In a recent paper, Dring et al. (1) showed that natural killer (NK) cell-associated killer cell Ig-like receptors (KIR) gene KIR2DS3 and IL28B (IFNλ3) T allele are associated with chronic hepatitis C, confirming earlier reports (2, 3). Interestingly, they showed that the presence of both genetic markers synergistically increased the risk of chronic infection over either factor alone (1).
As a potential explanation for this finding, they presented experimental data suggesting that IL28A inhibits IFN-γ secretion by NK cells (1). Thus, Dring et al. (1) proposed a functional link between NK cells and λ-IFNs.
This finding is unexpected because IFN-λR1, the natural receptor of λ-IFNs, has been shown to display a rather restricted expression pattern, limiting the response to λ-IFNs to primarily epithelium-like tissues, whereas leukocytes do not respond to IFN-λ (2).
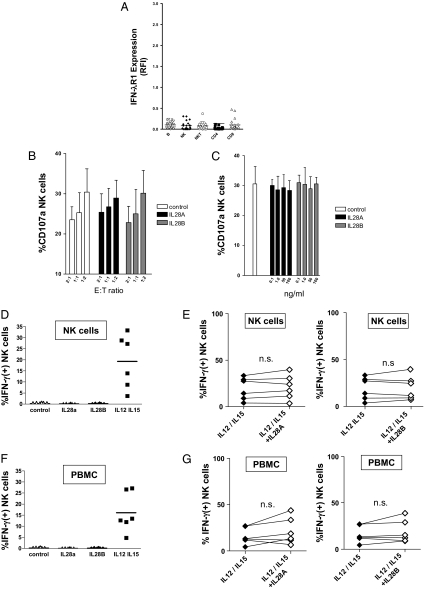
In line with the previously reported absence of IFN-λR1 on hemopoietic cells (4), we could not detect IFN-λR1 surface expression on freshly isolated NK cells from healthy donors (Fig. 1A). Furthermore, neither recombinant IL28A nor IL28B revealed any detectable effects on cytolytic activity of purified NK cells against MHC I-deficient K562 target cells, even at high concentrations (Fig. 1 B and C). In addition, we could not observe any significant effects of IL28A or IL28B on IFN-γ secretion in either resting or IL12/15-stimulated NK cells, respectively (Fig. 1 D and E).
Fig. 1.
Effect of λ-IFNs on NK cell functions. (A) Surface expression of IFN-λR1 was analyzed by flow cytometry on different lymphocyte subsets [B, B lymphocytes; NK, NK cells; NKT, NK T cells; CD4, CD4(+) T cells; CD8, CD8(+) T cells] obtained from healthy donors (n = 21). Data are presented as fluorescence intensity relative to the isotype control (RFI). (B) Cytolytic activity of purified NK cells (n = 6) was studied after coculture with MHC I-deficient K562 at different effector-to-target ratios (E:T ratio) in the presence or absence of either IL28A or IL28B. Columns represent mean and SEM. (C) NK cell cytotoxicity against K562 cells was tested in the presence of increasing concentrations of IL28A and IL28B, respectively. Columns represent mean and SEM. (D) Purified NK cells were stimulated with IL28A, IL28B, or a combination of IL12 (30 ng/mL) and IL15 (100 ng/mL) and then, were studied for IFN-γ production. (E) IFN-γ secretion of purified, IL12/15-stimulated NK cells was analyzed by flow cytometry in the absence or presence of IL28A (Left) and IL28B (Right), respectively. (F) PBMCs were stimulated with IL28A, IL28B, and a combination of IL12 (30 ng/mL) and IL15 (100 ng/mL), respectively. Then, IFN-γ production was determined in the NK cell subset by FACS analysis as described in D. (G) PBMCs were cultured with IL12/15 in the absence or presence of IL28A (Left) and IL28B (Right), respectively. Then, IFN-γ production of NK cells was studied as described in E.
Thus, our experimental data do not support the concept that λ-IFNs might directly act on NK cells to modify their functional activity.
How might the findings of Dring et al. (1) and our divergent results become reconciled? Dring et al. (1) studied peripheral blood monocytes (PBMCs), whereas we had analyzed purified NK cells. A possible scenario could involve the possibility that type III IFNs do not directly act on NK cells but act on other leukocyte subsets, which then indirectly modulate the activity of NK cells. Therefore, we repeated our experiments with PBMCs instead of purified NK. However, again, we were unable to detect any effects of recombinant IL28A and IL28B IFN-γ secretion in the NK cell subset (Fig. 1 F and G). These findings exclude the possibility that any major subpopulations within the PBMCs (e.g., T or B lymphocytes) indirectly modify NK activity in response to type III IFNs. Of note, Dring et al. (1) found that only 8 of 11 donors were sensitive to IL28B. This detail, together with our findings, seems to suggest that only cells present at low frequency in PBMCs remain candidates for a λ-IFN–sensitive subpopulation with regulatory potential against NK cells. At present, it remains unclear whether such a rare cell population actually exists, because we failed to detect any substantial surface expression of IFN-λR1 on the studied lymphocyte subpopulations. Thus, identification and functional characterization of such a special lymphocyte subset is of pivotal importance to better understand the role of the immune system in hepatitis C. Otherwise, an alternative mechanism should be proposed to explain how type III IFNs modify cellular functions on a cell type that apparently does not express the cognate receptor.
Acknowledgments
We thank Claudia Zwank for perfect technical assistance. This work was supported by German Research Foundation Grant DFG SFB/TR 57 and H. W. and J. Hector Foundation Grant M42.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dring MM, et al. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc Natl Acad Sci USA. 2011;108:5736–5741. doi: 10.1073/pnas.1016358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DL, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]