Abstract
Mouse mast cells (MCs) express a large number of serine proteases including tryptases, mouse mast cell protease (mMCP)-6 and -7; chymases, mMCP-1, -2, and -4; and an elastase, mMCP-5; along with carboxypeptidase-A3 (CPA3). In helminth-infected mouse intestine, distinct protease phenotypes are observed for connective tissue MCs (CTMCs) (mMCP-4+–7+, and CPA3+) and mucosal MCs (MMCs) (mMCP-1+ and 2+). To determine whether the protease phenotype was regulated by the tissue, we compared the phenotype of constitutive CTMCs and induced MMCs in trachea and large airways in antigen-sensitized unchallenged and challenged mice to MCs in skin and helminthic-infected intestine. We found that in the trachea, unlike in skin and intestine, CTMCs and MMCs both express all six serine proteases and CPA3 (mMCP-1+, -2+, 4+–7+, CPA3+). This phenotype also holds for the lung CTMCs in the proximal bronchi, whereas the induced MMCs express only four proteases, mMCP-1, -2, -6, and -7. Thus, the T-cell–dependent induction of MMCs in trachea, large bronchi, and small intestine provides numbers but does not determine the protease phenotype. Furthermore, the CTMCs, which are constitutive, also show striking differences at these tissue sites, supporting the view that the differences in expression are tissue directed and not dependent on inflammation.
A phenotypic difference between constitutive connective tissue mast cells (CTMCs) and helminth-induced T-cell–dependent mucosal MCs (MMCs) was first described in rats by Enerback, on the basis of their different fixation properties in the jejunum (1). Decades later, the constitutive CTMCs in small intestine, peritoneum, and skin of mice were shown to express the chymase mMCP-4, the elastase mMCP-5, and two tetrameric tryptases, mMCP-6 and -7, whereas the helminth-induced MMCs of the small intestine expressed only two chymases, mMCP-1 and -2 (2–4). However, it was noted that the MMCs in the lamina propria during the resolution phase of a Trichinella spiralis infection underwent a modulation of the protease phenotype so as to express mMCP-5 or -6 along with mMCP-2 (4, 5). A MC-specific carboxypeptidase, CPA3, is also associated with murine CTMCs in the serosal cavity and skin and absent in MMCs (6, 7). The mucosal protease, mMCP-1 has been shown to increase from a constitutive plasma baseline by 80- to 100-fold during expulsion of the adult T. spiralis in concert with a similar expansion of MMCs in WT mice and clearance of the adult worms is impaired in a mMCP-1–deficient strain, revealing a role in host defense (8). Physiologic roles for the chymase mMCP-4 include regulating intestinal permeability (9) and turnover of fibronectin (10). In a sensitization and pulmonary challenge model, a deficiency of mMCP-4 increased some features of airway remodeling (11). In a pharmacologic approach, administration of the tryptase mMCP-6 to WT mice was shown to enhance neutrophil extravasation and innate host defense against a Klebsiella pneumonia infection of the lung and peritoneal cavity (12, 13). Thus, distinct in vivo functions of specific mouse MC proteases are now being recognized in models of host defense and inflammatory injury.
The human MC expresses a tryptase, beta 1, a chymase, and a carboxypeptidase that are homologous to mMCP-6, mMCP-4, and mouse CPA3, respectively (10, 14). These proteases are expressed in intestinal and skin CTMC (often termed MCTC), but only tryptase is present in most MMCs (termed MCT) of intestine and lung (15, 16). The MMCs are considered to be T-cell dependent, on the basis of their absence in the gastrointestinal mucosa of individuals with acquired severe immune deficiency (17). Recently, the reported protease exclusivity of these two phenotypes in human MCs has been challenged by the findings of increased transcripts for tryptase and CPA3 but relatively fewer chymase transcripts in bronchial mucosal biopsies from asthmatic patients showing increased MMCs and a high Th2 transcript phenotype (18). Similarly, in patients with eosinophilic esophagitis, the associated MC hyperplasia correlated with increased transcripts for tryptase and CPA3 but not chymase (19).
An increase in lung MCs is well documented in mouse models of allergic pulmonary inflammation (20, 21); however, there has been no previous attempt to describe the overall protease phenotype of constitutive and induced MCs in the trachea and proximal airways. We now find that the definitions of constitutive CTMCs (mMCP-4+–7+ and CPA3+) and induced MMCs (mMCP-1+ and -2+), which are based on their protease phenotypes in intestine (4, 5) and supported by that of CTMCs in skin (6), do not hold for lung. Rather, in mouse trachea, both CTMCs and MMCs express both tryptases, mMCP-6 and -7; three chymases, mMCP-1, -2, and -4; an elastase, mMCP-5; and CPA3, revealing no distinction between constitutive and induced subclasses. In the proximal bronchi, the protease profile of CTMCs is like that of the trachea, whereas the induced MMCs express both tryptases and the chymases, mMCP-1 and -2, but do not express mMCP-4, mMCP-5, or CPA3. Most importantly, the induced profile of proteases by allergic inflammation in trachea or large airway is distinct from that of the T. spiralis-infected small intestine, indicating that in the BALB/c strain, Th2 inflammation alone does not define this expression. Rather, T-cell–driven inflammation predominantly determines the induction of the MMC hyperplasia from a negligible/minimal background number. Equally compelling is the finding that in sensitized, unchallenged mice, the T-cell–independent CTMCs in trachea and large airways express chymases not present in CTMCs in small intestine or skin. Together, these findings support a tissue lineage for MCs that, whether constitutive or expanded by T-cell host responses, assumes a secretory granule protease phenotype that is highly regulated by the host tissue site. Understanding the functional implications of these prominent tissue-specific MC phenotypes of constitutive and induced MCs is a compelling future direction.
Results
Increase in Lung MC Progenitor (MCp) Numbers and in Mature MMCs in the Trachea and Large Bronchi After Seven Daily Challenges with Aerosolized Ag in OVA-Alum Sensitized BALB/c Mice.
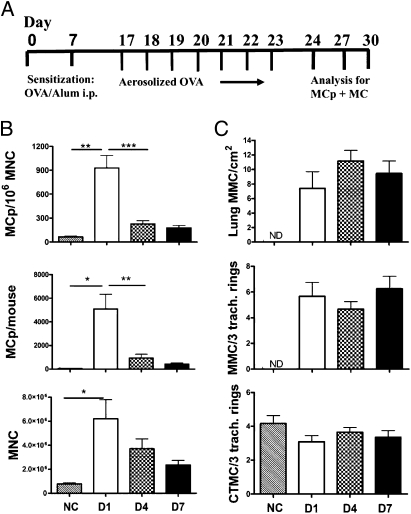
In our previous studies focused on the recruitment of MCp to lung, we sought to avoid their maturation to induced MMCs by using only three challenges of sensitized mice with aerosolized Ag. We routinely assessed the MCp 1 d after the last challenge by limiting dilution and clonal expansion, a high throughput assay that we had confirmed by identification of MCp by flow cytometry (22). We did not find any mature MCs in the lung mononuclear cell (MNC) preparations by cytochemistry. We also did not observe any increase in MMCs in the large bronchi or trachea by histochemistry 1 d after the last challenge (22–24) but did note a small increase in tracheal intraepithelial MMCs 7 d after the last challenge (22). Although others have provided protocols of longer duration that increase numbers of pulmonary MMCs (20, 21, 25), we sought to develop a protocol of less than 1 mo in duration. Thus, in the same cohort of BALB/c mice, we monitored both the recruitment of MCp within the MNC population isolated from lung and the appearance of MMCs in the lung and trachea. We arrived at a protocol in which we again sensitized mice with OVA-alum on days 0 and 7 but followed with seven daily aerosolized Ag challenges (days 17–23) with assessments on days 24, 27, and 30 (Fig. 1A). We assessed for both MCp per 106 lung MNCs and for CTMCs and MMCs in trachea and large bronchi in parallel cohorts at each of the three time points. MCp were assessed by limiting dilution and clonal expansion and mature MCs by histochemistry with chloroacetate esterase (CAE) reactivity. Values from both assays for sensitized and challenged mice were compared with sensitized but unchallenged controls.
Fig. 1.
Time course showing the changes in the numbers of lung MCp's and MCs in trachea and large airways of sensitized BALB/c mice on various days after the last of seven daily challenges. (A) The protocol of OVA-induced airway inflammation. BALB/c mice were sensitized with OVA adsorbed to alum on days 0 and 7 and either not challenged (NC) or challenged with aerosolized OVA daily on days 17–23. Mice from the original cohort were analyzed on day 24 (D1 postchallenge), day 27 (D4 postchallenge), or day 30 (D7 postchallenge). (B) The number of lung MCp's per 106 MNCs (Top), total lung MCp's per mouse (Middle), and isolated lung MNCs per mouse (Bottom) from NC mice or from challenged mice evaluated on D1, D4, or D7 postchallenge. The challenged values are the mean (± SE) from four separate experiments at each time point with 12 mice/group and the NC values are from 6 mice (two experiments). (C) The number of intraepithelial MMCs per cm2 in large bronchi (greater than 200 μm, Top), the number of MMCs per three tracheal rings (Middle), and the number of submucosal CTMCs per three tracheal rings (Bottom). Values are the mean (± SE) from NC: 6 mice, two experiments; D1: 12 mice, four experiments; D4: 30 mice, nine experiments; and D7: 20 mice, six experiments. ND, none detected; *P < 0.05, **P < 0.01, ***P < 0.001.
One day after seven challenges, there was a robust increase in pulmonary MCp concentration (MCp/106 MNC) (∼19-fold), in total lung MCp per mouse (∼136-fold) and in the total number of MNCs recovered from the lung (∼6.2-fold) relative to the sensitized but not challenged controls (Fig. 1B). In another cohort, 1 d after the seventh challenge, there was a striking appearance of intraepithelial MMCs in the trachea and large bronchi (200–250 μm) from a zero baseline in sensitized but unchallenged mice (Fig. 1C). The concentration of MCp and their numbers per mouse lung fell off significantly and selectively by postchallenge day 4, compared with the concomitant reduction of lung MNCs, which did not reach statistical significance even at day 7 postchallenge. At day 4 postchallenge, the numbers of induced MMCs in trachea and large bronchi were still comparable to the increment at day 1, and these numbers were still present at day 7 in another cohort (Fig. 1C). The significant decrease in the concentration of lung MCp/106 MNCs at the time of induction of intraepithelial MMCs in the trachea and large airways of parallel cohorts suggests that a rapid maturation of recruited MCp may account for the increase in the number of MMCs, which then persisted through the 7 d of study.
In contrast, there was no increment in numbers of submucosal CTMCs in the trachea at day 1 postchallenge but rather a slight decrease. The decrease is likely due to degranulation, although no extracellular granules were detected. Assessing MC degranulation by monitoring the appearance of extracellular granules is difficult even in passive cutaneous anaphylaxis and recording the response as reduced total MC numbers is reasonable (26). We cannot exclude the possibility that some of the decrease in CTMCs that occurs at the same time as the increase in MMCs may be due to migration of these CTMCs into the epithelial compartment. We also saw no degranulation of MMCs as evidenced by extracellular granules around the cells, and the absence of any MMCs in the unchallenged mice eliminates the possibility of seeing a reduction in number. Overall, the findings for induced MMCs in our protocol were similar to those reported with extended protocols for inflammation, although the remodeling observed in these longer protocols in terms of smooth muscle hypertrophy/hyperplasia, subepithelial fibrosis, collagen deposition, and increased angiogenesis, was absent (20, 21).
Specificity of the Abs Used to Define the Protease Phenotype of CTMCs and MMCs.
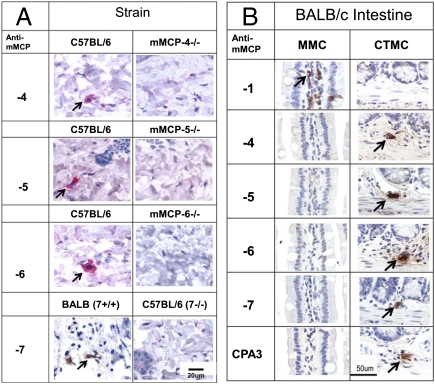
To determine the protease phenotype of the MCs found in trachea and large airways, we first evaluated the specificity of the various antipeptide IgGs using the appropriate protease-deficient mice that have become available since these Abs were first designed. We used dorsal skin from WT and protease-deficient mice to establish that our Abs directed to mMCP-4–7 did not recognize some other secretory granule protease. All samples contained CAE-reactive MCs. As shown in Fig. 2A, anti–mMCP-4–6 reacted with CTMCs in C57BL/6 skin but not with CTMCs in the skin of mice specifically deficient in these proteases. The specificity of mMCP-7 was confirmed by showing the reactivity in skin of BALB/c mice, which express mMCP-7, whereas there was no reactivity in the skin of C57BL/6 mice, which do not express this protease (27). The specificity of mAb to mMCP-1 has been previously reported (28) and was confirmed by showing reactivity in the helminth-induced MMCs of the intestine of BALB/c mice but not in the CTMCs in the intestinal submucosa (Fig. 2B) or in skin of C57BL/6 mice. Mice lacking mMCP-2 are not available. For the helminth-infected intestine, the MMCs showed only mMCP-1, whereas the CTMCs of the submucosa showed mMCP-4–7 and CPA3, but not mMCP-1. These findings with reagents of proven specificity confirmed the earlier data of a segregated protease profile for MMCs and CTMCs in the helminth-infected BALB/c small intestine (4, 5).
Fig. 2.
Evaluation of the specificity of the Abs directed to mMCP-4–7 peptides and of the mAb to mMCP-1. (A) Demonstration of the immunodetection of proteases mMCP-4–6 in dorsal skin of C57BL/6 mice and the absence of each protease in the respective strain with a targeted protease deficiency. To evaluate the specificity of the anti–mMCP-7 IgG, dorsal skin from mMCP-7+ BALB/c mice was compared with that from the naturally mMCP-7–deficient C57BL/6 strain. Magnification is indicated by the bar in Lower Right corner. (B) Demonstration of the immunodetection of mMCP-1 in intestinal MMCs and its absence in the intestinal submucosal CTMCs of T. spiralis-infected BALB/c mice and conversely, the lack of mMCP-4–7 and CPA3 in the MMCs and their presence in the CTMCs. Arrows indicated MCs positive for the various proteases.
Broad and Identical Protease Phenotype of CTMCs and MMCs in the Trachea.
The trachea is a mucosal-lined hollow tubular structure consisting of 15–18 evenly spaced parallel C-shaped rings of hyaline cartilage within and separated by fibroelastic tissue. The free dorsal ends of the cartilages are connected by bands of smooth muscle and connective tissue fibers. The wall of the trachea consists of mucosa, submucosa, cartilage-associated smooth muscle, and surrounding adventitia. The adventitia is loose connective tissue that is primarily composed of blood vessels, nerves, and adipose tissue surrounding the trachea.
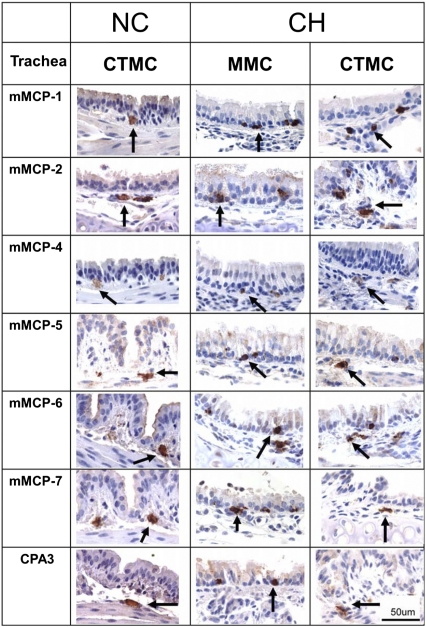
In sensitized but not challenged BALB/c mice, the CAE positive MCs of the trachea were located in the connective tissue of the submucosa and adventitial layers and there were none in the mucosa. The submucosal tracheal CTMCs of unchallenged mice expressed each of the seven proteases studied, namely the chymases mMCP-1, -2, and -4; the elastase mMCP-5; both tryptases, mMCP-6 and -7; and the carboxypeptidase, CPA3 (NC, not challenged; Fig. 3). The protease phenotype of CTMCs in adventitia of these unchallenged mice was the same as that in the submucosa. The CTMC in the submucosa and adventitia in the challenged mice showed the same seven proteases as in unchallenged mice (CH, challenged; Fig. 3). Thus, CTMCs of the trachea of sensitized BALB/c mice with or without challenge express the chymase mMCP-1, which is not seen in submucosal CTMCs of the small intestine or skin of the BALB/c strain and has been considered the hallmark of the jejunal MMCs (28, 29).
Fig. 3.
Both the constitutive CTMCs and the induced MMCs of the trachea express all seven secretory granule proteases. The Left column indicates the antiprotease specificity used to define the protease phenotype of tracheal MCs. Mice were either sensitized and not challenged (NC) or challenged (CH) for 7 d with analysis on day 4 postchallenge. Arrows indicate the protease reactive CTMCs in the submucosa of the trachea from NC and CH mice (Left and Right, respectively) and the induced intraepithelial tracheal MMCs from CH mice (Middle) that each expressed mMCP-1, -2, -4–7, and CPA3. No MMCs were found in the epithelium of trachea from NC mice. Images are representative of the results of analysis of three mice per group from three separate experiments. (Scale bar, 50 μm.)
Even more surprising was the finding of all seven of these proteases in the induced intraepithelial MMCs of the trachea (CH, Fig. 3). These MMCs were situated between tracheal epithelial cells above the basement membrane and were somewhat less granulated than the CTMCs, suggesting that they either had experienced some degranulation following Ag provocation or that they were newly arisen. Their expression of both tryptases, mMCP-6 and -7, the chymase mMCP-4, the elastase mMCP-5, and the carboxypeptidase CPA3, contrasts sharply with the prior characterization of mouse MMCs in the jejunum (Fig. 2B), which lack these five enzymes and express only the chymases, mMCP-1 and -2 (4, 5, 29).
Broad Protease Phenotype of the CTMCs and MMCs of the Large Airways in the Lung.
The trachea terminates at a main bifurcation (the carina) and gives rise to two main extra pulmonary bronchi that enter the left and right lung lobes and ramify in a monopodial branching pattern. There are no cartilaginous rings in the walls of bronchi within the lung. The pulmonary arteries accompany the airways in a sheath of connective tissue, which is defined as a bronchovascular bundle. The wall of an intrapulmonary bronchus consists of mucosa, a narrow layer of smooth muscle, submucosa, and adventitia. In the lungs of sensitized but unchallenged BALB/c mice, the CAE-positive MCs were distributed in the adventitial connective tissue surrounding the primary left and right lobar bronchi of at least 200 μm diameter, as observed for large bronchi in the rat (30). These CTMCs in the bronchi of unchallenged (NC, Fig. 4) or challenged mice (CH, Fig. 4), were positive for mMCP-1, -2, -4–7, and CPA3. Again, the finding of the chymase mMCP-1 in CTMCs was not expected, on the basis of the literature for CTMCs in jejunum and skin.
Fig. 4.
The constitutive CTMCs of the large bronchi in the lung express all seven secretory granule proteases, whereas the induced MMCs do not express mMCP-4, -5, or CPA3. The Left column indicates the antiprotease specificity used to define the protease phenotype of the MCs in the large bronchi in the lung. Images are from the bronchovascular bundles from the lung of the same animals depicted in Fig. 3. Arrows indicate the MCs positive for mMCP-1, -2, -4–7, and CPA3 in CTMCs around the large bronchi in the lung of unchallenged (NC) and challenged (CH) mice (Left and Right, respectively). Lung MMCs in the epithelium of the large airways of CH mice (Middle) expressed mMCP-1, -2, -6, and -7, and do not express mMCP-4, -5, or CPA3. The cellular infiltrates (red arrowhead) found in the bronchovascular bundles of the CH mice are highlighted in a single panel. No MMCs were found in the bronchial epithelium of NC mice. Images are representative of the results of analysis of three mice per group from three separate experiments. (Scale bar, 50 μm.)
In sensitized but not challenged mice, no intraepithelial MMCs were observed in the airways of the bronchovascular bundles in lung by either CAE reactivity or by immunohistochemistry using Abs specific to these seven different MC proteases. After challenge, the MMCs appearing in the epithelial lining of the large bronchi and assessed at day 4 expressed the chymases mMCP-1 and -2 and the tryptases mMCP-6 and -7 but not the chymase mMCP-4, the elastase mMCP-5, or CPA3 (CH, Fig. 4). The bronchovascular bundles were surrounded by cellular infiltrates with prominent eosinophils and lymphocytes and the bronchi showed desquamated epithelium, goblet cell metaplasia, and mucus plugging. The cellular infiltrate is noted by a red arrow in one of the panels showing MMCs (CH, Fig. 4). We did not observe any typical features of airway remodeling, such as thickening of basement membranes or hypertrophy of the bronchial wall muscle.
Discussion
Our findings that CTMCs and MMCs of lung and trachea exhibit a broad protease phenotype, which does not match that observed for CTMCs in mouse intestine and skin or for MMCs in intestine, imply several different lessons of which the foremost is that the protease phenotype of constitutive and T-cell–induced MCs is determined by the local host tissue. We had previously emphasized the distinction in protease profiles of intestinal CTMCs and MMCs as further defining the existence of these two subclasses by location and as reflecting the role of T cells in providing the MMCs but not CTMCs. However, we had recognized that these findings could reflect the broader role of the microenvironment on the basis of observing a mixed protease phenotype of MMCs in the intestinal lamina propria during resolution of a T. spiralis infection in which various lamina propria MMCs gained expression of mMCP-5 or mMCP-6 while retaining expression of mMCP-2 (4, 5). The role of the microenvironment was further supported by experiments using a cloned cell line developed by infection of bone marrow cells with a replication-deficient v-abl virus. In BALB/c mice injected i.v. with the transformed progenitor line expressing mMCP-5 and -6, the cells that developed into mature MCs in liver and spleen expressed these proteases and mMCP-2 and -7, whereas those expanding just below the intestinal epithelium in the small intestine turned off expression of mMCP-5 and -6 and expressed only mMCP-2 (7). The current findings now establish different protease phenotypes for CTMCs and induced MMCs at different tissue sites revealing local regulation at baseline and even in the presence of T-cell–dependent expansion. Thus, on the basis of comparative studies of gut and lung in the same strain, it appears that the immune response of T cells drives the increased appearance of MMCs, whereas the local tissue is the more important regulator of protease expression.
A possible second lesson relates back to the inability of investigators to derive intestinal-type MMCs expressing only mMCP-1 and -2 by culture of MCs from mouse bone marrow (BM). Immature mouse BM-derived MCs (BMMCs) developed by culture with stem cell factor, expressed mMCP-4–6 transcripts but not mMCP-1 and -2. Culture of BMMCs with IL-9 or IL-10 induced expression of mMCP-1 and -2, but without deletion of mMCP-5 or -6 (31–33), providing a protease phenotype not seen in the intestine at rest or with T. spiralis-induced inflammation. We now see that a similar complex protease profile holds for CTMCs of mouse trachea and large airways in lung and for MMCs in trachea. That mMCP-1 and -2 were present in the CTMCs of sensitized but unchallenged mouse trachea indicates that an induced local Th2 response was not needed for their expression. Miller and colleagues have found that TGFβ1 can induce mMCP-1 in BMMCs and that the inducing action of IL-9 for mMCP-1 is TGFβ1 dependent (34). As TGFβ1 is constitutively present in the lung, is up-regulated by allergic inflammation, and can be activated by the induced expression of αvβ6 integrins, this may be the mechanism by which the CTMCs and MMCs in this tissue express this chymase (35). However, the sustained expression of the other proteases is likely a function of stem cell factor and other local cytokines. Early studies demonstrated that BMMCs cocultured with a mouse fibroblast line increased heparin synthesis, histamine storage, and granule maturation, and this was ascribed to fibroblast production of stem cell factor (36, 37). More recently, a second fibroblast-derived factor, IL-33, which is an amplifier of innate mucosal immunity, was shown to augment mMCP-6 expression (38). Although T-cell–derived factors can generate and expand MMCs, it appears that stromal factors are the key regulators of the tissue protease phenotype. Addressing the mechanisms of the differential protease expression of pulmonary MCs is difficult, as the absence of the stromal factors implicated from in vitro studies, stem cell factor, and TGFβ1, results in a lack of all MCs and the presence of basal pulmonary inflammation, respectively (39, 40).
A third point is that the expression of the tryptases in lung CTMCs and MMCs is consistent with a role for these proteases in previous studies of host defense to bacterial infection and allergic pulmonary inflammation. Both human beta 1 tryptase and its murine ortholog, mMCP-6, caused a selective influx of neutrophils when instilled into the lung of BALB/c or C57BL/6 mice, and MC-deficient mice treated with active but not inactive human tryptase beta 1 were protected when challenged with K. pneumoniae 24 h later (14). This host defense function of mMCP-6 was supported by the selective recruitment of neutrophils into the peritoneal cavity after direct instillation (12) and by the impaired resolution of K. pneumoniae infection of the peritoneal cavity in mMCP-6–deficient mice (13). In contrast, in an immune complex-based arthritis model, a target of tryptase cleavage with pathologic consequences was the proteoglycan of the articular cartilage, aggrecan (41). Further, in two different mouse models of antigen sensitization and challenge-induced pulmonary inflammation and in atopic asthmatic humans, tryptase inhibitors have been shown to reduce various aspects of the airway inflammation. In a model of OVA/alum sensitization followed by four intratracheal OVA challenges, administration of a selective tryptase inhibitor intranasally 30 min before the last two challenges reduced the influx of eosinophils and the levels of IL-4 and IL-13 in the bronchoalveolar lavage (BAL) (42). There was also a decrease in the cellular infiltrate in the bronchovascular bundles and in goblet cell hyperplasia and mucus secretion. In a model using mice sensitized and challenged with intratracheal house dust mite extract, DerP, systemic administrations of inhibitors of limited selectively decreased the numbers of eosinophils and neutrophils in BAL (43). Furthermore, Krishna et al. (44) using a selective tryptase inhibitor found decreased late-phase bronchoconstriction in allergen-challenged atopic asthmatics. Thus, our observation of a robust constitutive and induced expression of the tryptases in the pulmonary tract likely underlies the prominent role these proteases play in innate responses linked to microbial host defense and allergic inflammation in the mouse airways.
A final point from these studies relates to our findings for mMCP-1 in CTMCs in submucosa and adventitia of trachea and large airways. The seminal studies of Miller and colleagues identified expression of this protease in jejunal MMCs and its liberation into the serum with helminth infection was consistent with this interpretation (28, 29). More recently, it was noted that mMCP-1 could be found in the MMCs induced in the airways of mice subjected to chronic allergen challenge (20) and that allergic reactions occurring in the lung with conventional protocols also increased serum mMCP-1 levels (43). We even noted mMCP-1 accumulation on alveolar macrophages as an innate systemic effect of hind limb reperfusion injury, wherein there was complement activation and transient permeability changes in the lung (45). In the absence of MMCs, we now suggest that the mMCP-1 was derived from peribronchial CTMCs. Further, mMCP-1 may have contributed to the change in lung permeability in this trauma model by analogy to its role in the intestine during worm expulsion (46).
Materials and Methods
Animals.
BALB/c and C57BL/6 mice were obtained from Taconic Laboratories. The protease-deficient mice have been described (8, 10, 13, 47). See SI Materials and Methods for more complete descriptions.
Ag Sensitization, Aerosol Challenge, T. spiralis Infection, and Pulmonary MCp Assessment.
These procedures have been described previously (4, 23, 48).
Histochemical and Immunohistochemical Evaluations of MCs.
For the histochemical evaluation of mature MCs, the tissues were prepared as described previously (4, 22). For immunohistochemical evaluation of the proteases, mMCP-1 was performed using a mAb from R&D Systems (28). The other proteases were detected using polyclonal antipeptide IgGs prepared in rabbits (7, 27, 49–52).
Supplementary Material
Acknowledgments
This work was supported by Grant R01-AI083516 (to M.F.G.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111048108/-/DCSupplemental.
References
- 1.Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds DS, et al. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci USA. 1990;87:3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens RL. Gene expression in different populations of mouse mast cells. Ann N Y Acad Sci. 1991;629:31–37. doi: 10.1111/j.1749-6632.1991.tb37958.x. [DOI] [PubMed] [Google Scholar]
- 4.Friend DS, et al. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friend DS, et al. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160:5537–5545. [PubMed] [Google Scholar]
- 6.Stevens RL, et al. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci USA. 1994;91:128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurish MF, et al. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995;3:175–186. doi: 10.1016/1074-7613(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 8.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groschwitz KR, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waern I, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- 13.Thakurdas SM, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, et al. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 15.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J Immunol. 1991;147:247–253. [PubMed] [Google Scholar]
- 17.Irani AM, et al. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 18.Dougherty RH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abonia JP, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–149. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pae S, et al. Chronic allergen challenge induces bronchial mast cell accumulation in BALB/c but not C57BL/6 mice and is independent of IL-9. Immunogenetics. 2010;62:499–506. doi: 10.1007/s00251-010-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu M, et al. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallgren J, et al. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abonia JP, et al. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones TG, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009;183:5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda RK, et al. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 26.Wershil BK, Murakami T, Galli SJ. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988;140:2356–2360. [PubMed] [Google Scholar]
- 27.Ghildyal N, et al. Lack of expression of the tryptase mouse mast cell protease 7 in mast cells of the C57BL/6J mouse. J Immunol. 1994;153:2624–2630. [PubMed] [Google Scholar]
- 28.Scudamore CL, et al. Mast cell heterogeneity in the gastrointestinal tract: Variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode-infected and normal BALB/c mice. Am J Pathol. 1997;150:1661–1672. [PMC free article] [PubMed] [Google Scholar]
- 29.Huntley JF, et al. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990;12:85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilkes LK, McMenamin C, Holt PG. Postnatal maturation of mast cell subpopulations in the rat respiratory tract. Immunology. 1992;75:535–541. [PMC free article] [PubMed] [Google Scholar]
- 31.Eklund KK, Ghildyal N, Austen KF, Stevens RL. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol. 1993;151:4266–4273. [PubMed] [Google Scholar]
- 32.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem. 1992;267:8473–8477. [PubMed] [Google Scholar]
- 33.Ghildyal N, et al. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992;149:2123–2129. [PubMed] [Google Scholar]
- 34.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: Upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–3486. [PubMed] [Google Scholar]
- 35.Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 36.Dayton ET, et al. 3T3 fibroblasts induce cloned interleukin 3-dependent mouse mast cells to resemble connective tissue mast cells in granular constituency. Proc Natl Acad Sci USA. 1988;85:569–572. doi: 10.1073/pnas.85.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurish MF, et al. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J Exp Med. 1992;175:1003–1012. doi: 10.1084/jem.175.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaieda S, et al. Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1) J Biol Chem. 2010;285:21478–21486. doi: 10.1074/jbc.M110.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura Y, Hirota S, Nishida T. A loss-of-function mutation of c-kit results in depletion of mast cells and interstitial cells of Cajal, while its gain-of-function mutation results in their oncogenesis. Mutat Res. 2001;477:165–171. doi: 10.1016/s0027-5107(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 40.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 41.Shin K, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh SW, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 43.Chen CL, et al. Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma. J Allergy Clin Immunol. 2006;118:105–112. doi: 10.1016/j.jaci.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 44.Krishna MT, et al. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. J Allergy Clin Immunol. 2001;107:1039–1045. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- 45.Mukundan C, Gurish MF, Austen KF, Hechtman HB, Friend DS. Mast cell mediation of muscle and pulmonary injury following hindlimb ischemia-reperfusion. J Histochem Cytochem. 2001;49:1055–1056. doi: 10.1177/002215540104900813. [DOI] [PubMed] [Google Scholar]
- 46.McDermott JR, et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens RL, et al. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene cannot store carboxypeptidase A in their granules. FASEB J. 1996;10:1307. [Google Scholar]
- 48.Gurish MF, et al. Intestinal mast cell progenitors require CD49dbeta7 (α4β7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghildyal N, Friend DS, Nicodemus CF, Austen KF, Stevens RL. Reversible expression of mouse mast cell protease 2 mRNA and protein in cultured mast cells exposed to IL-10. J Immunol. 1993;151:3206–3214. [PubMed] [Google Scholar]
- 50.Forsberg E, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 51.McNeil HP, Frenkel DP, Austen KF, Friend DS, Stevens RL. Translation and granule localization of mouse mast cell protease-5. Immunodetection with specific antipeptide Ig. J Immunol. 1992;149:2466–2472. [PubMed] [Google Scholar]
- 52.Ghildyal N, et al. Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis: Prolonged retention of exocytosed mMCP-6 in connective tissues, and rapid accumulation of enzymatically active mMCP-7 in the blood. J Exp Med. 1996;184:1061–1073. doi: 10.1084/jem.184.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.