Abstract
Protected areas are used to sustain biodiversity and ecosystem services. However, protected areas can create tradeoffs spatially and temporally among ecosystem services, which can affect the welfare of dependent local communities. This study examines the effect of a protected area on the tradeoff between two extractive ecosystem services from mangrove forests: cutting mangroves (fuelwood) and harvesting the shrimp and fish that thrive if mangroves are not cut. We demonstrate the effect in the context of Saadani National Park (SANAPA) in Tanzania, where enforcement of prohibition of mangrove harvesting was strengthened to preserve biodiversity. Remote sensing data of mangrove cover over time are integrated with georeferenced household survey data in an econometric framework to identify the causal effect of mangrove protection on income components directly linked to mangrove ecosystem services. Our findings suggest that many households experienced an immediate loss in the consumption of mangrove firewood, with the loss most prevalent in richer households. However, all wealth classes appear to benefit from long-term sustainability gains in shrimping and fishing that result from mangrove protection. On average, we find that a 10% increase in the mangrove cover within SANAPA boundaries in a 5-km2 radius of the subvillage increases shrimping income by approximately twofold. The creation of SANAPA shifted the future trajectory of the area from one in which mangroves were experiencing uncontrolled cutting to one in which mangrove conservation is providing gains in income for the local villages as a result of the preservation of nursery habitat and biodiversity.
Keywords: poverty trap, conservation and development, East Africa, coastal
Mangrove forests comprise only 0.12% of the world’s total land area, but are highly productive ecosystems that underpin a major portion of the world’s fisheries (1, 2). Mangroves thrive where many other species cannot survive, and are important habitats for associated flora and aquatic and terrestrial fauna (1, 3–5), with more than 1,500 faunal species inhabiting mangroves in the Indo-Malaysian region (3, 4).
Many coastal communities in developing countries, especially the rural poor, rely upon extraction of mangrove forests for their subsistence and livelihoods (6, 7). Overexploitation for fuelwood, charcoal, and timber production has degraded more than one quarter of the world’s mangrove habitats (8). The direct harvest of mangroves not only affects biodiversity levels and species interactions, but also causes physical changes that can cause propagules and saplings to be washed away with the retreating tides. Mangrove extraction adversely impacts nursery habitat for fish and shrimp vital to the subsistence and livelihoods of coastal communities. Approximately 80% of worldwide fish catches are estimated to depend directly or indirectly on mangroves (9), and almost 100% of the shrimp catch in the Association of Southeast Asian Nations countries depend on mangroves for at least part of their life cycle (10). Penaeid shrimp production decreases precipitously as the remaining mangrove area is reduced (11).
The rapid destruction of mangrove forests has spawned a host of protected areas across the world. However, given the reliance of many local communities on mangrove forests for fuelwood, charcoal, and other uses from harvested mangroves, protection efforts that sustain the long-term viability of these ecosystems—including their value for fisheries—could pose an immediate threat to livelihoods of the rural poor. Without some mechanism to compensate the affected households, protected areas can place them in a poverty trap, i.e., a mechanism that causes poverty to persist (12). However, if protected areas can enhance long-run livelihood opportunities for the poor, they can potentially be a win/win solution for conservation and poverty alleviation. This question underlies the literature in integrated conservation and development projects and their variants, which are recent efforts to conserve biodiversity and alleviate poverty together (13–15). However, there has been little empirical evidence of successful delivery of both goals (16).
This article demonstrates that improvements in mangrove ecosystems that result from a protected area have resulted in tangible improvements in incomes for the poor. The impact of protected areas on the natural resources and the local communities’ livelihood, and the variation of the impact among households in different wealth groups remain largely unexplored (17–19). Protected areas often create tradeoffs among multiple ecosystem services, making it challenging to quantify and assess the linkage between the human and natural systems. Previous studies do not show strong linkages between changes in natural resources and use patterns at the household level. In the context of mangrove conservation, although previous studies linked variations in mangrove areas to potential benefits from fisheries (e.g., refs. 20–23), they do not observe actual changes in mangroves and their effects on tangible benefits in the form of income or consumption. Moreover, most studies do not clearly identify the causal link between protected areas and poverty because they fail to use direct measures of well-being and fail to control for potential confounding effects of baseline characteristics (17, 18). Protected areas in developing countries are often established in remote areas with high poverty rates and few alternative livelihood strategies (24). To identify whether protected areas create tradeoffs among different benefits from mangrove forests, the appropriate comparison would be between households living near protected areas and households with similar characteristics and trends that are not affected by protected areas (18).
The overall goal of this study is to assess the environmental and economic impacts of a major mangrove protection effort undertaken to preserve biodiversity in Saadani National Park (SANAPA) in Tanzania. This region has mangrove forests, which sustain a rich biodiversity, but the local communities suffer from persisting poverty. Specifically, we examine the effect of strengthened enforcement of prohibition of mangrove harvesting in the protected area on the tradeoff between short-term benefits from cutting mangroves and long-term benefits from harvesting the fish and shrimp that thrive if mangroves are not cut, and whether households fell in a poverty trap as a result. There are several mechanisms through which SANAPA can affect the livelihoods of the local households. First, after the establishment of SANAPA, they are prohibited from harvesting mangroves for fuelwood and other uses. Second, there are penalties imposed for infringing within the park boundaries. Third, park protection and monitoring of mangroves increase the mangrove cover, causing recovery of shrimp and fish populations, and hence increasing incomes from shrimping and fishing activities. Finally, there are opportunities for new nonagricultural employment (largely with SANAPA). The first two impose negative effects on villagers and the last two generate positive gains, at least for those who fish or shrimp or attain jobs with the park service.
To meet these objectives, we coupled geospatial and georeferenced household survey data to examine local changes in mangrove cover and socioeconomic impacts of SANAPA. In an effort to overcome some of the previous limitations in protected areas and poverty studies, we assessed the components of income that are directly linked to ecosystem services from mangrove forests. We also used econometric techniques to explore causal linkages between mangrove protection and poverty. In addition, we extended the model to understand how the establishment of the protected area affected households from the three wealth segments (poorer, middle, richer), which were defined based on the total value per capita of productive and consumable asset levels in 2004.
Site Description and Mangrove Protection Efforts
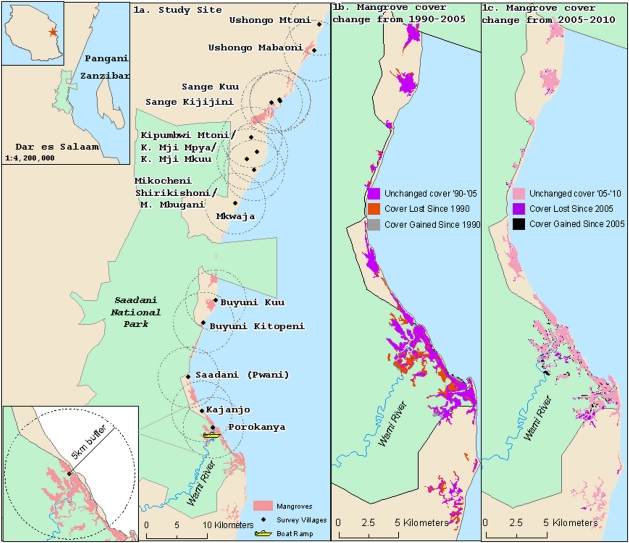
SANAPA, Tanzania’s only coastal national park, is located approximately 80 km north of Dar es Salaam and 27 km west of Zanzibar within the districts of Pangani and Bagamoyo (latitude 5° 20′ to 6° 17′ S; longitude 38° 45′ to 39° 02′ E). It was established in 2005, and spans across 1,100 km2 (Fig. 1A) (25, 26). It protects a range of different habitats, including coastal forests, mangroves, and coral reefs, and encompasses the Wami River Estuary, a critical habitat for many species of fish, shrimp, and birds (25). The estuary provides extensive lengths of mangrove-lined habitat edge, where juvenile shrimp have access to the mangroves. This type of configuration has been shown to be a more important indicator of shrimp densities, as there is a direct relationship between length of mangrove-lined habitat edge and density of juvenile shrimp (27). Also, the abundant and diverse bird population associated with these mangrove forests are a draw for ecotourism.
Fig. 1.
(A) Study site of SANAPA, Tanzania, and villages used in econometric analyses. Inset: 5-km radius around each village used to assess mangrove cover change per village within and outside SANAPA. (B and C) Change in mangrove forest cover from 1990 to 2005 (B) and from 2005 to 2010 (C).
Before the establishment of the park, very high levels of mangrove cutting for charcoal production, firewood, and building materials threatened both the local artisanal fisheries and the biodiversity of the area (7, 25, 26). This rapid degradation of mangrove forests was in part caused by weak property rights and enforcement (28). Between 1995 and 2005, the total mangrove area within the current park boundaries decreased by 27%. The creation of SANAPA prohibited the consumptive use of all mangrove resources within the park’s boundaries (26). Authority vested to SANAPA enforcement personnel allows them to arrest and fine any individuals caught harvesting mangroves. The penalties are strict: imprisonment for 3 to 5 y and fines of 50,000 Tanzanian Shillings (Tsh; approximately $34). Park personnel actively enforce any charcoal-related activity in the general vicinity of SANAPA, and will stop and arrest crews that are transporting charcoal between the mainland and Zanzibar. Based on our interviews with SANAPA enforcement officials, approximately 60 individuals were fined and/or arrested between 2005 and 2010. Based on surveys with numerous village residents, it appears that enforcement of the ban on mangrove fuelwood harvest occurs beyond park boundaries; many villagers are now afraid to harvest mangroves from areas within and surrounding SANAPA. In addition to enhanced enforcement, some collaborative community mangrove forest management initiatives outside of SANAPA’s boundaries, but within our study area, commenced in the mid-1990s (29).
SANAPA is surrounded by rural villages with persisting high poverty rates (7, 30). In Bagamoyo district, 40% of the village inhabitants lived below the poverty line in 2000. The region lacks basic needs (89% do not have access to a piped or protected water source and 94% do not have electricity) and suffers from one of the highest infant mortality rates in Tanzania. Additionally, there is high population growth [i.e., total population increased on average by >2% per year between 1998 and 2009 (7, 31)] and low investment, and most households lack access to credit and insurance markets. The rural poor living in the vicinity of SANAPA largely depend on and earn their livings from natural resources, and their livelihoods are tightly linked to the ecosystem services provided by the mangrove forests. For example, focus groups conducted in our study area revealed that, for many households, shrimping and fishing were the only lucrative income activities, and in some areas, mangroves are still the only fuel source.
Results
Changes in Mangrove Cover.
The loss of mangroves within SANAPA slowed considerably after the park’s establishment in 2005 (Fig. 1C and Table 1). The mean loss from 1990 to 2005 was 27.3 ha/y, versus 1.8 ha/y from 2005 to 2010. The rate of harvest also decreased outside the park’s boundaries, and a mean regrowth of 11.9 ha/y was observed. Four additional mangrove patches were observed within the park’s boundaries in 2010, whereas no additional patches were observed during that time period outside of the park’s boundaries. Loss caused by natural events may have contributed to the changes observed, but we note that there were no tropical cyclones in the study region between 1990 and 2010 (32, 33).
Table 1.
Changes in mangrove forest area within and outside of SANAPA borders, 1990 to 2010
| Time period | Annualized mangrove change within SANAPA, ha/y | Annualized mangrove change within SANAPA, %/y | Annualized mangrove change outside SANAPA, ha/y | Annualized mangrove change outside SANAPA, %/y |
| 1990–2005 | −27.3 | −1.79% | −20.8 | −0.66% |
| 2005–2010 | −1.8 | −0.16% | +11.9 | 0.42% |
Although we have clear evidence that management practices are protecting and enhancing mangrove cover within SANAPA, more site specific data on improvements in biodiversity and the response of dependent fauna within the Wami River Estuary will require concentrated monitoring efforts (SI Published Literature, Table S1).
Changes in Mangrove Use for Fuel Source.
The most direct and common use of mangroves in the study area is for cooking and heating fuel (Table 2). Between 1990 and 2009, the use of mangroves as primary household fuel decreased from 42% to 34%, but the largest decrease took place between 2004 (39%; before SANAPA) and 2009 (34%; after SANAPA). These figures suggest that, with SANAPA, a number of households in the area lost a key extractive ecosystem service from mangroves. Still, more than one third of the households in the sample rely on mangroves as the primary fuel source. The actual figure could even be higher, as households may have been reluctant to report mangrove extraction in the survey (SI Survey). Most households that no longer use mangroves have switched to other trees, which may result in biodiversity impacts yet to be explored.
Table 2.
Changes in proportion of households that used mangroves as a primary source of cooking/heating fuel, 1990 to 2009
| Group | 1990 | 2000 | 2004 | 2009 |
| Total, % | 42 | 43 | 39 | 34 |
| Poorer group, % | 35 | 35 | 35 | 33 |
| Middle group, % | 38 | 38 | 35 | 29 |
| Richer group, % | 52 | 57 | 46 | 40 |
Group category is based on tercile of total value per capita of productive and consumable assets in 2004.
When we stratify the sample households into three wealth groups based on terciles of per capita assets, a larger proportion of the richer group has switched to other fuel sources (12%). In contrast, only 2% of the households in the poorer group changed to other fuel sources, suggesting that the poor may have limited alternative fuel sources. In addition to subsistence uses, there is a high urban demand for mangrove charcoal (7, 34, 35), but few households in our sample reported engagement in charcoal production. The charcoal market requires well organized networks with boats and trade connections that may be centered outside of the local villages.
Changes in Mangrove-Related Income.
To assess the impact of SANAPA on income, we focus on two major income sources related to mangroves: shrimping and fishing. Combined, they were the most important income source in 2009 for nearly 40% of the sample, far exceeding the proportion of households who reported that agriculture or off-farm occupations were their most important income source. Moreover, households are increasingly engaged in shrimping and fishing (Table 3, columns 1 and 2). Households engaging in shrimping increased from 16% of the sample in 2004 to 23% in 2009. Households engaging in fishing increased even more, from 27% in 2004 to 43% in 2009. Interestingly, the majority of the households that started shrimping and fishing between 2004 and 2009 were from the poorest segment of our sample, suggesting that these mangrove-related income sources are pro-poor. Our data also show an increase in the proportion of households engaged in agriculture, charcoal production, and other income sources, suggesting that households are diversifying their income sources. Some of the occupations in “other sources” include ecotourism, which are jobs associated with SANAPA.
Table 3.
Income source and changes in real income per capita, 2004 and 2009
| Engaging in mangrove related and other income activities, % |
Changes in real income per capita* |
||||
| Income activity | 2004 (before SANAPA) | 2009 (after SANAPA) | 2004 (before SANAPA) | 2009 (after SANAPA) | Mean change, 2004–2009 |
| Shrimping | 16 | 23 | 944.03 (1,014.49) | 674.03 (930.90) | +7.43 (848.34) |
| Fishing | 27 | 43 | 686.93 (826.14) | 599.21 (851.35) | +160.96 (1,043.24) |
| Agriculture | 19 | 34 | 146.39 (158.31) | 972.88 (124.24) | +12.14 (148.46) |
| Aquaculture | 1 | 1 | — | — | — |
| Charcoal (mostly not mangrove) | 6 | 11 | 534.76 (647.74) | 354.93 (743.06) | +41.24 (881.28) |
| Firewood (mostly not mangrove) | 3 | 3 | 756.10 (1,495.94) | 289.34 (470.89) | −225.39 (1,287.68) |
| Other sources | 45 | 79 | 202.54 (358.67) | 189.47 (308.20) | +72.98 (181.11) |
Mean of changes between the two years are calculated by first subtracting the 2004 value from the 2009 value for each household and then taking the mean. Values for 2009 are adjusted for inflation using consumer price index generated by the National Bureau of Statistics. Values in parentheses are SDs.
*Unit of measurement is 1,000 Tanzanian Shillings; $1 is equivalent to approximately 1,500 Tanzanian Shillings.
The household data show that shrimping and fishing incomes have increased over time (Table 3, column 5). In particular, annual fishing income increased on average by 161,000 Tsh (approximately $107) per household per year; shrimping income also showed a modest increase of 7,000 Tsh (approximately $12) per household per year. Importantly, the magnitude of increase in both shrimping and fishing incomes was the largest for the poorest segment of the sample, again underscoring the importance of mangrove-related income sources for the poor.
Effect of SANAPA on Mangrove-Related Income.
Point estimates from the regression models reveal that the establishment of SANAPA increased mangrove-related incomes (Table 4). As mangrove cover increased within SANAPA, there was an increase in incomes from shrimping (Table 4, models 1–3) and from fishing (Table 4, models 4–6). Specifically, a 1-km2 increase in mangrove cover within SANAPA increased the shrimping income by 19.5 million Tsh (approximately $13,000) per year, an estimate that is significant at the 5% level (Table 4, model 3, row 1). We found that the average SANAPA mangrove cover in a 5-km2 radius around each village in 2005 was 0.71 km2. Thus, our model result implies that an approximate 10% increase in SANAPA mangrove cover within a 5-km2 radius of the villages increases shrimping income by twofold. In contrast, a 1-km2 increase in mangrove cover outside SANAPA increased shrimping income by only 626,000 Tsh (approximately $417; Table 4, row 2). Qualitatively, we find a similar result for fishing income (Table 4, models 4–6). A 1-km2 increase in mangrove cover within SANAPA increased fishing income by 13.87 million Tsh (approximately $9,450 USD). On the contrary, a 1-km2 increase in mangrove outside SANAPA increased fishing income by only 323,000 Tsh (approximately $220). The changes in these incomes are a result of an increase in number of shrimping and fishing days, earnings per day, and, in the case of fishing, increase in consumption per day as well. The differences in the results between mangrove cover within and outside SANAPA may also reflect the greater fisheries productivity expected from mangroves located along the edge of riverine estuaries as occurs with the Wami River Estuary of SANAPA. We acknowledge, however, that, in theory, the same effect may also arise independently of the protected area, e.g., as a result of a price increase or improvements in harvesting technology, for which we cannot control in our analysis because of a lack of data. (SI Materials and Methods).
Table 4.
Regression results of the primary equation
| Dependent variable |
||||||
| Change in shrimping income per capita |
Change in fishing income per capita |
|||||
| Explanatory variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
| Change in mangrove area within SANAPA | 6,052.34 (1.75)† | 14,872.23 (2.88)‡ | 19,429.28 (2.83)§ | 5,475.83 (2.37)§ | 9,366.99 (2.10)§ | 13,873.98 (2.12)§ |
| Change in mangrove area outside SANAPA | 127.78 (1.56) | 510.11 (2.99)‡ | 626.15 (2.88)‡ | 85.67 (2.14)§ | 178.757 (1.32) | 322.99 (1.70)† |
| Change in mangrove area outside SANAPA * distance to boat ramp | — | −8.16 (2.57)§ | −12.46 (2.51)§ | — | −2.73 (1.05) | −5.55 (1.36) |
| Distance to boat ramp | — | −3.23 (0.64) | 0.62 (0.07) | — | 7.817 (1.65) | 13.41 (1.25) |
| Poorer group | — | — | −269.37 (0.57) | — | — | −368.68 (0.83) |
| Middle group | — | — | 22.224 (0.04) | — | — | −404.59 (0.83) |
| Change in mangrove area within SANAPA * poorer group | — | — | −12,924.48 (1.76)† | — | — | 3,664.04 (0.45) |
| Change in mangrove area within SANAPA * middle group | — | — | 3,277.62 (0.40) | — | — | 881.57 (0.11) |
| Change in mangrove area outside SANAPA * poorer group | — | — | 4.59 (0.02) | — | — | −0.31 (0.00) |
| Change in mangrove area outside SANAPA * middle group | — | — | 125.93 (0.80) | — | — | 33.66 (0.19) |
| R2 | 0.26 | 0.46 | 0.56 | 0.39 | 0.43 | 0.46 |
| N | 31 | 31 | 31 | 59 | 59 | 59 |
Robust t statistics are in parentheses. All regression models also control for IMR in 2004 and 2009 and income levels in 2004 of respective income sources.
Significant differences at †10%, §5%, and ‡1%.
The results also reveal that degree of monitoring for enforcement, as proxied by the distance to boat ramp, has had an effect on shrimping income, but not on fishing income. Specifically, the interaction term between change in mangrove area outside SANAPA and distance to boat ramp is negative and significant for changes in shrimping income per capita, meaning that the closer the mangrove area is to the enforcement officers’ base, the larger the increase in shrimping income. This finding suggests that there may be some spillover effect of enforcement beyond the park boundaries. This coefficient was negative but insignificant for fishing income.
In addition, we find that, although the new entrants to shrimping and fishing were in the poorest group, the effect of the increase in mangrove area within SANAPA on incomes does not particularly favor the poor (Table 4, models 3 and 6). Although most coefficients related to the wealth groups are insignificant (Table 4, rows 5–10), the effect of SANAPA on shrimping income is lower for the poorest third of the sample compared with the richest third of the households. Wealth represents a few factors that affect incomes from shrimping and fishing, such as quantity/size of shrimping gear and boats, search capacity, and, potentially, skills. There is no difference across wealth groups for the effect on fishing income.
Overall, the households that have stopped using mangroves for firewood can be considered the “losers” from establishment of SANAPA, whereas those who started fishing/shrimping (or making more revenue out of it) are the “winners.” Our data suggest that there are more winners than losers: the proportion of households that newly engaged in mangrove-related income activities after SANAPA outweighs the proportion of households that no longer used mangroves for their firewood. In our sample, the proportion of households that used mangroves for firewood decreased by 5%. In contrast, during the same time period, households that newly engaged in shrimping increased by 7% and those who engaged in fishing increased by 16%.
Mangrove Protection vs. Poverty Trap
The expansion of mangrove protection through the creation of SANAPA and enhanced enforcement led to a markedly different future for the mangrove forest species and the biodiversity within that habitat. It also influenced the welfare of the adjacent communities that have been relying on these forests for their livelihood. The trajectory shifted from one in which the mangroves were experiencing uncontrolled cutting, which was destroying the foundation of a critical ecosystem, to one in which mangrove conservation is providing gains in income for the local communities through the preservation of nursery habitat and biodiversity. Our findings suggest that SANAPA has created a tradeoff between the short-run benefits from cutting mangrove forests and potential long-run benefits from not cutting mangroves—and these tradeoffs appear to differ somewhat by household wealth. Many households have experienced an immediate loss in the consumption of mangrove firewood, with the loss most prevalent in richer households.
The households that have entered the fisheries since 2005 were in the poorest group of our sample, suggesting that they have benefited considerably from protection of mangrove forests. At the same time, all wealth classes appear to benefit from long-term sustainability or gains in shrimping and fishing that result from mangrove protection in the Wami River Estuary. This is in contrast to other studies that found that the impact of protected areas was not uniform across households, or that nonpoor households captured most of the welfare gains (7, 17, 36).
However, it is not clear whether the continued protection of mangrove cover would avoid a poverty trap in the long run. Only 2% of the households in the poorer group changed to a different source of fuel since 2005, suggesting the need for some support to transition to alternative fuel sources. Another concern is that there exists no formal mechanism for the winners of the protected area (i.e., those who enjoy increased fishing opportunities) to compensate the losers (i.e., those who lost access to mangroves for firewood and other uses). Without such mechanism, tensions may arise in the future. Furthermore, the sparse data environment for artisanal fisheries in Tanzania precludes us from assessing whether the current rate of harvest is sustainable. Even if it were at a sustainable level, the long-term sustainability of shrimp and other fisheries is contingent not only upon the continued existence of nursery habitat, but also sustainable levels of harvest, which requires appropriate institutions and property rights to manage the fisheries effectively. Although the artisanal fisheries have been given a temporary lifeline as a result of mangrove protection and the recent countrywide banning of commercial trawlers in 2008, there is a strong need for sustainable fisheries management, as well as improvements in storage facilities within the villages and greater accessibility to markets (SI Fisheries). To help prevent excessive pressure on the fisheries, especially if the population levels continue to increase, efforts may be needed to further generate other livelihood options such as ecotourism, which is now possible as a result of the creation of SANAPA. In fact, several respondents said that their job in ecotourism was now their most important income source.
Our field work and survey data show that SANAPA already generates a number of new direct and indirect benefits to the local communities. If these benefits grow with the expansion of ecotourism, there is potential for further poverty alleviation (Table S2). As an example of direct benefits, SANAPA directs a portion of the park fees to local communities for building schools, dispensaries, and mosques. In addition, park personnel assist in supplying drinking water to the communities through the construction of pumps and collection of nonsaline river water, and help to transport ill community members to regional hospitals. SANAPA can also provide indirect benefits to the communities through improving roads and cellular phone towers and the creation of temporary and permanent employment opportunities in tourism. Our survey confirmed that these factors were perceived as benefits by the local communities, especially among those who live closer to SANAPA. Together with increases in mangrove related incomes, these benefits may turn SANAPA into a win/win strategy.
Materials and Methods
Geospatial Data and Household Surveys.
The present study focused on mangrove habitat cover in 1990 (before park establishment), 2005 (time of park establishment), and 2010 within and immediately adjacent to SANAPA (Fig. 1). Landsat images were manually interpreted and delineated within ArcGIS (ESRI) at a scale of 1:17,000 (SI Materials and Methods). ArcGIS was used to calculate mangrove area per time period inside and outside of the SANAPA boundaries. It was also used to identify the mean center point for each subvillage and create circular land cover analysis zones. The latter extended in a 5-km radius around each mean center point to quantify mangrove forest cover located within these zones that was inside or outside the boundaries of SANAPA in 2005 and 2010 (Fig. 1A). We selected an area encompassed within a 5-km radius of each subvillage to reflect the likely travel distance for subvillage fishermen. The continental shelf in this area extends less than 5 km offshore, and most small-scale fishermen do not have access to the technology (e.g., outboard or inboard engines and cooling or freezing facilities) and the capital needed to fish in waters greater than 5 km offshore (7, 37).
We next combined the geographic information systems mangrove data with a survey data set obtained from georeferenced households. We administered the survey in April 2010 to evaluate the livelihood impact of SANAPA. The survey instrument was approved by the University of Rhode Island Institutional Review Board on Human Subjects. The household survey used a stratified sampling strategy designed to collect data on a random sample of 150 households in the SANAPA area. From 15 subvillages in the SANAPA area (Fig. 1A), which are of varying distances from the park boundary, 10 households per subvillage were randomly selected. Our sampling frame includes only subvillages that have some access (i.e., by road or water) to mangroves, some of which are within the park boundaries. By using the survey data, we were able to produce information on mangrove-related income (shrimping and fishing) for both before (in 2004) and after (in 2009) the establishment of SANAPA. The survey also included detailed information on primary fuel source for 1990, 2000, 2004, and 2009, asset holdings and income earnings for 2004 and 2009, and perceptions of the positive and negative impacts of SANAPA (SI Survey).
To identify the impact of SANAPA on mangrove-related incomes from fishing and shrimping, we used the variation across households in the changes in mangrove area within SANAPA boundaries. Specifically, we first used the GPS coordinates of the central location of each subvillage to draw a 5-km radius circle around each subvillage (Fig. 1A). We then calculated the changes in mangrove cover (in km2) in each 5-km-radius circle between 2005 and 2010. If enforcement is effective, we should expect an increase in mangrove-related incomes (from fishing and shrimping) where mangrove cover within SANAPA boundaries has increased. We used this variable as the key treatment variable and as a tool for identifying the effect of SANAPA.
Econometric Methods.
In identifying a causal linkage between the establishment of SANAPA and mangrove-related incomes, we used econometric methods to address concerns that changes in mangrove-related incomes could be caused by factors other than the establishment of SANAPA and stronger enforcement of regulations on mangrove harvest (SI Materials and Methods). For example, stocks of shrimp and fish could have increased between 2004 and 2009 all along the coast of the study area as a result of more favorable weather or ecological conditions. Changes in mangrove-related incomes could also be caused by changes in mangrove areas outside SANAPA. Moreover, they also could result from unobservable factors that affect both mangroves and mangrove-related income (e.g., a community’s ability in managing mangroves) and location-specific factors that affect productivity of mangroves. To evaluate convincingly the impact of the protected area on mangrove-related incomes, we needed to control for time effect and unobservable factors to the extent possible. We also had a sample selection issue in which a large proportion of respondents reported zero income for certain income categories. If we did not deal with these issues, the estimates of the impact of establishing SANAPA could have been biased.
Our identification strategy attempted to deal with these issues through several different econometric methods. First, we used data on two periods—before and after the establishment of SANAPA—and applied a method to control for sample selection for panel data (38). Specifically, we used a first-differenced model, which is equivalent to a fixed-effects model with two periods, with inverse Mills ratios (IMRs) for each period (SI Materials and Methods). This approach allowed us to control for time trends, time-invariant unobservable factors, and sample selection. We acknowledge the shortcoming, however, that this approach does not allow us to control for time-varying factors that could affect fishing and shrimping income, such as prices and fish stock.
Second, to address the potential confounding effect of changes in mangrove cover outside the protected area, we controlled for changes in mangrove cover outside SANAPA within 5 km from each subvillage. We expected a smaller coefficient on this variable compared with within-SANAPA mangrove cover for the following two reasons. First, there is a placement effect, i.e., SANAPA protects the areas that are key shrimp and fish breeding areas. Second, there could be quality differences in mangroves; presumably, mangroves within the park boundaries have better protection and hence are more productive as a habitat. We also create a variable to proxy the degree of enforcement by calculating the distance between each subvillage and the park’s boat ramp at which the park enforcement agents periodically reside. We explored whether subvillage proximity to the boat ramp is associated with stronger enforcement. As anecdotal evidence suggests there could be some spillover effect of enforcement to areas outside the park boundaries, we attempted to capture this effect by interacting the distance to the boat ramp and the mangrove area outside the park boundaries. A positive coefficient would indicate that an increase in mangrove area outside the park boundaries is associated with a larger increase in shrimping or fishing income if the subvillage is closer to the boat ramp and is subject to stronger enforcement.
In sum, we estimate the following empirical model:
where yit is the outcome variables of interest (i.e., shrimping and fishing income) for individual i in year t; xit is a vector of time-variant observables, including the distance from the boat ramp (measure of enforcement after establishment of SANAPA) and the interaction term between mangrove cover outside the park boundaries and the distance from the boat ramp; αi is an individual fixed effect; λit is a vector of IMR from a probit model for each year; and εit is the error term. We report a robust SE that corrects for heteroscedasticity (SI Materials and Methods, Table S3).
In addition, we extended the model to understand how the establishment of the protected area affected households from the three wealth segments (poorer, middle, richer) differently. Specifically, we divided the sample into terciles (i.e., three groups of equal size) based on the value of productive and consumable asset per capita (SI Survey). We then added to Eq. (1) dummy variables for the poorer and middle groups (richer group as the base category) and the interaction terms between the dummy variables and the variables for mangrove areas. Intuitively, coefficients on these variables measure how the impact of increased area in mangroves in SANAPA differs for the two groups relative to the richer group. Descriptive statistics for the variables are available in Table S4.
Supplementary Material
Acknowledgments
We thank C. Barrett and A. Travis for organizing the Biodiversity Conservation and Poverty Traps workshop. We greatly appreciate the assistance of J. Daffa, A. Mandari, W. Mkama, S. Ngomuo, J. Mohamed, S. Msuya, and other individuals for their dedicated effort in fieldwork. Our appreciation extends to the survey respondents for sharing their time and knowledge, A. Kafle and H. Wakamatsu for their research assistance, S. Cobb, K. Castro, and Coastal Resources Center staff for sharing their expertise, and the editor and the four anonymous reviewers for their helpful comments. This work was partially funded through the Coastal Institute of the University of Rhode Island.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.J.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101825108/-/DCSupplemental.
References
- 1.Polidoro BA, et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE. 2010;5:e10095. doi: 10.1371/journal.pone.0010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitch WJ, Gosselink JG. Wetlands. New York: Wiley; 2000. Mangrove swamps; pp. 335–373. [Google Scholar]
- 3.Kathiresan K, Rajendran N. Mangrove ecosystems of the Indian Ocean region. Indian J Mar Sci. 2005;34:104–113. [Google Scholar]
- 4.Spalding MD, Blasco F, Field CD. World Mangrove Atlas. Okinawa, Japan: International Society for Mangrove Ecosystems; 1997. [Google Scholar]
- 5.Nagelkerken I, et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat Bot. 2008;89:155–185. [Google Scholar]
- 6.Walters BB, et al. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat Bot. 2008;89:220–236. [Google Scholar]
- 7.Semesi AK, et al. Coastal resources utilization and conservation issues in Bagamoyo, Tanzania. Royal Swedish Acad Sci. 1998;27:635–644. [Google Scholar]
- 8.Valiela I, Bowen JL, York JK. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience. 2001;51:807–815. [Google Scholar]
- 9.Ellison AM. Managing mangroves with benthic biodiversity in mind: Moving beyond roving banditry. J Sea Res. 2008;59:2–15. [Google Scholar]
- 10.Singh HR, Chong VC, Sasekumar A, Lim KH. Value of mangroves as nursery and feeding grounds. Status reviews. Proceedings of the third ASEAN-Australia symposium on living coastal resources. In: Wilkinson CR, Suraphol S, Chou LM, editors. Vol 1. Bangkok: Chulalongkorn Univ; 1994. pp. 105–122. [Google Scholar]
- 11.Robertson AL, Blaber SJM. Plankton, epibenthos and fish communities. In: Robertson AI, Alongi DM, editors. Tropical Mangrove Ecosystems. Washington, DC: American Geophysical Union; 1992. pp. 173–224. [Google Scholar]
- 12.Azariadis C, Stachurski J. Poverty traps. In: Aghion P, Durlauf S, editors. Handbook of Economic Growth. Amsterdam: Elsevier; 2005. pp. 295–384. [Google Scholar]
- 13.Agrawal A, Redford K. Poverty, Development, and Biodiversity Conservation: Shooting in the Dark? Bronx, NY: Wildlife Conservation Society; 2006. [Google Scholar]
- 14.Hughes R, Flintan F. Integrating Conservation and Development Experience: A Review and Bibliography of the ICDP Literature. London: International Institute for Environment and Development; 2001. [Google Scholar]
- 15.Salafsky N, Wollenberg E. Linking livelihoods and conservation: A conceptual framework and scale for assessing the integration of human needs and biodiversity. World Dev. 2000;28:1421–1438. [Google Scholar]
- 16.Campbell B, et al. Assessing the performance of natural resource systems. Conserv Ecol. 2001;5:22. [Google Scholar]
- 17.Andam KS, Ferraro PJ, Sims KRE, Healy A, Holland MB. Protected areas reduced poverty in Costa Rica and Thailand. Proc Natl Acad Sci USA. 2010;107:9996–10001. doi: 10.1073/pnas.0914177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkie DS, et al. Parks and people: Assessing the human welfare effects of establishing protected areas for biodiversity conservation. Conserv Biol. 2006;20:247–249. doi: 10.1111/j.1523-1739.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 19.Adams WM, et al. Biodiversity conservation and the eradication of poverty. Science. 2004;306:1146–1149. doi: 10.1126/science.1097920. [DOI] [PubMed] [Google Scholar]
- 20.Aburto-Oropeza O, et al. Mangroves in the Gulf of California increase fishery yields. Proc Natl Acad Sci USA. 2008;105:10456–10459. doi: 10.1073/pnas.0804601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miththapala S. Mangroves: Coastal Ecosystem Series. Vol 2. Colombo, Sri Lanka: IUCN Ecosystems and Livelihoods Group; 2008. [Google Scholar]
- 22.Baig SP, Iftikhar UA. Are the Mangroves for the Future? Empirical Evidence of the Value of Miani Hor Mangrove Ecosystem as the Basis for Investments. Karachi, Pakistan: International Union for Conservation of Nature; 2005. [Google Scholar]
- 23.Iftikhar U. Sea Intrusion in the Coastal and Riverine Tracts of the Indus Delta - A Case Study. Karachi, Pakistan: International Union for Conservation of Nature; 2002. [Google Scholar]
- 24.de Sherbinin A. Is poverty more acute near parks? An assessment of infant mortality rates around protected areas in developing countries. Oryx. 2008;42:26–35. [Google Scholar]
- 25.Baldus RD, Beddoe V, Jafferji J. Saadani National Park. Arusha, Tanzania: Gallery Publications; 2007. [Google Scholar]
- 26.Tanzania National Parks Department of Planning and Projects Development . Saadani National Park Management Zone Plan. Arusha, Tanzania: 2003. [Google Scholar]
- 27.Staples DJ, Vance DJ, Heales DS. Habitat requirements of juvenile penaeid prawns and their relationship to offshore fisheries. In: Rothlisberg PC, Hill BJ, Staples DS, editors. Second Australian National Prawn Seminar. Cleveland, Australia: CSIRO; 1985. pp. 47–54. [Google Scholar]
- 28.Katoomba Ecosystem Services Incubator . Getting Started on REDD in Tanzania: A Scoping Study for the Katoomba Ecosystem Services Incubator. Washington, DC: Forest Trends; 2009. [Google Scholar]
- 29.Nurse M, Kabamba J. Defining Institutions for Collaborative Mangrove Management: A Case Study from Tanga, Tanzania. Nairobi: International Union for Conservation of Nature Eastern African Regional Office; 2000. [Google Scholar]
- 30.Research and Analysis Working Group of the United Republic of Tanzania . Poverty and Human Development Report. Dar es Salaam, Tanzania: Mkuki na Nyota Publishers; 2005. [Google Scholar]
- 31.Bagamoyo District Planning Department . Bagamoyo, Tanzania: Bagamoyo District Planning Department; 2006. Bagamoyo District Profile. [Google Scholar]
- 32.Wang YQ, et al. In: Remote Sensing of Coastal Environments. Wang YQ, editor. Boca Raton: CRC Press; 2010. pp. 395–411. [Google Scholar]
- 33.World Meteorological Organization WMO annual statements on the status of the global climate . Geneva: World Meteorological Organization; pp. 1995–2010. Available at www.wmo.int/pages/prog/wcp/wcdmp/statement/wmostatement_en.html. Accessed June 8, 2011. [Google Scholar]
- 34.Ahrends A, et al. Predictable waves of sequential forest degradation and biodiversity loss spreading from an African city. Proc Natl Acad Sci USA. 2010;107:14556–14561. doi: 10.1073/pnas.0914471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwampamba TH. Has the woodfuel crisis returned? Urban charcoal consumption in Tanzania and its implications to present and future forest availability. Energy Policy. 2007;35:4221–4234. [Google Scholar]
- 36.Bandyopadhyay S, Tembo G. Household consumption and natural resource management around national parks in Zambia. J Nat Resources Policy Res. 2010;2:39–55. [Google Scholar]
- 37.Jiddawi NS, Öhman MC. Marine fisheries in Tanzania. Ambio. 2002;31:518–527. [PubMed] [Google Scholar]
- 38.Wooldridge JM. Selection corrections for panel data models under conditional mean independence assumptions. J Econom. 1995;68:115–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.