Abstract
The activity of Cdk5 and its regulatory subunit p35 is thought to be important in both normal brain function and neurodegenerative disease pathogenesis. Increased Cdk5 activity, via proteolytic cleavage of p35 to a p25 fragment by the calcium-activated protease calpain or by phosphorylation at Cdk5(Tyr15), can contribute to neurotoxicity. Nonetheless, our knowledge of regulation of Cdk5 activity in disease states is still emerging. Here we demonstrate that Cdk5 is activated by S-nitrosylation or reaction of nitric oxide (NO)-related species with the thiol groups of cysteine residues 83 and 157, to form SNO-Cdk5. We then show that S-nitrosylation of Cdk5 contributes to amyloid-β (Aβ) peptide-induced dendritic spine loss. Furthermore, we observed significant levels of SNO-Cdk5 in postmortem Alzheimer’s disease (AD) but not in normal human brains. These findings suggest that S-nitrosylation of Cdk5 is an aberrant regulatory mechanism of enzyme activity that may contribute to the pathogenesis of AD.
Cdk5 is a cyclin-dependent kinase that is activated by proteins p35, p25, and p39 (1–3). As a predominantly neuronal-specific kinase, Cdk5 lacks a role in cell-cycle control but has been implicated in an array of neuronal functions, including cell survival, axon guidance, neuronal migration, and regulation of synaptic spine density (4–6). Dysregulation of Cdk5 activity may play a role in the pathogenesis of stroke and several neurodegenerative disorders, including Alzheimer’s disease (AD), amyotrophic lateral sclerosis, Parkinson’s disease, and Huntington’s disease (7–11). Cdk5 is also hyperactivated in response to oxidative stress, mitochondrial dysfunction, excitotoxicity, amyloid-β (Aβ) exposure, calcium overload, and neuroinflammation, thus contributing to neuronal damage. These neurotoxic stimuli activate calpain, which cleaves the Cdk5 activator p35 (or p39) into p25 (or p29); p25 accumulation thus contributes to Cdk5 activation (12–16). These changes trigger various events associated with neurodegeneration. Although increased Cdk5 activity has been observed in AD brains compared with nondemented control brains, the mechanism remains contentious (17).
Similarly, nitric oxide (NO) and related species contribute to a number of neurodegenerative diseases. The major source of NO in neurons is neuronal nitric oxide synthase (NOS1). Excitotoxic stress increases intracellular Ca2+, which in turn activates NOS1, thus generating NO. In general, NO can stimulate soluble guanylate cyclase to form cGMP or can S-nitrosylate critical cysteine residues to regulate the activity of multiple target proteins, in some sense akin to phosphorylation. Indeed, S-nitrosylation may explain many cGMP-independent mechanisms of NO action in neurodegenerative diseases (18). Our group has demonstrated that NO contributes to neurodegenerative disorders by redox reaction consisting of S-nitrosylation; in some cases this reaction is followed by further oxidation. Proteins affected in this manner include the gelatinase enzyme matrix metalloprotinease-9 (MMP-9), the protein folding/chaperone enzyme protein disulfide isomerase (PDI), the ubiquitin E3 ligase/neuroprotectant protein parkin, the antioxidant enzyme peroxiredoxin II (PrxII), and the mitochondrial fission-inducing protein dynamin-related protein 1 (Drp1) (18–20). Because Cdk5-p35 in adult brain occurs predominantly as a membrane-bound complex with a subcellular localization quite similar to NOS1 (21), we reasoned that NO generated by NOS1 may cause dysregulation of Cdk5 function by direct modification, thereby contributing to neuronal damage in AD. Moreover, a recent protein microarray-based analysis suggested that Cdk5 could be S-nitrosylated (22). Additionally, while the current investigation was in progress, another report showed that very high (nonphysiological) levels of exogenous NO could S-nitrosylate Cdk5, apparently inhibiting its activity (23). In contrast, in the present study we demonstrate that under pathophysiologically relevant conditions, S-nitrosylation of Cdk5 stimulates enzymatic activity via reaction of NO at cysteine residues 83 and 157. This nitrosylation reaction leads to increased phosphorylation of substrates, including the proapoptotic serine/threonine-specific protein kinase, ataxia telangiectasia mutated (ATM) (6, 24). Furthermore, we demonstrate that S-nitrosylation of Cdk5 occurs in human brains with AD and contributes to Aβ- and N-methyl-d-aspartate (NMDA)-induced dendritic spine loss. These findings elucidate a unique regulatory mechanism of Cdk5 that may be involved in the etiology of Alzheimer’s disease-related synaptic pathology.
Results
NO Enhances Cdk5 Kinase Activity.
The contribution of nitrosative stress to neurodegeneration and the involvement of Cdk5 in various neurodegenerative diseases led us to ask whether Cdk5 is S-nitrosylated under pathophysiologically relevant conditions and whether enzymatic activity is thus affected. We first looked at the crystal structure of human Cdk5 (PDB ID: 1H4L) and found eight cysteine residues, four of them on the protein surface. Among these, Cys83 is located in a classical S-nitrosylation motif, surrounded by D, Q, and E (Fig. S1) (25, 26). Additionally, C83 is spatially close to D145, a critical amino acid for Cdk5 kinase activity. Therefore, we reasoned that S-nitrosylation of this residue might affect Cdk5 kinase activity.
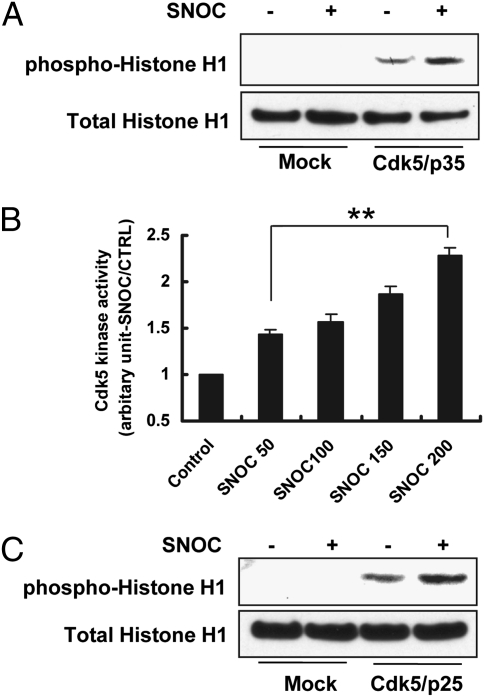
To test this hypothesis, we transfected HEK293 cells with Cdk5 and p35 expression constructs, exposed the cells to S-nitrosocysteine (SNOC, a physiological NO donor) or S-nitrosoglutathione (GSNO, a second physiological NO donor), and assessed Cdk5 activity by histone H1 phosphorylation (Fig. 1A). Exposure to 200 μM SNOC or GSNO enhanced Cdk5 kinase activity (Fig. 1A and Fig. S2A). However, at 1 mM SNOC or GSNO, Cdk5 kinase activity was inhibited (Fig. S2A), as recently reported (23). Because millimolar concentrations of NO donors are never reached in vivo, in our study we considered further only the effects of lower, (patho)physiologically relevant concentrations of NO in the micromolar range.
Fig. 1.
NO Enhances Cdk5 Kinase Activity. (A) Effect of SNOC on Cdk5/p35 phosphorylation of histone H1. HEK293 cells transfected with Cdk5 and p35 plasmids were exposed to 200 μM SNOC and cell lysates prepared for immunoblot analysis. Control cells were exposed to old SNOC, from which NO had been dissipated. The experiments were repeated four times. (B) Effect of SNOC on Cdk5 kinase activity. HEK293 cells transfected with Cdk5 and p35 plasmids were exposed to various concentrations of SNOC (50–200 μM) and cell lysates prepared for measurement of kinase activity using a luciferase assay. Control cells were treated with old SNOC. Kinase activity is normalized to control. Values are mean + SEM (n > 3 for each group, **P < 0.01 by ANOVA). (C) SNOC enhances Cdk5/p25 activity, monitored by histone H1 phosphorylation. HEK293 cells transfected with Cdk5 and p25 plasmids were exposed to 200 μM SNOC and cell lysates prepared for immunoblot analysis. Control cells were exposed to old SNOC.
Concerning the mechanism of activation of Cdk5 by NO, p35 is reportedly unstable in the face of oxidative or nitrosative stress. This finding suggested that enhancement of Cdk5 activity by SNOC might be due to cleavage of p35 to the more stable p25 rather than direct S-nitrosylation of Cdk5 itself. However, we did not observe effects on p35 protein levels in the presence of Cdk5 after exposure to SNOC (Fig. S2B). Moreover, in an in vitro kinase activity assay, we showed that SNOC enhanced Cdk5 activity in a dose-dependent manner under conditions not requiring p35 cleavage (Fig. 1B). Additionally, when we transfected HEK293 cells with expression constructs for Cdk5 and p25, SNOC still enhanced Cdk5 activity with p25 as the coactivator (Fig. 1C). Thus, S-nitrosylation of Cdk5 by NO was deemed a more likely mechanism for Cdk5 activation.
Next, we tested whether endogenously generated NO could enhance Cdk5 kinase activity. For this purpose, we used an HEK293 cell line stably expressing NOS1 (designated HEK/NOS1). This cell-based system thus mimicked physiological conditions by producing endogenous NO. We found that transfection of these cells with Cdk5 and exposure to A23187 to activate NOS1 via Ca2+ resulted in enhanced phosphorylation of histone H1 (Fig. S3A). This finding is consistent with the notion that endogenously generated NO can activate Cdk5.
S-Nitrosylation of Cdk5 at Cys Residues 83/157.
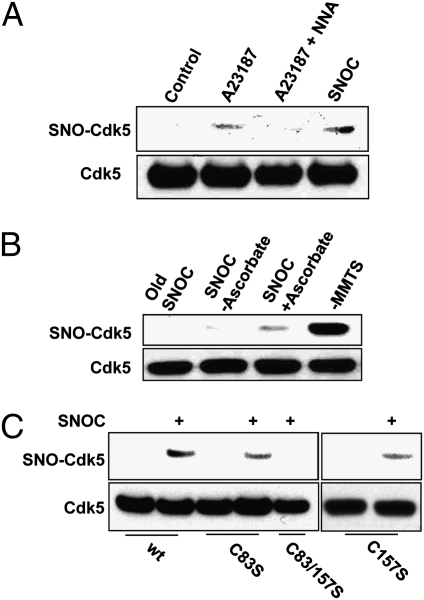
To demonstrate S-nitrosylation of Cdk5 by NO, we used the biotin-switch method (27). We found that activation of NOS1 in HEK/NOS1 cells resulted in formation of S-nitrosylated Cdk5 (SNO-Cdk5). This effect was abrogated by the NOS inhibitor, N-nitro-l-arginine (NNA) (Fig. 2A), consistent with the notion that endogenously generated NO was capable of S-nitrosylating Cdk5. Additionally, we found that endogenous Cdk5 could be S-nitrosylated by 50 μM SNOC in SH-SY5Y cells (Fig. 2B).
Fig. 2.
S-Nitrosylation of Cdk5 in vitro and in vivo. (A) NO derived from NOS1 S-nitrosylates Cdk5 in HEK/NOS1 cells. HEK293 cells stably expressing NOS1 were assayed for endogenous SNO-Cdk5 by the biotin-switch method. NOS1 was activated by Ca2+ ionophore A23187 (5 μM) in the presence or absence of 1 mM NNA. (B) S-Nitrosylation of endogenous Cdk5. SH-SY5Y cells were exposed to 50 μM SNOC for 30 min and subjected to the biotin-switch assay. Control cells were exposed to SNOC without ascorbate or to old SNOC. As a positive control, methyl methane thiosulfonate (MMTS) was omitted so that free thiol groups were not alkylated and could thus react with biotin-HPDP. (C) Mutation of Cdk5 critical cysteine thiols (C83/157S) prevents S-nitrosylation by SNOC. HEK293 cells were transfected with wild-type (wt) or mutated Cdk5 and then exposed to 50 μM SNOC. Cells were then lysed and subjected to biotin-switch assay.
Next, we identified the S-nitrosylated cysteine residue(s) in Cdk5. We mutated Cys83 because it is located on the protein surface within a presumptive consensus motif for S-nitrosylation, consisting of acidic and basic amino acids flanking the critical cysteine residue (25). This mutation partially abolished S-nitrosylation of Cdk5 by 50 μM SNOC (Fig. 2C). We then mutated the other surface cysteines (C94, C157, and C269) both individually and in conjunction with C83S. C157 is surrounded by K and R residues, which may also facilitate S-nitrosylation (25, 26). We found that the C83S/C157S double mutation totally abrogated S-nitrosylation of Cdk5 (Fig. 2C and Fig. S3 B and C).
It is also possible that p35 and p25 may be S-nitrosylated under some conditions. In fact, we detected SNO-p35 after exposure of cells or recombinant protein to 50–100 μM SNOC (Fig. S4). However, unlike SNO-Cdk5, we did not detect SNO-p35 or SNO-p25 after exposure to endogenous NO in HEK/NOS1 cells, in various animal disease models, or in human diseased brains, making it unlikely to be responsible for the effects of NO observed here on Cdk5 activity.
S-Nitrosylation of Cdk5 Enhances Its Kinase Activity.
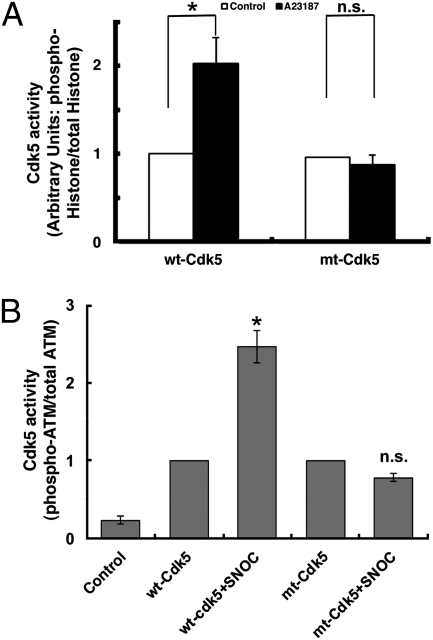
To more directly prove that the increase in Cdk5 kinase activity in response to NO (Fig. 1) was due to formation of SNO-Cdk5, we compared the activity of wt-Cdk5 and its non-nitrosylatable mutant (mt) after exposure to endogenous NO. We immunoprecipitated wt- or mt-Cdk5/p35 from HEK/NOS1 cells exposed to A23187 (to increase NOS1 activity) or control solution and then assessed kinase activity using two substrates, histone H1 and ATM. The non-nitrosylatable mutation per se (in the absence of A23187) did not affect Cdk5 phosphorylation of histone H1 because the kinase activity of the mutant was equal to that of wt-Cdk5 (Fig. 3A). In contrast to wt-Cdk5, however, the mutant protein did not display increased kinase activity after A23187 exposure, consistent with the notion that S-nitrosylation of Cdk5 mediated the increase in kinase activity. Similar results were observed with p25 as the coactivator of Cdk5 in HEK/nNOS cells (Fig. S3A).
Fig. 3.
Mutation of critical cysteine residues in Cdk5 prevents enhancement of kinase activity by NO. (A) Effect of endogenous NO on Cdk5 kinase activity. HEK/NOS1 cells transfected with wt-Cdk5/p35 or mt-Cdk5/p35 were incubated in 5 μM A23187 plus calpeptin (20 μM) for 4 h and lysed, and kinase activity was quantified by histone-H1 phosphorylation. Control cells were incubated in calpeptin only. (B) Density ratio of phospho-ATM to total ATM from experiments shown in Fig S6. Preparations treated with SNOC are normalized to their respective wt- and mt-Cdk5 ratios. Values are mean + SEM (t test, n > 3 in each group; *P < 0.01; n.s., nonsignificant).
To further test the enhancement of Cdk5 kinase activity by S-nitrosylation, we monitored phosphorylation of a second Cdk5 substrate, ATM, representing a pathway involved in neuronal cell death signaling (6, 24). Similar results were obtained with ATM to those with histone H1; Cdk5 kinase activity increased after S-nitrosylation (Fig. 3B and Fig. S5).
SNO-Cdk5 Contributes to NMDA-Induced Dendritic Spine Loss and Neuronal Apoptosis.
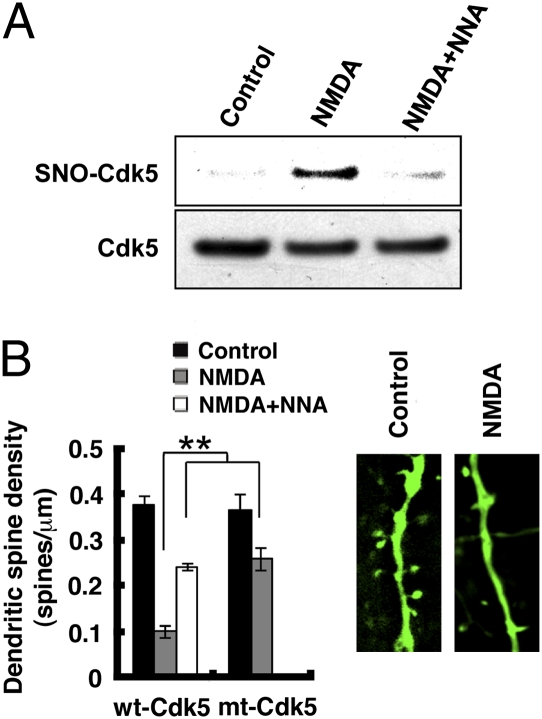
Overstimulation of NMDA-type glutamate receptors (NMDARs) generates excessive NO via NOS1, induces loss of dendritic spines, and eventually results in neuronal cell death (19, 20). Because Cdk5 has been reported to be an important modulator of NMDAR signaling (28), we asked whether SNO-Cdk5 plays a role in NMDAR-mediated dendritic spine loss. We found that exposure to NMDA led to S-nitrosylation of wt-Cdk5, which was abrogated by pretreatment with NNA, implicating mediation by NO (Fig. 4A). We then tested the Cdk5 inhibitor, Roscovitine (29), on NMDA-induced toxicity in rat cerebrocortical cultures. Roscovitine partially prevented NMDA-induced synaptic spine loss (Fig. S6). Although not perfectly specific for Cdk5, Roscovitine’s effect was consistent with the notion that Cdk5 plays a role in NMDA-induced neurotoxicity, as previously reported (30). Next, we transfected neurons with control vector pcDNA3.0, WT-Cdk5/p35 or non-nitrosylatable mt-Cdk5/p35 and then measured dendritic spine density after exposure to NMDA. Neurons transfected with wt-Cdk5/p35 suffered >70% loss of dendritic spines. In contrast, neurons transfected with mt-Cdk5/p35 lost only 32% of their dendritic spines, similar to vector-transfected control neurons (Fig. 4B). These data suggest that SNO-Cdk5 contributes to NMDA-induced dendritic spine loss and neuronal damage.
Fig. 4.
SNO-Cdk5 contributes to NMDA-induced dendritic spine loss. (A) SNO-Cdk5 in primary cortical neurons after exposure to 100 μM NMDA for 4 h in the presence or absence of NNA. (B) SNO-Cdk5 partially mediates NMDA-induced dendritic spine loss. Cortical neurons were transfected with pEGFP plus pcDNA3 (empty vector control, not shown), pEGFP plus wt-Cdk5/p35 or pEGFP plus mt-Cdk5/p35, and then exposed to NMDA (25 μM for 14 h) in the presence or absence of NNA. Neurons were then fixed and stained with anti-MAP2 antibody to identify neurons. Spines were visualized with EGFP (Insets) and quantified per micrometer of dendritic length. Values are mean + SEM (t test with Bonferroni correction, n > 6; **P < 0.01).
SNO-Cdk5 Contributes to Aβ-Induced Dendritic Spine Loss.
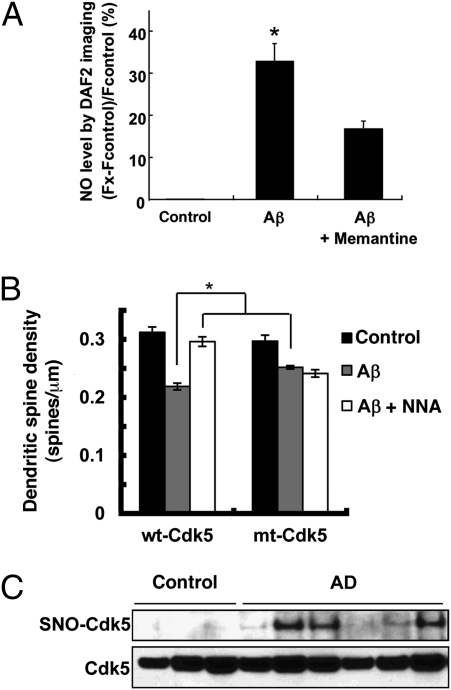
Brains affected by AD manifest a significant increase in Aβ peptide and Cdk5 activity compared with control brains (31). Because we found that SNO-Cdk5 contributes to NMDA-induced spine retraction, and NMDAR antagonists are known to prevent dendritic spine loss and neuronal damage induced by oligomers of Aβ peptide (32–34), we reasoned that SNO-Cdk5 might be an important mediator of this form of toxicity. After exposure to oligomerized Aβ, rat cortical neuronal/glial cultures manifested a dramatic increase in neuronal NO that could be suppressed by memantine, a specific NMDAR antagonist used in the treatment of AD (Fig. 5A) (34, 35). Additionally, Aβ exposure induced NMDAR-dependent S-nitrosylation of Cdk5 in cultured cortical neurons (Fig. S7A). Moreover, S-nitrosylation of Cdk5 was also observed in the brains of Tg2576 transgenic mice, an AD model overexpressing Aβ (Fig. S7B).
Fig. 5.
S-Nitrosylation of Cdk5 in response to Aβ in vitro and in brains with Alzheimer’s disease in vivo. (A) Aβ oligomers induce NO in cortical neurons, at least in part, via NMDAR activation. Cortical neurons were preloaded with 4 μM DAF-2 to detect NO and exposed to 5 μM oligomerized Aβ1–42 in the presence or absence of the NMDAR inhibitor memantine (10 μM); similar results were obtained with Aβ25–35, but not with Aβ35–25 or nonoligomerized Aβ1–42. By dynamic light scattering analysis, the oligomerized preparation of Aβ1–42 contained 250 nM of oliogmers. DAF-2 fluorescence was measured using deconvolution microscopy. (B) SNO-Cdk5 mediates in part Aβ-induced dendritic spine loss. Cortical neurons were transfected with pEGFP plus pcDNA3, pEGFP plus wt-Cdk5/p35 or pEGFP plus mt-Cdk5/p35 and then exposed to Aβ25–35 (10 μM for 4 d) or control Aβ35–25. Cells were then fixed and stained with anti-MAP2 antibody to identify neurons. Dendritic spines were visualized by the presence of EGFP protuberances and quantified per micrometer of dendritic length. For A and B, values are mean + SEM (t test with Bonferroni correction, n > 3; *P < 0.05). (C) Postmortem human brain tissues from control subjects or patients with Alzheimer’s disease were subjected to the biotin-switch assay to detect SNO-Cdk5.
In both animal models and human AD brains, synaptic damage represents an early neuropathological manifestation that correlates with the degree of cognitive decline (36–38). Here, we confirmed that exposure to Aβ resulted in loss of dendritic spines. In neurons transfected with wt-Cdk5/p35, exposure to Aβ resulted in ∼33% loss of spine density compared with control, and transfection with nonnitrosylatable mt-Cdk5/p35 partially abrogated this effect (Fig. 5B). Moreover, pretreatment with NNA completely abolished this effect of Aβ in the presence of wt-Cdk5/p35. These results are consistent with the notion that formation of SNO-Cdk5 contributes to Aβ-induced loss of synaptic spines.
S-Nitrosylation of Cdk5 in Human Brains with Alzheimer’s Disease.
The effect of Aβ on S-nitrosylation of Cdk5 and its influence on spine loss led us to ask whether Cdk5 might be S-nitrosylated in AD. We obtained human brain tissue with short postmortem intervals (Table S1) and, as with the Tg2576 AD mice, found significantly increased SNO-Cdk5 levels in AD brains compared with control brains (Fig. 5C). Importantly, SNO-Cdk5 was not detectable in control human brains, consistent with the notion that S-nitrosylation of Cdk5 represents an aberrant nitrosylation event occurring predominantly in the diseased state. To determine whether the level of SNO-Cdk5 in human brain with Alzheimer’s disease was of pathophysiological significance, we calculated the ratio of SNO-Cdk5 (by biotin-switch assay) to total Cdk5 (as quantified from immunoblots), as we have previously described, and found that this ratio was comparable to or greater than that encountered in our neuronal cell-based models manifesting a decrease in Aβ/SNO-Cdk5–induced spine density (Fig. S8) (19, 20, 39). These findings are consistent with the notion that SNO-Cdk5 may be a contributing factor to the synaptic pathology observed in AD.
Transnitrosylation of Drp1 by SNO-Cdk5.
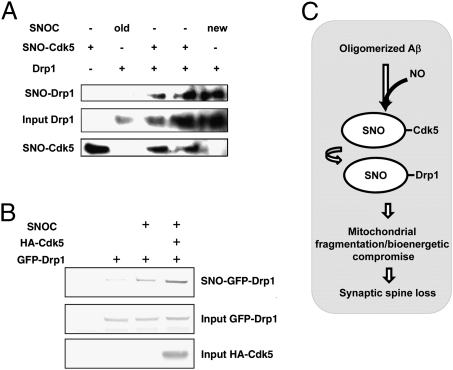
Recently, we reported that exposure of neurons to oligomerized Aβ peptide resulted in S-nitrosylation of the mitochondrial fission protein, dynamin-related protein1 (to form SNO-Drp1). In turn, we showed that SNO-Drp1 triggered massive mitochondrial fragmentation, bioenergetic compromise, and synaptic spine loss (20, 40). Therefore, we asked whether SNO-Cdk5 in brains with Alzheimer’s disease might possibly contribute to this pathway to synaptic damage via transfer of the NO group to Drp1 (to form SNO-Drp1) by a reaction mechanism termed transnitrosylation (39, 41, 42). Previously, it was postulated that Cdk5 could activate Drp1, but the pathway remained obscure because direct phosphorylation of Drp1 by Cdk5 was not observed (43). Here, using the biotin-switch assay, we initially found that SNO-Cdk5 could indeed S-nitrosylate Drp1 in vitro via transnitrosylation, with consequent formation of SNO-Drp1 (Fig. 6A). Furthermore, using a technique that we had previously reported (39), we obtained evidence that transnitrosylation from Cdk5 to Drp1 would favorably proceed in intact cells by calculating the relative redox potential for the reaction (ΔEo′). This technique uses a modification of the Nernst equation that we developed to calculate the associated change in Gibbs free energy (ΔGo′) and hence predict whether the reaction will indeed proceed in vivo (Fig. S9 and SI Materials and Methods). Accordingly, our biotin-switch data showed that SNO-Drp1 levels are elevated in Cdk5-transfected cells compared with control plasmid-transfected cells (Fig. 6B). These findings suggest that transnitrosylation of NO from Cdk5 to Drp1 might be involved in the pathway to SNO-Cdk5–mediated spine loss (Fig. 6C).
Fig. 6.
Transnitrosylation of Drp1 by SNO-Cdk5. (A) In vitro transnitrosylation of Drp1 by SNO-Cdk5. Transnitrosylation reactions were performed as described in SI Materials and Methods. Amounts (input) of Cdk5 and Drp1 were verified in each reaction. S-Nitrosylated proteins were detected by biotin-switch assay. (B) Overexpression of Cdk5 increases the formation of SNO-Drp1. HEK293 cells overexpressing GFP-Drp1, with or without coexpression of HA-Cdk5, were subjected to the biotin-switch assay. (C) Schema of dendritic spine injury in AD triggered by oligomeric Aβ peptide, SNO-Cdk5 formation, and possible transnitrosylation to Drp1.
Discussion
Cdk5 kinase activity requires binding to neuron-specific p35 and has been implicated in neuronal cell death pathways during both normal development and neurodegenerative diseases (44). In the present study, we demonstrate aberrant redox regulation of Cdk5 activity by S-nitrosylation in AD models and in human brain. NOS1 and Cdk5 are both localized at the cell surface (45, 46), and the close proximity of NOS1 to Cdk5 may thus make endogenous NO available for reaction to form SNO-Cdk5. We found that S-nitrosylation increases Cdk5 kinase activity, identified the cysteine residues susceptible to (patho)physiological regulation by NO, and showed that this redox effect was abrogated by nonnitrosylatable mutant Cdk5. Also, NO enhanced both Cdk5/p25 and Cdk5/p35 activity equally well. These data support the premise that S-nitrosylation can directly regulate Cdk5 activity.
Cdk5 activity is known to regulate the morphogenesis of dendritic spines, the major sites of excitatory synaptic transmission in the CNS, thus influencing the function of neuronal circuits (6, 47, 48). Under pathological conditions, dendritic spine loss, representing synaptic damage, is known to correlate with the degree of cognitive decline in AD (36–38). We report here that SNO-Cdk5 contributes to Aβ/NMDAR-mediated spine loss. Moreover, we found that expression of mutant, nonnitrosylatable Cdk5 significantly ameliorated spine retraction during Aβ exposure or excitotoxic stress. These findings suggest that SNO-Cdk5 may represent a unique therapeutic target for ameliorating spine damage in AD and other neurodegenerative conditions. Prior reports showed that Cdk5 can trigger excessive mitochondrial fission involving Drp1 and suggested that Drp1 may be a substrate for Cdk5 regulation (43). Such a relationship may link our recent findings that Aβ-induced activation of Drp1 via S-nitrosylation causes excessive mitochondrial fragmentation, with consequent bioenergetic compromise and dendritic spine loss (20, 40). Indeed, we show in the present study that SNO-Cdk5 can transfer the NO group by transnitrosylation to Drp1 (forming SNO-Drp1), in this manner possibly mediating the synaptic damage that we observed.
Previous studies showed that nitrosative stress contributes to a number of neurodegenerative disorders. These findings prompted us to ask whether SNO-Cdk5 could be detected in human brains with AD. On the basis of the ratio of SNO-Cdk5 to total Cdk5, we found pathophysiologically relevant amounts of the S-nitrosylated kinase in human AD brains (19, 20, 39). In contrast, the virtual absence of detectable SNO-Cdk5 in normal human brains suggested that S-nitrosylation of Cdk5 may be an aberrant event dependent on excessive NO generation, as seen in neurodegenerative disorders. Our finding that Cdk5 is activated by Aβ-induced S-nitrosylation suggests that this mechanism may contribute to the pathogenesis of AD. The enhanced kinase activity of SNO-Cdk5 that we observed might also be expected to contribute to tau hyperphosphorylation and other phosphorylation events relevant to Alzheimer’s disease pathology (49). In conclusion, the elucidation of this SNO-Cdk5–mediated pathway, which contributes to synaptic damage, may facilitate development of unique therapeutic approaches for AD and other neurodegenerative diseases associated with abnormal protein phosphorylation and nitrosative/oxidative stress.
Materials and Methods
Detailed procedures are provided in SI Materials and Methods.
Reagents.
All chemicals were purchased from Sigma. Cdk5 substrate and Aβ25–35, Aβ35–25, and Aβ1–42 peptides were from Anaspec.
Cell Culture and Transfection.
We performed cell transfections with Lipofectamine 2000 (Invitrogen), including primary mixed cerebrocortical cultures.
Immunoblot Analysis.
Proteins were run on gradient, denaturing gels, blotted, and probed with appropriate antibodies.
Site-Directed Mutagenesis.
Mutations in Cdk5 were made using the Quick Change (Stratagene) kit.
Biotin-Switch Assay.
Analysis of SNO-Cdk5 by the biotin-switch assay was performed as described (19, 20). In brief, cells were lysed, free thiols blocked, and proteins precipitated and resuspended. S-nitrosothiols were selectively reduced by ascorbate and biotinylated with biotin-HPDP (Pierce Biotechnology). Biotinylated proteins pulled down with streptavidin–agarose beads (Thermo Scientific) were analyzed by immunoblotting.
Cdk5 Kinase Luciferase Assay.
Cdk5 protein was precipitated from whole-cell lysates and the Cdk5 kinase luciferase assay performed using histone H1 peptide (PKTPKKAKKL). Remaining ATP was determined by Kinase-Glo Plus Luminescent Kinase Assay (Promega). Phosphorylated histone H1 was detected by anti–phospho-histone H1 antibody.
Quantification of Dendritic Spine Density and Neuronal Apoptosis.
Primary mixed cerebrocortical cultures were transfected with EGFP and Cdk5 and exposed to Aβ peptides or NMDA to initiate NO production by NOS. Cultures were stained for neuron-specific microtubule-associated protein 2 (MAP2), images acquired by deconvolution microscopy, and secondary or tertiary dendrites randomly selected for masked counting of dendritic spine protuberances. Neuronal apoptosis was quantified by pyknotic, Hoechst-stained nuclei in MAP2-positive cells.
Transnitrosylation Assay.
Recombinant Cdk5 from bacteria was incubated with SNOC, and S-nitrosylated proteins were used as NO donors to test for transnitrosylation of recombinant Drp1. Assay was by NO–biotin switch, as described (27, 39). The relative redox potential as well as the change in Gibbs free energy for the transnitrosylation reaction was calculated as previously described (39).
Supplementary Material
Acknowledgments
We thank Traci Newmeyer for preparation of primary cultures and Dr. Eliezer Masliah for kindly providing human brain tissues. This work was supported in part by National Institutes of Health Grants P01 HD29587, P01 ES016738, R01 EY09024, R01 EY05477, and P30 NS057096 (to S.A.L.).
Footnotes
Conflict of interest statement: S.A.L. is the named inventor on numerous patents in territories worldwide for the use of memantine in neurodegenerative disorders. He has no direct ownership in the drug, which is currently clinically approved and marketed for moderate to severe Alzheimer’s disease under the name Namenda. Under the rules of Harvard University, his former institution where the work was initially performed, S.A.L. participates in a royalty-sharing plan administered by Harvard Medical School and Children’s Hospital, Boston.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105172108/-/DCSupplemental.
References
- 1.Lew J, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 2.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 3.Tang D, et al. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 4.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 7.Patrick GN, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MD, Larivière RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: Toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 9.Smith PD, et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu D, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Paoletti P, et al. Dopaminergic and glutamatergic signaling crosstalk in Huntington’s disease neurodegeneration: The role of p25/cyclin-dependent kinase 5. J Neurosci. 2008;28:10090–10101. doi: 10.1523/JNEUROSCI.3237-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 13.Strocchi P, Pession A, Dozza B. Up-regulation of cDK5/p35 by oxidative stress in human neuroblastoma IMR-32 cells. J Cell Biochem. 2003;88:758–765. doi: 10.1002/jcb.10391. [DOI] [PubMed] [Google Scholar]
- 14.Zambrano CA, Egaña JT, Núñez MT, Maccioni RB, González-Billault C. Oxidative stress promotes tau dephosphorylation in neuronal cells: The roles of cdk5 and PP1. Free Radic Biol Med. 2004;36:1393–1402. doi: 10.1016/j.freeradbiomed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahlgren CM, et al. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Lipton SA. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 19.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 20.Cho DH, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asada A, et al. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J Neurochem. 2008;106:1325–1336. doi: 10.1111/j.1471-4159.2008.05500.x. [DOI] [PubMed] [Google Scholar]
- 22.Foster MW, Forrester MT, Stamler JS. A protein microarray-based analysis of S-nitrosylation. Proc Natl Acad Sci USA. 2009;106:18948–18953. doi: 10.1073/pnas.0900729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, et al. S-nitrosylation of cyclin-dependent kinase 5 (cdk5) regulates its kinase activity and dendrite growth during neuronal development. J Neurosci. 2010;30:14366–14370. doi: 10.1523/JNEUROSCI.3899-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian B, Yang Q, Mao Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat Cell Biol. 2009;11:211–218. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 26.Marino SM, Gladyshev VN. Structural analysis of cysteine S-nitrosylation: A modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 29.Meijer L, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 30.Hawasli AH, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai LH, Lee MS, Cruz J. Cdk5, a therapeutic target for Alzheimer’s disease? Biochim Biophys Acta. 2004;1697:137–142. doi: 10.1016/j.bbapap.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harkany T, et al. beta-amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–2745. doi: 10.1046/j.1460-9568.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- 34.Xia P, Chen HSV, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 36.Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, et al. Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol Cell. 2010;39:184–195. doi: 10.1016/j.molcel.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsoum MJ, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 43.Meuer K, et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- 44.Ko J, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed TM, et al. Specific role of neuronal nitric-oxide synthase when tethered to the plasma membrane calcium pump in regulating the beta-adrenergic signal in the myocardium. J Biol Chem. 2009;284:12091–12098. doi: 10.1074/jbc.M809112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minegishi S, et al. Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry. 2010;49:5482–5493. doi: 10.1021/bi100631f. [DOI] [PubMed] [Google Scholar]
- 47.Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- 48.Fu WY, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- 49.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.