [DOI] [PubMed] [Google Scholar]
- 18.Zuleger N, Korfali N, Schirmer EC. Inner nuclear membrane protein transport is mediated by multiple mechanisms. Biochem Soc Trans. 2008;36:1373–1377. doi: 10.1042/BST0361373. [DOI] [PubMed] [Google Scholar]
- 19.McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytol. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- 20.Downie L, Priddle J, Hawes C, Evans DE. A calcium pump at the higher plant nuclear envelope? FEBS Lett. 1998;429:44–48. doi: 10.1016/s0014-5793(98)00564-x. [DOI] [PubMed] [Google Scholar]
- 21.Ané JM, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. doi: 10.1126/science.1092986. [DOI] [PubMed] [Google Scholar]
- 22.Coombes S, Hinch R, Timofeeva Y. Receptors, sparks and waves in a fire-diffuse-fire framework for calcium release. Prog Biophys Mol Biol. 2004;85:197–216. doi: 10.1016/j.pbiomolbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Tang AH, Wang SQ. Transition of spiral calcium waves between multiple stable patterns can be triggered by a single calcium spark in a fire-diffuse-fire model. Chaos. 2009;19:037114. doi: 10.1063/1.3207814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcke M. Reading the patterns in living cells—the physics of Ca2+ signaling. Adv Phys. 2004;53:255–440. [Google Scholar]
- 25.Alberts B, et al. Molecular Biology of the Cell. 5th Ed. New York: Garland Science; 2008. [Google Scholar]
- 26.Bruno L, Solovey G, Ventura AC, Dargan S, Dawson SP. Quantifying calcium fluxes underlying calcium puffs in Xenopus laevis oocytes. Cell Calcium. 2010;47:273–286. doi: 10.1016/j.ceca.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brière C, Xiong TC, Mazars C, Ranjeva R. Autonomous regulation of free Ca2+ concentrations in isolated plant cell nuclei: A mathematical analysis. Cell Calcium. 2006;39:293–303. doi: 10.1016/j.ceca.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Wais RJ, et al. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engstrom EM, Ehrhardt DW, Mitra RM, Long SR. Pharmacological analysis of nod factor-induced calcium spiking in Medicago truncatula. Evidence for the requirement of type IIA calcium pumps and phosphoinositide signaling. Plant Physiol. 2002;128:1390–1401. doi: 10.1104/pp.010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capoen W, et al. Calcium spiking patterns and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata. Plant Cell. 2009;21:1526–1540. doi: 10.1105/tpc.109.066233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charron D, Pingret JL, Chabaud M, Journet EP, Barker DG. Pharmacological evidence that multiple phospholipid signaling pathways link Rhizobium nodulation factor perception in Medicago truncatula root hairs to intracellular responses, including Ca2+ spiking and specific ENOD gene expression. Plant Physiol. 2004;136:3582–3593. doi: 10.1104/pp.104.051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fusi F, Tzankova V, Valoti M, Pessina F, Sgaragli G. 3,5-di-t-butyl-4-hydroxyanisole (DTBHA) activation of rat skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase. Biochem Pharmacol. 2001;62:1613–1619. doi: 10.1016/s0006-2952(01)00794-8. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, Shoji N, Ohizumi Y. Gingerol, a novel cardiotonic agent, activates the Ca2+-pumping ATPase in skeletal and cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1987;903:96–102. doi: 10.1016/0005-2736(87)90159-3. [DOI] [PubMed] [Google Scholar]
- 34.Baxter I, et al. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003;132:618–628. doi: 10.1104/pp.103.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miwa H, Sun J, Oldroyd GE, Downie JA. Analysis of calcium spiking using a cameleon calcium sensor reveals that nodulation gene expression is regulated by calcium spike number and the developmental status of the cell. Plant J. 2006;48:883–894. doi: 10.1111/j.1365-313X.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 36.Groth M, et al. NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell. 2010;22:2509–2526. doi: 10.1105/tpc.109.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison MJ, Dewbre GR, Liu J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell. 2002;14:2413–2429. doi: 10.1105/tpc.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humbert JP, Matter N, Artault JC, Köppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- 39.Imaizumi-Anraku H, et al. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature. 2005;433:527–531. doi: 10.1038/nature03237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.