Abstract
During egress from the nucleus, HSV capsids that contain DNA (termed C capsids) are preferentially enveloped at the inner nuclear membrane over capsid types lacking DNA. Using coimmunoprecipitation and biochemical analyses of wild-type and mutant capsids, we identify an interaction between a complex of pUL17/pUL25, termed the C capsid-specific complex (CCSC), and pUL31, a component of the nuclear egress complex (NEC). We also show that the interactions between these components are dependent on expression of all three proteins but occur independently of the pUL31 interacting protein and NEC component pUL34, as well as a kinase encoded by US3 that phosphorylates both pUL31 and pUL34. The interaction between the CCSC and pUL31 in the NEC suggests a mechanism to conserve viral resources by promoting assembly of only those viral particles with the potential to become infectious.
Keywords: virus assembly, virus egress
Herpesvirus nucleocapsids (type C capsids) are assembled in the nucleoplasm and are preferentially selected over other capsid types, such as type B that lack DNA, to undergo an initial or primary envelopment reaction at the inner nuclear membrane (INM) (reviewed in refs. 1 and 2). Primary envelopment requires the products of genes UL31 and UL34 in the HSV system (3–5). Orthologs of UL31 and UL34 are present in all known herpesviruses, and for those systems in which it has been studied, the requirement for these proteins in primary envelopment is conserved (6–9). UL31 encodes a nucleoplasmic phosphoprotein, pUL31 (10), that is maintained in close approximation to the INM by association with pUL34, a type II integral membrane protein (5, 11–13). The bulk of pUL34 is located within the nucleoplasm, with only five amino acids predicted to lie within the perinuclear space (14, 15). Although it has been established that pUL31 and pUL34 are incorporated into perinuclear virions (16, 17), whether the capsid engages the pUL31/pUL34 complex (also known as the nuclear envelopment complex or NEC) directly or indirectly is not known.
The UL17 and UL25 gene products interact, forming a stable complex (18). DNA-containing C capsids contain ≈75 copies of pUL25, whereas B capsids contain ≈20 copies (10). Because of its enrichment in C capsids, the UL25/UL17 complex was named the C capsid-specific complex or CCSC (19). The CCSC bridges pentameric vertices to the adjacent 20 planar faces on the capsid surface (10, 19, 20). One hypothesis to explain how C capsids are selected for envelopment is that the products of UL25 and UL17 bind more efficiently to the surface of type C capsids after DNA packaging is complete (19), and these capsids subsequently engage the NEC complex either directly or indirectly. Consistent with this hypothesis is the observation that UL17 and UL25 null capsids do not become enveloped (21, 22). However, CCSC components have additional functions that may contribute indirectly to envelopment. For example, UL17 is necessary for DNA to be cleaved and packaged (21), and UL25 is required for optimal production of genomic termini and retention of cleaved DNA in the capsid (22–24). Thus, it is unclear whether lack of envelopment of pUL17 or pUL25 null capsids at the INM reflects the respective roles of these genes in assembly of DNA-containing capsids, or more directly as ligands to attach capsids to the NEC.
Supporting the latter possibility, the present study identifies an interaction between the UL31 component of the NEC and CCSC components in infected cells. The data support a model in which the CCSC is added to capsids after DNA is inserted and engages pUL31 either in the nucleoplasm or within the NEC at the INM to effectively select DNA-containing capsids for envelopment. Preferential envelopment of DNA-containing capsids is an elegant method to conserve cellular resources such that only capsids with the potential to produce infectious virions undergo subsequent steps in the virion assembly pathway.
Results and Discussion
Components of the CCSC Interact with pUL31.
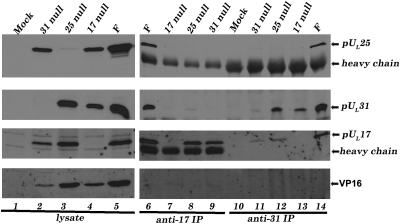
Preliminary evidence suggested that the UL31 proteins coimmunoprecipitated with pUL25 and pUL17 (Fig. S1). To investigate the pUL17/pUL25/pUL31 interaction further, CV1 cells were mock infected or infected with viruses individually lacking the UL17, UL25, or UL31 genes, and lysates of these cells were clarified, denatured, and subjected to gel electrophoresis. The electrophoretically separated proteins were then probed with antibodies to pUL31, pUL25, and pUL17. This analysis showed (Fig. 1) that neither pUL17, pUL25, nor pUL31 required any of the other proteins for accumulation in the clarified lysate. Moreover, antibody reactivity was specific, inasmuch as bands comigrating with each protein were greatly reduced or not detected in lanes containing lysates of cells infected with the respective null virus (e.g., the pUL17-specific band was not detected in lysates of cells infected with the UL17 null virus).
Fig. 1.
Coimmunoprecipitation of pUL17, pUL25, and pUL31 requires expression of all three proteins. CV1 cells were infected with HSV-1(F), UL17 null, UL25 null, or UL31 null viruses, or were mock infected. At 18 h after infection cells were lysed, and soluble lysates (Left) or immunoprecipitation reactions (Right) using anti-pUL17 or anti-pUL31 antibodies were electrophoretically separated and immunoblotted with anti-pUL25, anti-UL31, anti-pUL17, or anti-VP16 antibodies.
Clarified cellular lysates were also subjected to immunoprecipitation with pUL31- and pUL17-specific antibodies. Immunoprecipitated material was separated on denaturing polyacrylamide gels, transferred to nitrocellulose, and probed with antibodies to pUL17, pUL25, and pUL31. As shown in Fig. 1, both pUL31- and pUL17-specific antibodies immunoprecipitated or coimmunoprecipitated pUL31, pUL17, and pUL25 from lysates of cells infected with wild-type virus HSV-1(F). Moreover, loss of expression of any one protein precluded coimmunoprecipitation of the others. Specifically, whereas the pUL17-specific antibody immunoprecipitated pUL17 from lysates of cells infected with the UL31 and UL25 null viruses, pUL31 was not coimmunoprecipitated with this antibody from lysates of cells infected with the UL25 null mutant, and pUL25 was not coimmunoprecipitated from lysates of cells infected with the UL31 mutant. Similar results were obtained using pUL31-specific antibody: whereas this antibody efficiently immunoprecipitated pUL31 from lysates of cells infected with wild-type virus or the UL25 or UL17 deletion viruses, pUL17 was not coimmunoprecipitated as efficiently with this antibody from lysates of UL25 null infected cells (Fig. 1, lane 12). Moreover, pUL25 did not coimmunoprecipitate with pUL31 from lysates of cells infected with the UL17 deletion mutant that were reacted with the UL31-specific antibody (Fig. 1, lane 13).
The following was observed in control reactions. (i) Faint bands comigrating with pUL31-specific bands were occasionally detected in electrophoretically separated anti-pUL31 immunoprecipitations from mock and UL31 null virus-infected cell lysates (Fig. 1, lanes 10 and 11, respectively), but these bands were much less prominent than pUL31 immunoprecipitated from lysates of cells infected with HSV-1(F), UL17 null, or UL25 null viruses. Moreover, these faint bands were inconsistent in size and intensity from experiment to experiment (for example, Fig. S1, lane 6, Bottom). (ii) Bands attributable to pUL25 were not detected in immunoprecipitations from lysates of UL31 null virus-infected cells reacted with the UL31 antibody, nor from lysates of UL17 null virus-infected cells reacted with the pUL17-specific antibody. (iii) Some pUL17 immunoreactivity was observed in immunoprecipitation reactions from UL31 null virus-infected cells reacted with pUL31 specific antibody, suggesting that some pUL17 was immunoprecipitated nonspecifically (Fig. 1, lane 11). Nevertheless, intensity of this band was lower than was detected in similar reactions from HSV-1(F) or UL25 null virus-infected cell lysates and was inconsistent from experiment to experiment (for example, Fig. S1, lane 6, Middle). (iv) Immunoblotting electrophoretically separated immunoprecipitated material with antibody to VP16, an abundant HSV-1 protein, indicated that this protein was not coimmunoprecipitated with anti-pUL31 or anti-pUL17 antibodies despite readily detectable amounts in the various cellular lysates.
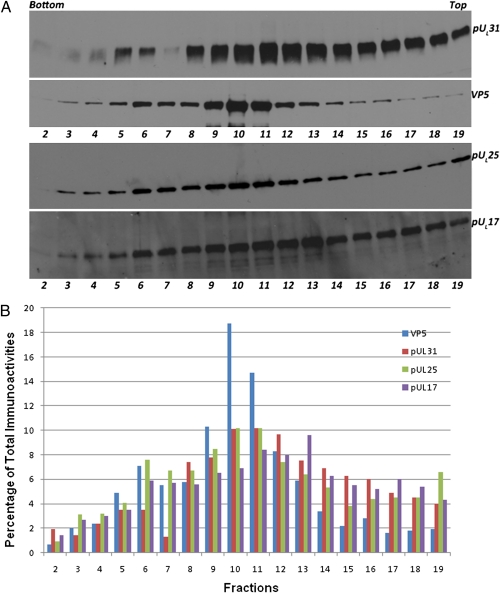
Given the observation (Fig. 1, lanes 6 and 14) that pUL31 associated with pUL17 and pUL25, which constitute the CCSC, we next asked whether pUL31 associated with capsids. Capsids were purified from lysates of cells infected with wild-type HSV-1(F) on sucrose gradients using standard protocols. Fractions from the gradients were collected, and protein in each fraction was trichloroacetic acid (TCA) precipitated, denatured, electrophoretically separated, transferred to nitrocellulose, and probed with antibodies to VP5 (the major capsid protein), pUL25, pUL17, or pUL31. The results are shown in Fig. 2A. Protein-specific signals were quantified by densitometry, and the percentage of immunoreactivity in each fraction relative to total immunoreactivity with the same antibody is shown in Fig. 2B.
Fig. 2.
Immunoblot of sucrose gradient-fractionated wild-type capsids probed with anti-pUL31, anti-pUL25, or anti-VP5 or anti-pUL17 antibodies. CV1 cells were infected with HSV-1(F) at a multiplicity of infection of 5 pfu/cell. At 20 h after infection cells were collected and lysed. Capsids were pelleted by centrifugation through a sucrose cushion and were then resuspended and separated on a continuous sucrose gradient (Materials and Methods). Approximately 0.5-mL fractions as determined by eye were collected from the bottom of the gradient (fraction 1) to the top (fraction 20) using a Buchler Auto Bensi-Flow IIC gradient collector. Proteins in fractions were TCA precipitated, and pellets were denatured and solubilized in SDS. Fractions 2 through 19 were separated on an SDS polyacrylamide gel and analyzed by immunoblotting, followed by reaction with appropriate conjugates, application of chemiluminescence substrate, exposure to X-ray film, and digital scanning. (A) Top and Upper Middle: Images of the same blot first probed with pUL31 then stripped and reprobed with VP5 antibodies. Lower Middle and Bottom: A second blot containing identical samples probed with pUL25 antibody. Immunoreactivity was then stripped, and the blot was probed with pUL17 antibody. (B) Percentage of immunoreactivity of a given antibody in different fractions as quantified by Image J software.
pUL31 was detected in most fractions to varying extents but accumulated in two peaks, a smaller peak in fraction 5 and 6 and a second peak containing lighter material with maximal levels in fractions 10 and 11. A minor VP5 peak corresponding to the presence of C capsids was observed in fraction 6. A second, more intense VP5 peak corresponding to B capsids was observed with maximum immunoreactivity in fraction 10. Fractions containing material less dense than capsids (fractions 13–19) also contained substantial amounts of pUL31 immunoreactivity, with amounts decreasing in each fraction as they approached the top of the gradient. Although overall immunoreactivity was lower with the pUL25-specific antibody, fractions that contained pUL31 also contained pUL25. pUL25 immunoreactivity peaked in fractions 6 and 10–11, thus corresponding to C and B capsids, respectively. High levels of pUL17 also associated with capsid-containing fractions. However, peak amounts of pUL17 accumulated in fractions slightly less dense than capsids (with a maximal amount in fraction 13). These data suggest that pUL31 associates with wild-type capsids but also pUL17 and pUL25 that are free of capsids.
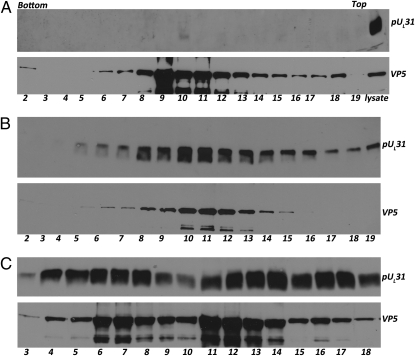
To determine whether the capsid association of pUL31 was dependent on pUL25, capsids were isolated from lysates of cells infected with a UL25 deletion mutant and purified on a continuous sucrose gradient. Proteins within the fractions were precipitated with TCA and analyzed on immunoblots probed with VP5 and pUL31. As shown in Fig. 3A, the absence of pUL25 greatly affected the distribution of pUL31 in the gradient. As opposed to gradients containing wild-type capsids (Fig. 2A), pUL31 was not detected to a great extent in any fraction from UL25 deletion mutant-infected cell lysates, although there was barely detectable immunoreactivity in fraction 10. As a control, a small amount of UL25 deletion mutant-infected cell lysate on the same immunoblot was probed with pUL31 antibody and revealed ample immunoreactivity (Fig. 3A, lane labeled lysate). We conclude that pUL31 association with capsid-containing fractions requires pUL25.
Fig. 3.
pUL31 capsid association requires pUL25 but not pUL17 nor pUL34. CV1 cells were infected with 5.0 pfu/cell of (A) UL25 null, (B) UL17 null, or (C) UL34 null viruses. Cellular lysates prepared at 20 h after infection were pelleted through a sucrose cushion. Material in the pellets was resuspended and separated by ultracentrifugation through 10 mL continuous sucrose gradients. Twenty ≈0.5-mL fractions were collected from bottom to top of each gradient. Material in fractions was TCA precipitated and solubilized in SDS. Fractions 2–19 (A and B) from the UL25 null and UL17 null gradients and fractions 2–18 (C) of the UL34 null gradient were denatured in SDS, electrophoretically separated, and subjected to immunoblotting with VP5 and pUL31 specific antibodies.
Because pUL17 interacts with pUL25, efforts were made to determine the contribution of pUL17 to pUL31 capsid association. Capsids were therefore purified from cells infected with the UL17 deletion virus (this virus only produces B capsids), and immunoblots of gradient fractions were probed with VP5- and pUL31-specific antibodies. As shown in Fig. 3B, the absence of pUL17 did not eliminate association of pUL31 with capsid-containing fractions. On the other hand, considerably less pUL31-specific immunoreactivity was observed in fractions containing material lighter than capsids than was apparent upon similar analyses of lysates infected with wild-type virus. We conclude that pUL17 is dispensable for association of pUL31 with capsids but augments association of pUL31 with material less dense than capsids.
Efforts were then expended to characterize the material other than capsids with which pUL31 associated. Because the known interacting partner of pUL31 is the integral membrane protein pUL34, and this would conceivably tether pUL31 to membranes, capsids were purified from lysates of cells infected with the UL34 deletion mutant and immunoblotted with the UL31-specific antibody. As shown in Fig. 3C, the absence of UL34 caused increased pUL31 immunoreactivity in the gradient relative to other capsid preparations. Two peaks of pUL31 immunoreactivity were observed, with maximal levels in fractions 6–8 and 12–14 in the particular gradient shown in Fig. 3C. However, substantial pUL31 immunoreactivity was also detected near the top of the gradient. We attribute the prominence of the peak in fractions 6–8 relative to other capsid preparations to the enrichment of C capsids in nuclei of cells infected with the UL34 null mutant, inasmuch as these capsids do not become enveloped and therefore do not exit the nucleoplasm. VP5 also accumulated in two peaks, with maximal amounts in fractions 7 and 11. Thus, although substantial pUL31 immunoreactivity was contained in capsid-containing fractions, considerable amounts were detected in fractions containing material less dense than capsids. These results confirm the association of pUL31 with capsids and indicate that capsid association and association with material lighter than capsids occurred independently of pUL34.
Another possibility was that the components less dense than capsids include remnants of the nuclear lamina because pUL31 has been shown to interact with lamin A, a major lamina component (25). Alternatively, this material may represent association with virion tegument proteins, inasmuch as pUL17 has been shown to interact not only with pUL25 but also major tegument components pUL46 and pUL47 (26). To address these possibilities, we probed immunoblots identical to those above with antibodies to either lamin A or pUL47. Despite some nonspecific background staining with pUL47 antibody in most fractions, discrete bands attributable to either pUL47 or lamin A/C were not detected in the gradient (Fig. S2), even though such bands were readily detected in the total cellular lysate. These data suggest that the less dense material lacking capsids but containing pUL17, pUL25, and pUL31 did not contain abundant tegument proteins or nuclear lamina components.
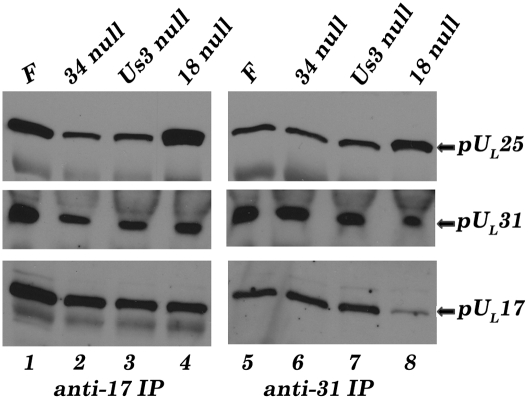
Given the observations that pUL31 associated with capsids, the observed coimmunoprecipitation of pUL17, pUL25, and pUL31 (Fig. 1) might simply reflect immunoprecipitation of the entire capsid with pUL17- or pUL31-specific antibodies. To rule out this possibility, cells were infected with a virus lacking UL18, which encodes VP23. Because VP23 is an essential component of capsid triplexes that link adjacent capsomeres, its absence precludes capsid formation (27–29). As shown in Fig. 4, pUL25 was coimmunoprecipitated efficiently with pUL31 antibody from lysates of cells infected with the UL18 deletion virus and from wild-type virus HSV-1(F), indicating that pUL31 and pUL25 could interact even in the absence of intact capsids. On the other hand, less pUL17 was immunoprecipitated with the pUL31-specific antibody from lysates of cells infected with the UL18 deletion virus compared with levels from HSV-1(F)–infected cell lysates. Thus, either the presence of capsids or VP23 enhanced the coimmunoprecipitation of pUL17 with pUL31 antibody. To distinguish between these possibilities, we reacted UL18 null virus-infected cell lysates with pUL17-specific antibody and found that this antibody efficiently coimmunoprecipitated both pUL25 and pUL31. Taken together, these data indicate that pUL17, pUL25, and pUL31 can interact even in the absence of intact capsids.
Fig. 4.
The pUL17/pUL25/pUL31 complex forms in the absence of pUL18, intact capsids, pUL34, or pUS3. CV1 cells were infected with HSV-1(F), UL18 null, UL34 null, or US3 null viruses. At 18 h after infection cells were lysed and immunoprecipitated with anti-pUL17 (Left) or anti-pUL31 (Right) antibodies. The immunoprecipitated material was then subjected to immunoblotting with anti-pUL25, anti-UL31, or anti-pUL17 antibodies.
We were also interested to determine whether pUS3, a kinase that phosphorylates pUL31 and thereby regulates virus egress from the nucleus (30), and pUL34, the integral membrane protein that normally interacts with pUL31 in the NEC, affected the pUL31/pUL17/pUL25 interaction. As shown in Fig. 4, neither the absence of US3 or of pUL34 precluded or even greatly affected coimmunoprecipitation of pUL31, pUL17, or pUL25 with antibodies to either pUL31 or pUL17.
The data in this work indicate an interaction between pUL31, a component of the NEC, and pUL25 and pUL17, constituting the CCSC. Evidence supporting the interaction comes from the observations that (i) coimmunoprecipitation of any two of the three proteins requires expression of all three; (ii) coimmunoprecipitation of pUL17, pUL25, and pUL31 can occur independently of capsid formation and pUL34, the interaction partner of pUL31; and (iii) that capsid association of pUL31 requires pUL25. It follows that pUL31 on the capsid would localize coincident with the CCSC [i.e., at vertices linking hexons and pentons (19, 20)].
The data presented herein suggest a model in which enrichment of pUL17 and pUL25 on C capsids helps select these capsids for envelopment by interacting with pUL31 of the NEC, eventually leading to capsid budding at the nuclear membrane. Data supporting this model include the observations that capsids do not exit the nuclei of cells infected with UL17 and UL25 null mutants, pUL25 is more abundant on DNA-containing capsids as opposed to other capsid types, and pUL31 is required for optimal envelopment of capsids at the INM (4, 5, 10, 21, 22, 31, 32).
UL25 null capsids of pseudorabies virus (PRV), a well-studied swine herpesvirus, accumulate against the nuclear membrane of infected cells, suggesting they are able to attach to the INM (33). Thus, components of the capsid other than pUL25 are sufficient to mediate INM tethering in this system. It follows that PRV UL25 is required to trigger capsid budding at the INM, perhaps by inducing a conformational change in the budding machinery, which likely includes the NEC. Supporting this role of the NEC is the observation that expression of PRV pUL31 and pUL34 in the absence of other viral proteins and capsids is sufficient to induce budding from the INM (34). In the HSV-1 system, pUL25-null capsids were not observed to attach excessively to the INM (22), but whether HSV-1 pUL25 is truly dispensable for attaching to the INM of HSV-infected cells requires further investigation.
The sequence of events governing UL31 association with the CCSC is not entirely clear. Any model must take into account previous observations that pUL31 can associate with pUL34 in the absence of other viral proteins (5, 35). Three possibilities differ in the timing of addition of pUL31 to capsids. Specifically, (i) the capsid lacking pUL31 migrates to the NEC, and the CCSC-NEC engagement occurs exclusively at the INM; (ii) pUL31 associates with capsid-bound CCSC in the nucleoplasm, to eventually bind to pUL34 or a complete NEC in the INM; or (iii) pUL31 associates with capsid-free pUL17/pUL25, and the three proteins eventually interact with capsids, followed by interaction between the pUL31 moeity and pUL34 at the INM.
Although the first of these possibilities was favored by us primarily because pUL31 accumulates at the nuclear membrane of infected cells (5), data in the present study suggest this model may be too simplistic. This conclusion is based on observations indicating that capsid-free pUL17/pUL25 can associate with pUL31. Specifically, pUL31 coimmunoprecipitates with CCSC components in the absence of pUL34 and capsids (Fig. 4), is required for optimal pUL25 and pUL17 coimmunoprecipitation (Fig. 1), and can associate with capsids independently of pUL34 (Fig. 3). Moreover, pUL17, pUL25, and pUL31 accumulate in fractions of sucrose gradients containing material less dense than capsids (Fig. 2), suggesting that these proteins can form a capsid-free complex. These putative complexes (i.e., in fractions containing material less dense than capsids) are dependent on pUL25, reduced in lysates of cells infected with a UL17 null virus, do not contain lamin A/C or the pUL17-interacting protein pUL47, and are increased in the absence of pUL34, presumably because more pUL31 is present in the nucleoplasm of these cells and is therefore more available for interaction with the capsid rather than with pUL34 at the INM (5). Thus, although pUL31’s migration in the sucrose gradient required pUL25 and was influenced by pUL17, association of pUL31 with the nuclear membrane or nuclear lamina was not relevant to this migration in the gradient. Caveats include the possibilities that these complexes represent capsid fragments released after cellular lysis or that some of the deletion mutant phenotypes reflect off-pathway effects. Further work will be necessary to determine the sequence of events involved in the NEC-CCSC interaction.
An interesting possibility is that consequential to higher pUL25/pUL17 occupancy, DNA-containing C capsids are more likely to bind pUL31, which in turn may bind the NEC. Thus, pUL31 on C capsids helps mediate their selection for envelopment at the INM to conserve resources such as sections of nuclear membrane embedded with viral glycoproteins and associated tegument proteins for envelopment and egress. Although selection of DNA-containing capsids for budding may conserve resources, it should be noted that the selection process is not foolproof, inasmuch as enveloped capsids lacking DNA can be observed occasionally in the perinuclear space of cells infected with HSV by electron microscopy. This may reflect the association of some pUL25 and pUL17 with capsids lacking DNA (Fig. 2 and refs. 10, 36, and 37). Some of these capsids lacking DNA become decorated with pUL31 (Fig. 2), making them competent to interact with pUL34 at the INM, which drives their attachment and occasional envelopment. Further work will be needed to determine the stoichiometry of pUL31 in different capsid types and under different conditions. An important caveat for such quantification is that the interaction between pUL31 and the CSSC is ultimately transient, inasmuch as it must be disrupted at the deenvelopment step in the egress pathway (Fig. 5, step 4). These observations suggest tight regulation of the interaction. Phosphorylation of pUL31 by the US3 viral kinase may represent one regulatory mechanism because this has been shown to promote virion egress from the perinuclear space (30).
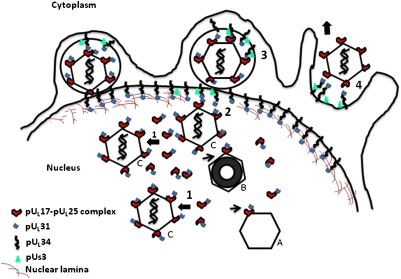
Fig. 5.
Model of nuclear capsid egress. Icosahedral C capsids contain DNA, B capsids lack DNA but contain an internal proteinaceous scaffold (black circle), and A capsids lack internal contents. The nuclear lamina is perforated by the action of cellular and viral kinases (such as viral US3) to allow nucleocapsids access to the INM. pUL17 and pUL25 interact with one another and with pUL31 in the nucleoplasm. The pUL17/pUL25/pUL31 complex then attaches to capsids (step 1). This complex is present on all capsid types but enriched on the surface of C capsids. The pUL31 moiety in this complex then interacts with pUL34 at the INM or a complete pUL31/pUL34 complex (the nuclear envelopment complex or NEC; step 2). The larger number of pUL17/pUL25/pUL31 complexes on C capsids may recruit locally high concentrations of NECs to trigger envelopment (step 3). During envelopment, the viral kinase pUS3 is also incorporated into the perinuclear virion. Deenvelopment (step 4) is triggered by pUS3-mediated phosphorylation of pUL31 and viral glycoprotein gB (gB is not diagrammed). pUL31 is retained in association with pUL34 at the outer nuclear membrane, whereas the capsid is released into the cytosol for eventual budding at cytoplasmic membranes.
Materials and Methods
Cells and Viruses.
CV1 and Vero cells were obtained from the American Type Culture Collection and were propagated in DMEM supplemented with 10% newborn calf serum and antibiotics as described previously (38, 39). HSV-1 F strain [HSV-1(F)] and R7027 (US3 null) were described previously and propagated in Vero cells (38, 39). Mutant viruses UL17 null, UL34 null, V3161 (UL31 null), KUL25 NS (UL25 null), UL18 null, and the complementing cell lines used to propagate them (CV1-17, R1310, UL31-CV1, C8-1, and G5, respectively) have been described previously (3, 4, 21, 27, 31, 37, 40). All of the mutant viruses were derived from HSV-1(F), except UL25 null virus KUL25 NS, which was derived from the HSV-1 strain KOS.
Sucrose Gradient Sedimentation and Capsid Purification.
Approximately 1.6 × 109 CV1 cells from two 850-cm 2 roller bottles were infected with HSV-1(F), UL17 null, UL25 null, or UL34 null viruses at a multiplicity of infection of 5 pfu/cell. At 20 h after infection, cells were collected and washed with PBS. The cell pellets were lysed in 25 mL of lysis buffer [20 mM Tris·Cl (pH 7.6), 500 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM DTT, and protease inhibitor], sonicated, and the lysates were precleared by centrifugation for 15 min at 11,400 × g. The supernatants were then subjected to ultracentrifugation at 104,000 × g for 1 h in a Beckman SW28 rotor through a 5.0-mL 35% (wt/vol) sucrose cushion prepared in TNE buffer [500 mM NaCl, 20 mM Tris (pH 7.6), and 1 mM EDTA]. The pellets containing capsids were resuspended in 300 μL of TNE by brief sonication on ice, layered on a 20–50% sucrose gradient, and centrifuged at 108,000 × g for 1 h in a Beckman SW41 rotor. Twenty fractions were collected from the gradients by eye from the bottom of the tube to the top using a Buchler Auto Densiflow IIC fraction collector. The fractions were precipitated by the addition of TCA to 200 mg/mL and incubation at 4 °C overnight, and pelleted by centrifugation at 13,400 × g for 10 min in a microfuge. The pellets were washed once with cold acetone, resuspended and boiled in SDS sample buffer, and proteins therein were separated on 10% polyacrylamide SDS gels and transferred electrically to nitrocellulose membranes for immunoblotting.
Immunoprecipitation.
Approximately 8 × 106 CV1 cells were infected with 5 pfu of various viruses per cell. Cells were collected at 18 h after infection, pelleted by centrifugation, and lysed by resuspension in 800 μL of immunoprecipitation buffer [1% Nonidet P-40, 20 mM Tris (pH 7.4), 150 mM NaCl, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL pepstatin, and 1 μg/mL leupeptin]. After clarification at 16,000 × g for 10 min in a microfuge, the supernatants were incubated with primary antibodies and Gamma Bind G Sepharose 4B beads (GE Healthcare) overnight at 4 °C with rotation. For immunoprecipitation with anti-pUL17 antibody, rabbit anti-chicken Ig Y was added to the primary antibodies and clarified lysates before addition of the Gamma bind G beads as previously described (18). The beads with bound proteins were pelleted and washed 4 times with ice-cold immunoprecipitation buffer, and protein was eluted from the beads in 2× SDS/PAGE buffer [100 mM Tris·HCl (pH 6.8), 4.0% SDS, 0.2% bromophenol blue, 20% glycerol, and 200 mM fresh DTT], separated on 10% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes for immunoblotting.
Immunoblotting.
The procedure was described previously (41). Primary antibodies were diluted in PBS containing 2% BSA. Primary antibodies were added to immunoblots for 2 h at room temperature or overnight at 4 °C at the following dilutions: chicken anti-pUL17 1:2,000 (37), rabbit anti-pUL31 1:1,000 (5), mouse anti-pUL25 monoclonal antibody 4A11 E4 1:1,000 (20), mouse anti-VP5 monoclonal antibody 1:1,000 (H1.4, BioDesign), rabbit anti-VP13/14 (pUL47) 1:1,000 (26), goat anti-VP16 (Santa Cruz Biotechnology, SC-1728) 1:500, and anti-lamin A/C mouse monoclonal antibody 1:200 (Santa Cruz Biotechnology, SC-7292). The bound immunoglobulins were detected by reaction with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG or anti-chicken IgY and visualized by enhanced chemiluminescence (Thermo Scientific) followed by exposure to X-ray film. In some experiments, the blot was stripped by incubating in buffer containing 62.5 mM Tris·HCl (pH 6.8), 2% SDS, and 100 mM B-mercaptoethanol at 50 °C for 30 min. Stripped blots were washed extensively, blocked, and reprobed by immunoblotting as described above. Chemiluminescent signals of individual bands were quantified with Image J software.
Supplementary Material
Acknowledgments
We thank Elizabeth Wills for reading the manuscript, Richard Roller for the UL34 null virus, Bernard Roizman for the US3 null virus, Fred Homa for the UL25 null virus and antibody to pUL25, and Preshant Desai for the UL18 null virus. These studies were supported by National Institutes of Health Grant R01 AI52341.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108564108/-/DCSupplemental.
References
- 1.Baines JD, Roberts KL. In: Alphaherpesviruses, Molecular Virology. Weller SK, editor. Norfolk, United Kingdom: Caister Academic Press; 2011. pp. 195–206. [Google Scholar]
- 2.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 3.Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang YE, Van Sant C, Krug PW, Sears AE, Roizman B. The null mutant of the U(L)31 gene of herpes simplex virus 1: Construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds AE, et al. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol. 2001;75:8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farina A, et al. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J Virol. 2005;79:3703–3712. doi: 10.1128/JVI.79.6.3703-3712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granato M, et al. Deletion of Epstein-Barr virus BFLF2 leads to impaired viral DNA packaging and primary egress as well as to the production of defective viral particles. J Virol. 2008;82:4042–4051. doi: 10.1128/JVI.02436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lötzerich M, Ruzsics Z, Koszinowski UH. Functional domains of murine cytomegalovirus nuclear egress protein M53/p38. J Virol. 2006;80:73–84. doi: 10.1128/JVI.80.1.73-84.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnee M, Ruzsics Z, Bubeck A, Koszinowski UH. Common and specific properties of herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J Virol. 2006;80:11658–11666. doi: 10.1128/JVI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newcomb WW, Homa FL, Brown JC. Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices. J Virol. 2006;80:6286–6294. doi: 10.1128/JVI.02648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YE, Roizman B. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol. 1993;67:6348–6356. doi: 10.1128/jvi.67.11.6348-6356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang L, Baines JD. Identification of an essential domain in the herpes simplex virus 1 UL34 protein that is necessary and sufficient to interact with UL31 protein. J Virol. 2005;79:3797–3806. doi: 10.1128/JVI.79.6.3797-3806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi Y, et al. Herpes simplex virus type 2 UL34 protein requires UL31 protein for its relocation to the internal nuclear membrane in transfected cells. J Gen Virol. 2001;82:1423–1428. doi: 10.1099/0022-1317-82-6-1423. [DOI] [PubMed] [Google Scholar]
- 14.Shiba C, et al. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J Gen Virol. 2000;81:2397–2405. doi: 10.1099/0022-1317-81-10-2397. [DOI] [PubMed] [Google Scholar]
- 15.McGeoch DJ, et al. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds AE, et al. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J Virol. 2002;76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholtes L, Baines JD. Effects of major capsid proteins, capsid assembly, and DNA cleavage/packaging on the pUL17/pUL25 complex of herpes simplex virus 1. J Virol. 2009;83:12725–12737. doi: 10.1128/JVI.01658-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trus BL, et al. Allosteric signaling and a nuclear exit strategy: Binding of UL25/UL17 heterodimers to DNA-Filled HSV-1 capsids. Mol Cell. 2007;26:479–489. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway JF, et al. Labeling and localization of the herpes simplex virus capsid protein UL25 and its interaction with the two triplexes closest to the penton. J Mol Biol. 2010;397:575–586. doi: 10.1016/j.jmb.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmon B, Cunningham C, Davison AJ, Harris WJ, Baines JD. The herpes simplex virus type 1 U(L)17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72:3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNab AR, et al. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cockrell SK, Sanchez ME, Erazo A, Homa FL. Role of the UL25 protein in herpes simplex virus DNA encapsidation. J Virol. 2009;83:47–57. doi: 10.1128/JVI.01889-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stow ND. Packaging of genomic and amplicon DNA by the herpes simplex virus type 1 UL25-null mutant KUL25NS. J Virol. 2001;75:10755–10765. doi: 10.1128/JVI.75.22.10755-10765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds AE, Liang L, Baines JD. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J Virol. 2004;78:5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholtes LD, Yang K, Li LX, Baines JD. The capsid protein encoded by U(L)17 of herpes simplex virus 1 interacts with tegument protein VP13/14. J Virol. 2010;84:7642–7650. doi: 10.1128/JVI.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai P, DeLuca NA, Glorioso JC, Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993;67:1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rixon FJ, Davison MD, Davison AJ. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990;71:1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb WW, et al. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 30.Mou F, Wills E, Baines JD. Phosphorylation of the U(L)31 protein of herpes simplex virus 1 by the U(S)3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J Virol. 2009;83:5181–5191. doi: 10.1128/JVI.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang L, Tanaka M, Kawaguchi Y, Baines JD. Cell lines that support replication of a novel herpes simplex virus 1 UL31 deletion mutant can properly target UL34 protein to the nuclear rim in the absence of UL31. Virology. 2004;329:68–76. doi: 10.1016/j.virol.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Sheaffer AK, et al. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J Virol. 2001;75:687–698. doi: 10.1128/JVI.75.2.687-698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klupp BG, Granzow H, Keil GM, Mettenleiter TC. The capsid-associated UL25 protein of the alphaherpesvirus pseudorabies virus is nonessential for cleavage and encapsidation of genomic DNA but is required for nuclear egress of capsids. J Virol. 2006;80:6235–6246. doi: 10.1128/JVI.02662-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klupp BG, et al. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci USA. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fossum E, et al. Evolutionarily conserved herpesviral protein interaction networks. PLoS Pathog. 2009;5:e1000570. doi: 10.1371/journal.ppat.1000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurlow JK, et al. The herpes simplex virus type 1 DNA packaging protein UL17 is a virion protein that is present in both the capsid and the tegument compartments. J Virol. 2005;79:150–158. doi: 10.1128/JVI.79.1.150-158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wills E, Scholtes L, Baines JD. Herpes simplex virus 1 DNA packaging proteins encoded by UL6, UL15, UL17, UL28, and UL33 are located on the external surface of the viral capsid. J Virol. 2006;80:10894–10899. doi: 10.1128/JVI.01364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purves FC, Longnecker RM, Leader DP, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 40.Bjerke SL, et al. Effects of charged cluster mutations on the function of herpes simplex virus type 1 UL34 protein. J Virol. 2003;77:7601–7610. doi: 10.1128/JVI.77.13.7601-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K, Baines JD. The putative terminase subunit of herpes simplex virus 1 encoded by UL28 is necessary and sufficient to mediate interaction between pUL15 and pUL33. J Virol. 2006;80:5733–5739. doi: 10.1128/JVI.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.