Abstract
The contributions of aryl hydrocarbon receptor (Ahr) to the pathogenesis of rheumatoid arthritis have not been elucidated. Here, we show that Ahr deficiency ameliorated collagen-induced arthritis, a mouse model of RA. Collagen-immunized Ahr KO mice showed decreased serum levels of such proinflammatory cytokines as IL-1β and IL-6. The Th17 and Th1 cell populations in lymph nodes from these mice decreased and increased, respectively, whereas the percentage of regulatory T cells was unchanged. Interestingly, a lack of Ahr specifically in T cells significantly suppressed collagen-induced arthritis development, whereas Ahr deficiency in macrophages had no effect. These finding indicate that the development of experimental autoimmune arthritis depends on the presence of Ahr in T cells, and that Th1/Th17 balance may be particularly important for this process.
Keywords: dioxin receptor, autoimmunity, immune regulation
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder that affects approximately 1% of the population. The disease markedly affects joints, which become characterized by inflammatory cell infiltration, synovial lining hyperplasia, pannus formation, and cartilage and bone destruction. Although the pathogenesis of the disease is not fully understood, available evidence supports a central role for CD4+ T cells in the initiation and persistence of the chronic autoimmune response characteristic of RA (1). At present, TNF-α and IL-6 are important therapeutic targets. In some patients, for example, blocking TNF-α signaling results in rapid and sustained improvements in clinical signs and symptoms (2–4). The anti–IL-6 receptor monoclonal antibody tocilizumab also inhibits the progression of joint damage and reduces disease activity, including in patients who do not show a sufficient response to anti–TNF-α therapy (5–7). Recent studies of animal models have suggested that IL-17A–secreting CD4+ T cells—called Th17 cells—may play a key role in the progression of RA and multiple sclerosis (8–12). Th17 cell differentiation is induced by TGF-β and IL-6 in mice, whereas, in humans, IL-1β but not TGF-β participates with IL-6 in the development of Th17 cells (13, 14). Consistent with these finding, IL-6 deficiency or treatment with anti–IL-6 receptor antibodies (MR16-1) has been shown to inhibit Th17 cell development and arthritis onset (12, 15, 16).
Collagen-induced arthritis (CIA) is a widely studied animal model that reproduces many of the pathogenic mechanisms identified in human RA (17). Both T and B cells are required for disease initiation (18), although administration of collagen type II-specific antibodies reproduces the arthritic features of the disorder without the need for either of these immune cell populations (19).
Ahr is a ligand-activated transcription factor that belongs to the basic helix–loop–helix/PER–ARNT–SIM family (20–22). During immune responses, Ahr is activated by various ligands to regulate the generation of regulatory T cells and Th17 cells (23, 24). Although Ahr is known to perform important roles in immune regulation, how Ahr modulates RA-related immune responses in individual cell populations is unknown. Our group and others demonstrated that Ahr is expressed in naive T cells in response to TGF-β and IL-6, and contributes to the differentiation of Th17 cells (24–26). More recently, Ahr was shown to play an important negative regulatory role in proinflammatory responses in macrophages stimulated with LPS (27).
In the present study, we use mouse models of RA to show that levels of proinflammatory cytokines, for example, IL-6 and the Th17 cell population were decreased in collagen-immunized Ahr KO mice. Importantly, we provide evidence that Ahr deficiency in T cells, but not macrophages, inhibits Th17 cell generation and the onset of CIA.
Results
Suppression of CIA in Ahr KO Mice.
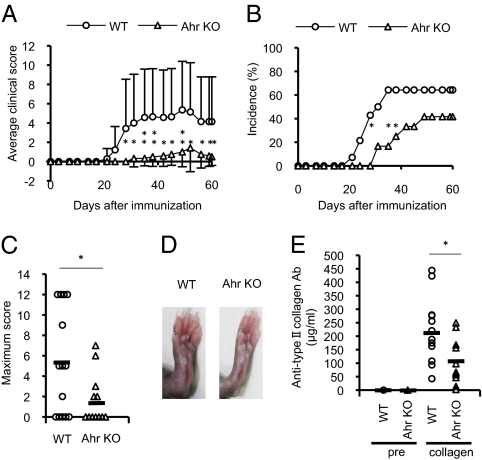
To determine the pathophysiologic role of Ahr in RA, we evaluated the effects of Ahr deficiency on CIA development. Arthritic symptoms were assessed in Ahr KO and WT mice after immunization with collagen on days 0 and 21. Arthritis severity was assessed using a visual scoring system. We investigated Ahr expression in inguinal lymph node cells from collagen-immunized mice by using real-time PCR. We found that Ahr expression was significantly higher in inguinal lymph node cells from mice with CIA than in those from control mice (Fig. S1). Clinical scores and disease incidence were markedly suppressed in collagen-immunized Ahr KO mice compared with WT mice with CIA (Fig. 1 A–D). Moreover, in the Ahr KO mice, we detected lower serum levels of anti-chicken type II collagen IgG (Fig. 1E), antibodies that are usually found at high levels during CIA development (28). These results indicate that Ahr is involved in development of CIA.
Fig. 1.
Ahr promotes CIA development. Mice were immunized with chicken type II collagen emulsified with CFA. Intradermal injections were made at several sites in the base of the tail on days 0 and 21. The mean clinical score (A), CIA incidence (B), and maximum clinical score (C) were recorded until day 60. Data are presented as the mean clinical and maximum scores ± SEM (WT, n = 14; Ahr KO, n = 12). P values were obtained with Student t tests. (D) Hind joints obtained 14 d after the second immunization. (E) Sera were collected from collagen-immunized mice on day 60 and serum levels of collagen-specific IgG were determined by ELISA (WT, n = 12; Ahr KO, n = 11; *P < 0.05; **P < 0.01).
Ahr Deficiency Blocks Cartilage Destruction and Reduces Matrix Metalloproteinase-3 Levels.
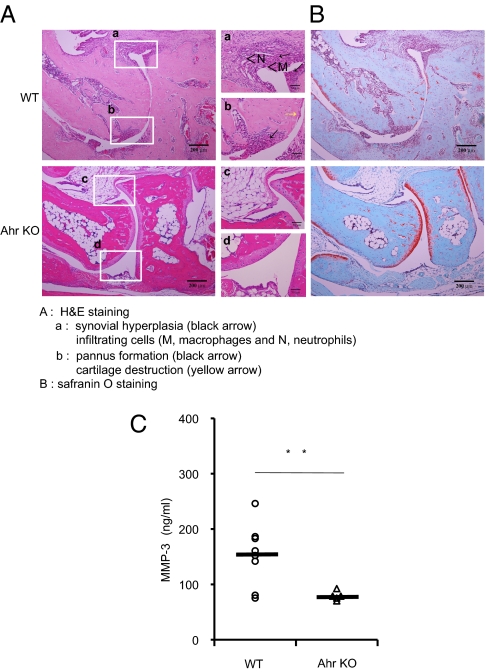
Mice lacking Ahr showed significantly suppressed CIA development, whereas WT joints showed severe pathology with cartilage destruction, bone erosion, cell infiltration, and pannus formation. Ahr KO mice showed notably suppression of these hallmarks of autoimmune arthritis (Fig. 2A). Safranin O-stained joints from collagen-immunized Ahr KO mice showed markedly reduced cartilage destruction compared with the joints of WT mice with CIA (Fig. 2B). It has been reported that serum matrix metalloproteinase (MMP)-3 is a good indicator in the inflammatory aspect and cartilage damage of CIA (29). Serum levels of MMP-3 are elevated in RA, providing a useful marker of synovial inflammatory activity (30). We found that serum MMP-3 levels in collagen-immunized Ahr KO mice were significantly reduced compared with WT mice with CIA (Fig. 2C). Therefore, decreased MMP-3 in serum may also reflect the lack of cartilage destruction in the collagen-immunized Ahr KO mice.
Fig. 2.
Ahr deficiency inhibits CIA-induced joint inflammation. (A and B) Representative sections of ankle joints from WT (a and b) and Ahr KO (c and d) mice 60 d after the second immunization were stained with H&E (A) or Safranin O (B). (a) Severe pathologic features were observed in the WT ankle joint, including synovial hyperplasia (black arrowheads) and infiltrating cells (M, macrophages; N, neutrophils). (b) Pannus formation (black arrowhead) and cartilage destruction (yellow arrowhead) were also observed in the WT sample. (c and d) Ankle joints from Ahr KO mice appeared normal. (C) Fourteen days after the second immunization, sera were collected and MMP-3 levels were measured by using a specific ELISA (WT, n = 8; Ahr KO, n = 6; **P < 0.01).
Reduced Proinflammatory Cytokine Levels and Th17 Cell Development in Collagen-Immunized Ahr KO Mice.
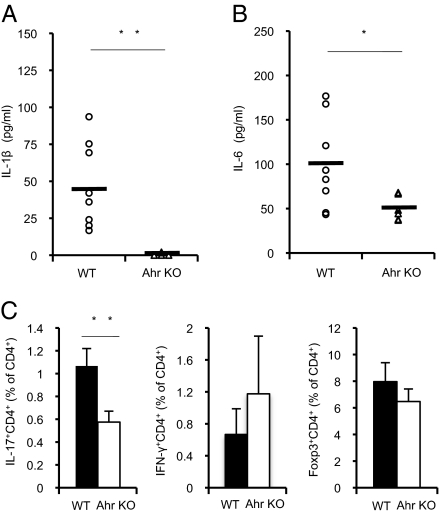
Although Ahr in combination with Stat1 negatively regulates proinflammatory cytokine production in macrophages (27), we detected reduced IL-1β and IL-6 levels in collagen-immunized Ahr KO mice compared with WT mice with CIA (Fig. 3 A and B). IL-6 is critical for Th17 cell generation, a process that is also mediated by IL-1β (13, 14). Recent studies with animal models have suggested that Th17 cells play key roles in the progression of RA and experimental autoimmune encephalomyelitis (EAE) (8–12). Furthermore, our group and others demonstrated that Ahr is expressed in naive T cells in response to TGF-β and IL-6, and involved in Th17 cell differentiation (24–26). To investigate Th17 cell generation during CIA, we isolated inguinal lymph node cells from collagen-immunized WT and Ahr KO mice and examined the populations of regulatory T (Treg), Th1, and Th17 cells by using fluorescence-activated cell sorting. The Ahr KO mice showed an approximate 50% decrease in the percentage of cells that were Th17 cells, whereas the population of Th1 cells increased by 1.8 fold (Fig. 3C). Although some evidence indicate that Ahr promotes TGF-β–induced Treg cell generation (24, 25), Ahr deficiency did not inhibit the development of Treg cells in collagen-immunized mice. These results suggest that Ahr deficiency suppressed the development of CIA possibly by inhibiting proinflammatory cytokine production and Th17 cell generation.
Fig. 3.
Ahr deficiency inhibits Th17 cell differentiation in response to collagen immunization. (A and B) Fourteen days after the second immunization, sera were collected and levels of the proinflammatory cytokines IL-1β (A) and IL-6 (B) were measured by using specific ELISAs (WT, n = 8; Ahr KO, n = 6). (C) CD4+ T cells selected from inguinal lymph node cells by magnetic-activated cell sorting (MACS) obtained from mice 14 d after the second immunization were counted after intracellular staining of IFN-γ, IL-17, or Foxp3 (WT, n = 3; Ahr KO, n = 4; *P < 0.05; **P < 0.01).
Lck-Cre Ahrflox/flox Mice Are Resistant to CIA Development.
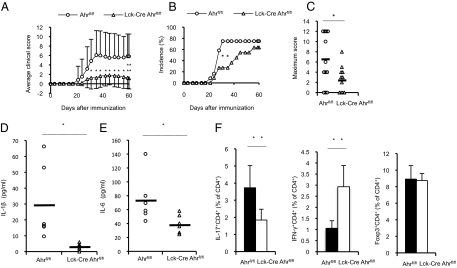
The roles of Ahr in various immune cells are still unknown. To determine which cell types are important for CIA inhibition in Ahr KO mice, we used conditional KO strains. Ahr was specifically deleted in T cells by crossing Ahrflox/flox mice with Lck-Cre mice (Fig. S2A). Interestingly, clinical scores and the disease incidence in collagen-immunized Lck-Cre Ahrflox/flox mice were markedly suppressed compared with control mice (Fig. 4 A–C). Whereas histologic analysis of joints from control mice with CIA revealed characteristic features of arthritis, collagen-immunized Lck-Cre Ahrflox/flox mice showed reduced cartilage destruction and serum MMP-3 levels (Fig. S3). Moreover, collagen-immunized Lck-Cre Ahrflox/flox mice showed significantly reduced serum levels of IL-1β and IL-6 (Fig. 4 D and E). We also examined the roles of Ahr in the development of various helper T-cell subsets during CIA development (Fig. 4F). Collagen-immunized Lck-Cre Ahrflox/flox mice showed an approximate 50% decrease in the percentage of Th17 cells, whereas the percentage of Treg cells was not affected. This result agrees with previous studies, which showed that Ahr is expressed in naive T cells in response to TGF-β and IL-6, and involved in Th17 cell differentiation (24–26). Moreover, consistent with Th1 cell differentiation in collagen-immunized Ahr KO mice, immunization with type II collagen tripled the percentage of Th1 cells in Lck-Cre Ahrflox/flox mice compared with control mice. An imbalance in the ratio of Th17 cells to Treg cells was recently proposed as an important pathogenic mechanism in RA development and progression (31, 32). However, we observed fewer Th17 cells and more Th1 cells in collagen-immunized Lck-Cre Ahrflox/flox mice, suggesting that a relative shift in these populations may promote CIA development. Thus, Ahr in T cells is critical for Th1 and Th17 cell generation and the onset of CIA.
Fig. 4.
CIA is significantly suppressed in Lck-Cre Ahrflox/flox mice. Ahrflox/flox and Lck-Cre Ahrflox/flox mice were immunized with collagen. Mice were assessed for the clinical arthritis score (A), disease incidence (B), and maximum CIA score (C) until day 60. Data are presented as the mean clinical and maximum scores ± SEM (Ahrflox/flox, n = 12; Lck-Cre Ahrflox/flox, n = 11). Fourteen days after the second immunization, sera were collected and levels of the proinflammatory cytokines IL-1β (D) and IL-6 (E) were measured using specific ELISAs (Ahrflox/flox, n = 6; Lck-Cre Ahrflox/flox, n = 6). (F) CD4+ T cells selected from inguinal lymph node cells by MACS obtained from mice 14 d after the second immunization were counted after IFN-γ, IL-17, or Foxp3 were intracellularly stained (Ahrflox/flox, n = 7; Lck-Cre Ahrflox/flox, n = 7; *P < 0.05; **P < 0.01).
CIA Development Is Not Suppressed in LysM-Cre Ahrflox/flox Mice.
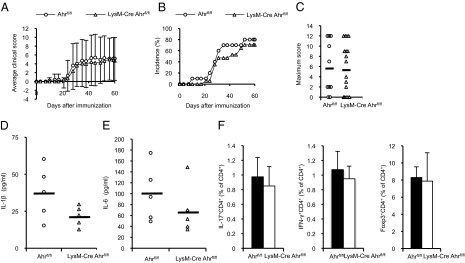
To examine macrophage-specific roles of Ahr in CIA, Ahr was specifically deleted in macrophages by crossing Ahrflox/flox mice with LysM-Cre mice (Fig. S2B). The incidence of disease and clinical scores were not significantly different in collagen-immunized LysM-Cre Ahrflox/flox and control mice (Fig. 5 A–C). Collagen-immunized LysM-Cre Ahrflox/flox mice also showed characteristic features of arthritis and elevated serum levels of MMP-3 (Fig. S4). We also measured serum levels of IL-1β and IL-6 in LysM-Cre Ahrflox/flox mice (Fig. 5 D and E); levels of these proinflammatory cytokines were lower in LysM-Cre Ahrflox/flox mice with CIA than in collagen-immunized control mice. This result did not agree with our previous finding, which showed that Ahr negatively regulates the LPS signaling pathway (27). The percentages of Th1, Th17, and Treg cells in lymph nodes from LysM-Cre Ahrflox/flox and control mice with CIA were similar (Fig. 5F). Thus, Ahr deficiency in macrophages reduced proinflammatory cytokine production, and had no effect on CIA development. Data reflecting the effects of Ahr deficiency in three types of collagen-immunized mice are summarized in Table 1. In collagen-immunized Ahr KO mice, reductions in disease incidence and clinical scores were similar to results from Lck-Cre Ahrflox/flox CIA mice, but not LysM-Cre Ahrflox/flox CIA mice.
Fig. 5.
CIA is not suppressed in LysM-Cre Ahrflox/flox mice. CIA was induced in Ahrflox/flox and LysM-Cre Ahrflox/flox mice as described in Fig. 1. The clinical arthritis score (A), disease incidence (B), and maximum CIA score (C) were recorded until day 60. Data are presented as the mean clinical and maximum scores ± SEM (Ahrflox/flox, n = 10; LysM-Cre Ahrflox/flox, n = 17). Fourteen days after the second immunization, sera were collected and levels of the proinflammatory cytokines IL-1β (D) and IL-6 (E) were measured by using specific ELISAs (Ahrflox/flox, n = 5; LysM-Cre Ahrflox/flox, n = 5). (F) CD4+ T cells selected from inguinal lymph node cells by MACS obtained from mice 14 d after the second immunization were counted after intracellular staining of IFN-γ, IL-17, or Foxp3 (Ahrflox/flox, n = 4; LysM-Cre Ahrflox/flox, n = 4).
Table 1.
Inhibition of the CIA development by Ahr deficiency in three types of mice
| Inhibition of CIA development vs. control, % |
||||||
| Day 35 |
Day 49 |
Day 60 |
||||
| Group | MCS | Incidence | MCS | Incidence | MCS | Incidence |
| Ahr KO VS WT | 92.7 | 74.1 | 81.3 | 35.2 | 87.9 | 35.2 |
| Lck-Cre Ahrfl/fl VS Ahrfl/fl | 79.1 | 63.6 | 69.5 | 27.3 | 79.7 | 15.2 |
| LysM-Cre Ahrfl/fl VS Ahrfl/fl | 13.6 | 32.8 | 13.6 | 7.6 | 9.5 | 11.8 |
To normalize the different genetic background between Ahr KO, Lck-Cre Ahrflox/flox and LysM-Cre Ahrflox/flox, the inhibition percentage of the CIA development based on the mean clinical score and CIA incidence in these mice was calculated at three time points (days 35, 49, and 60). These values were indicated by comparison with those of each control. The inhibition percentage is calculated as follows: (mean clinical score or CIA incidence of three types of Ahr deficient mice / mean clinical score or CIA incidence of each control mice) × 100%. MCS, mean clinical score.
Discussion
We showed that CIA development is strongly blocked in the absence of the dioxin receptor Ahr. Although a number of studies have recently detailed the roles of Ahr in such autoimmune diseases as EAE and inflammatory bowel disease, including contributions to Th17 and Treg cell development (24, 26, 33), how Ahr modulates the responses of various immune cell populations in autoimmune disease remained unclear. Furthermore, our group and others have demonstrated that Ahr KO mice are hypersensitive to LPS-induced septic shock, mainly as a result of macrophage dysfunction (27, 34). Because Ahr is highly expressed in synovial tissues of RA patients but not osteoarthritis patients, and 2,3,7,8-tetrachlorodibenzo-p-dioxin up-regulates IL-1β, IL-6, and IL-8 through binding to Ahr in RA synoviocytes, Ahr was suggested as a possible contributor to RA development (35). Here we examined the functions of Ahr in T cells and macrophages during CIA development by using two conditional Ahr KO mice: Lck-Cre Ahrflox/flox and LysM-Cre Ahrflox/flox.
CIA development was clearly blocked in Ahr KO and Lck-Cre Ahrflox/flox mice, but not in LysM-Cre Ahrflox/flox mice. Furthermore, the Th17 cell percentage was significantly reduced in collagen-immunized Ahr KO and Lck-Cre Ahrflox/flox mice. This result is consistent with previous findings of attenuated Th17 differentiation from naive T cells lacking Ahr (25, 26). Although Ahr appeared to participate in Th17 and Treg cell differentiation in vitro (25), Treg differentiation was not affected in collagen-immunized Ahr KO and Lck-Cre Ahrflox/flox mice in this study. On the contrary, Quintana et al. demonstrated that 2,3,7,8-tetrachlorodibenzo-p-dioxin–mediated activation of Ahr induced Treg cell differentiation, inhibited Th17 cell generation, and ameliorated EAE development. Our results, however, indicated that Ahr deficiency in T cells suppresses Th17 cell differentiation, but not Treg cell development, after immunization with type II collagen.
Of note, the Th1 cell population increased in collagen-immunized Ahr KO and Lck-Cre Ahrflox/flox mice. Although Th1 cells have been proposed to play a central role in RA, mice lacking IFN-γ, IFN-γ receptor, or IL-12p35 developed aggressive arthritis, despite defects in Th1 cells (36). In addition, IFN-γ was shown to suppress Th17 cell differentiation in vitro, potentially preventing or controlling autoimmune responses (9, 10). Furthermore, a recent study showed that autoimmune disease is regulated by the systemic ratio of IL-17 to IFN-γ rather than the absolute level of any single cytokine, further suggesting that the balance between Th1 cells and Th17 cells plays an important role in CIA (37).
Interestingly, disease incidence and CIA clinical scores were similar in LysM-Cre Ahrflox/flox and control mice, suggesting that Ahr in macrophages has little, if any, effect on disease induction. Strikingly, expression of proinflammatory cytokines, such as IL-6, decreased in LysM-Cre Ahrflox/flox mice with CIA, which is not consistent with previous reports of an anti-inflammatory function for Ahr in macrophages (27). Kimura et al. demonstrated that Ahr in combination with Stat1 negatively regulates LPS-induced inflammatory responses in macrophages (27). The authors also showed that Ahr interacted with Stat1 in LPS-stimulated peritoneal macrophages, but not with CpG oligodeoxynucleotides. Notably that the CpG oligodeoxynucleotide–TLR9 and LPS–TLR4 signaling pathways both induce proinflammatory cytokine expression via MyD88-NF-κB (38). These finding provide the possibility that may account for the different signaling pathway between the LPS and the process of CIA in regulation by Ahr in macrophages. Furthermore, an absence of Ahr in dendritic cells ameliorates IL-10 production, inhibits Treg cells development, and promotes Th17 cell generation in the presence of LPS or CpG in vitro (39). In this study, collagen-immunized Ahr KO mice showed a decrease in the percentage of Th17 cells, whereas the percentage of Treg cells was not affected, suggesting that Ahr in dendritic cells probably has no effect on the differentiation of Treg and Th17 cells in CIA. The roles of Ahr in various immune cells may differ in vitro and in vivo for a number of reasons. The immunopathogenesis of CIA involves both T-cell and B-cell responses to type II collagen (40). The pathogenesis of RA involves interactions among T cells, B cells, macrophages, dendritic cells, and fibroblasts, which create a regulatory feedback loop of cytokine signaling in vivo (41). In addition, CIA may cause chronic inflammation characterized by a different overall proinflammatory cytokine profile than that observed with LPS-induced acute inflammation. Nevertheless, our results showed that Ahr is not required in macrophages for CIA development.
To summarize, we have demonstrated that Ahr deficiency in T cells, but not macrophages, suppresses CIA development as was observed in Ahr KO mice. These effects may result from inhibited Th17 generation and proinflammatory cytokine production. In RA, Ahr mainly functions during Th1 and Th17 cell development, although roles in other cell types may contribute to autoimmune diseases. Future studies using conditional KO strains of Ahr mice will likely further define the roles of Ahr in other autoimmune diseases.
Materials and Methods
Mice.
Ahr KO mice (C57BL/6 background) were provided by Y. Fujii-Kuriyama (University of Tsukuba, Tsukuba, Japan). C57BL/6 WT mice were purchased from Charles River Japan. LysM-Cre Ahrflox/flox (macrophage-specific Ahr conditional KO) mice or Lck-Cre Ahrflox/flox (T-cell–specific Ahr conditional KO) mice were generated by breeding Ahrflox/flox mice and LysM-Cre or Lck-Cre knock-in mice, respectively. Six- to 8-wk-old female mice were used. All animal experiments were performed based on protocols approved by the institutional animal care and use committees of the Graduate School of Frontier Biosciences at Osaka University.
CIA.
CIA was performed as previously described (42). Complete Freund adjuvant (CFA) was prepared by grinding 100 mg of heat-killed Mycobacterium tuberculosis (H37Ra; Difco Laboratories) in 20 mL of incomplete Freund adjuvant (Sigma). An emulsion was formed by dissolving 2 mg/mL chicken type II collagen (Sigma) in 10 mM acetic acid overnight at 4 °C and combining it with an equal volume of CFA. The collagen-containing solution and the emulsion with CFA were always freshly prepared. Mice were injected intradermally at several sites into the base of the tail with a total of 100 μL of an emulsion containing 100 μg of type II collagen and 250 μg of M. tuberculosis. The same injection was repeated on day 21. Animals were clinically scored twice each week for as long as 60 d after the first immunization. For histologic analysis, mice were killed 60 d after the first immunization. Hind paws were collected for H&E and Safranin O staining. Cytokine levels were measured in serum prepared 35 d after the first immunization. Mouse IL-1β, IL-6, and MMP-3 levels from serum were measured by using an ELISA according to the manufacturer's instructions (R&D Systems). Measurement of anti-type II collagen IgG levels was performed with serum samples prepared 60 d after the first immunization, with an ELISA kit from Chondrex.
Evaluation of Arthritis Severity.
Clinical arthritis was assessed by using the following scale (42): grade 0, normal; grade 1, slight swelling and/or erythema; grade 2, extensive swelling and/or erythema; and grade 3, joint distortion and/or rigidity. The maximum score per mouse was 12. Mice with two consecutive positive evaluations were given a diagnosis of arthritis.
Flow Cytometry.
CD4+ T cells were purified from inguinal lymph node isolated from mice immunized with type II collagen by CD4+ T-cell isolation kit (Miltenyi). CD4+ T cells were stimulated with 50 ng/mL PMA (Calbiochem) and 800 ng/mL ionomycin (Calbiochem) for 5 h, with GolgiStop (BD PharMingen) added for the final 2 h. Samples were fixed and permeabilized with Cytofix/Cytoperm (BD PharMingen). Cells were stained with phycoerythrin-conjugated anti–IL-17 (BD PharMingen) and FITC-conjugated anti–IFN-γ (eBioscience) antibodies. For Foxp3 staining, cells were fixed and permeabilized by using fixation/permeabilization buffer (eBioscience) for 30 min at 4 °C before intracellular staining with FITC-conjugated anti-Foxp3 antibodies (eBioscience). Flow cytometric analysis was performed with a Cytomics FC500 system (Beckman Coulter).
qPCR.
Inguinal lymph node cells from C57BL6 mice 35 d after the first immunization were isolated. Naive T cells were purified from the spleens of Lck-Cre Ahrflox/flox and control mice and stimulated with Dynabeads Mouse CD3/CD28 T-cell Expander (Invitrogen), mouse IL-6 (20 ng/mL; R&D Systems), and human TGF-β1 (2 ng/mL; R&D Systems) for 2 d in RPMI medium. Thioglycollate-elicited peritoneal macrophages from LysM-Cre Ahrflox/flox and control mice were stimulated with 1 μg/mL LPS (Escherichia coli; Sigma) for 4 h. Total RNA was prepared using RNeasy (Qiagen) according to the manufacturer's protocol. Reverse transcription was performed in a thermal cycler (Applied Biosystems). A mouse Ahr probe (Gene Expression Assays, Mm00478930_m1; Applied Biosystems) was used. For reference, we quantified mouse GAPDH expression (Applied Biosystems). qPCR was carried out in a PRISM 7900 HT system (Applied Biosystems). Cycling conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. We used the comparative ΔΔCt method normalized to GAPDH expression to determine levels of Ahr mRNA.
Statistical Analysis.
Clinical score, serum levels of anti-chicken type II collagen IgG, IL-1β, IL-6, and MMP-3, and the percentage of each CD4+ T cell were analyzed with Student t tests. CIA incidence was analyzed with Fisher exact test. P values lower than 0.05 were regarded as significant.
Supplementary Material
Acknowledgments
This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences from the National Institute of Biomedical Innovation and by Chugai−Roche Pharmaceutical (Tokyo, Japan).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111786108/-/DCSupplemental.
References
- 1.Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 2.Maini R, et al. ATTRACT Study Group Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 5.Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biologics. 2008;2:75–82. doi: 10.2147/btt.s1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery P, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolen JS, et al. OPTION Investigators Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 8.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 10.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakae S, et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirota K, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto M, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 16.Iwanami K, et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 17.Trentham DE. Collagen arthritis as a relevant model for rheumatoid arthritis. Arthritis Rheum. 1982;25:911–916. doi: 10.1002/art.1780250801. [DOI] [PubMed] [Google Scholar]
- 18.Stuart JM, Townes AS, Kang AH. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- 19.Terato K, et al. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 20.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ema M, et al. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 22.Fujii-Kuriyama Y, Ema M, Mimura J, Sogawa K. Ah receptor: A novel ligand-activated transcription factor. Exp Clin Immunogenet. 1994;11:65–74. doi: 10.1159/000424195. [DOI] [PubMed] [Google Scholar]
- 23.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 25.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeuws S, et al. A multiparameter approach to monitor disease activity in collagen-induced arthritis. Arthritis Res Ther. 2010;12:R160. doi: 10.1186/ar3119. 10.1186/ar3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshihara Y, et al. Increased levels of stromelysin-1 and tissue inhibitor of metalloproteinases-1 in sera from patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:969–975. doi: 10.1002/art.1780380713. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, et al. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2011 doi: 10.1007/s00296-010-1710-0. 10.1007/s00296-010-1710-0. [DOI] [PubMed] [Google Scholar]
- 32.Boissier MC, Assier E, Falgarone G, Bessis N. Shifting the imbalance from Th1/Th2 to Th17/treg: the changing rheumatoid arthritis paradigm. Joint Bone Spine. 2008;75:373–375. doi: 10.1016/j.jbspin.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Takamura T, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 34.Sekine H, et al. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi S, et al. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1317–1322. doi: 10.1093/rheumatology/ken259. [DOI] [PubMed] [Google Scholar]
- 36.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S, et al. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: Roles of endogenous interferon-gamma and IL-4. Arthritis Res Ther. 2009;11:R158. doi: 10.1186/ar2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen NT, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 41.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.