Abstract
Maternal separation and poor maternal care in animals have been shown to have important effects on the developing hippocampus and amygdala. In humans, children exposed to abuse/maltreatment or orphanage rearing do not present changes in hippocampal volumes. However, children reared in orphanages present enlarged amygdala volumes, suggesting that the amygdala may be particularly sensitive to severely disturbed (i.e., discontinous, neglectful) care in infancy. Maternal depressive symptomatology has been associated with reductions in overall sensitivity to the infant, and with an increased rate of withdrawn, disengaged behaviors. To determine if poor maternal care associated with maternal depressive symptomatology has a similar pattern of association to the volumes of the hippocampus and amygdala in children, as is the case for severely disturbed infant care (orphanage rearing), we measured hippocampal and amygdala volumes as well as stress hormone (glucocorticoid) levels in children exposed (n = 17) or not (n = 21) to maternal depressive symptomatology since birth. Results revealed no group difference in hippocampal volumes, but larger left and right amygdala volumes and increased levels of glucocorticoids in the children of mothers presenting depressive symptomatology since birth. Moreover, a significant positive correlation was observed between mothers' mean depressive scores and amygdala volumes in their children. The results of this study suggest that amygdala volume in human children may represent an early marker of biological sensitivity to quality of maternal care.
Keywords: child, brain, development, parental care
In all mammalian species, infants are highly dependent on their mothers not only for nutrition, but also for physical and affective interactions (1). In rodents, one of the most potent stressors for pups is separation from the dam for long periods of time. Maternal separation activates the pups’ hypothalamic-pituitary-adrenal (HPA) axis, evidenced by increased circulating levels of adrenocorticotropic hormone and glucocorticoids (2). When they reach adulthood, rat pups subjected to maternal separation exhibit increased anxiety-like behavior, impaired cognitive capabilities, and dysregulation of the HPA axis (3). Similar outcomes have been reported in the offspring of mother rats that naturally display low levels of maternal care (4).
Maternal separation and poor maternal care in the animal neonatal period are associated with structural changes in brain regions linked to cognition and mood regulation, including the hippocampus and the amygdala. Poor or absent maternal care in rodents is associated with decreased hippocampal volume and function in adolescence (5) and adulthood (6, 7), and these delayed effects have been shown to be related to an attenuation of the rate of synaptic development in the hippocampus (8). In contrast to the hippocampus, which shows protracted effects, the amygdala shows rapid changes in response to maternal separation or poor maternal care. For example, poor caregiving in rodents results in acceleration of amygdala development (9, 10), increases in corticotrophin-releasing hormone-containing neurons (11), and sensitization of the amygdala in adolescence (12). In addition, nonhuman primate studies show that maternal separation alters amygdala development, and that this effect is more devastating when maternal separation occurs earlier in life (13).
In human research, adults who report parental loss or poor quality of maternal care during early childhood show higher basal levels of glucocorticoids (14, 15), increased glucocorticoid reactivity to a laboratory stressor (16), and reduced hippocampal volume [although only observed in women and in interaction with prenatal adversity (17)]. Exposure to childhood abuse and maltreatment is associated with reduced hippocampal volume in adults (8, 18-21), although it is not in children (22–24). At this point, there is no evidence for a link between amygdala volumes and abuse in children or in adults reporting exposure to childhood abuse (18, 24). Orphanage rearing, which may be a better human model of early maternal separation than abuse/maltreatment, is not associated with changes in hippocampal volumes in children (25, 26) but enlarged amygdala volumes have been reported in children reared in orphanages (25, 26). The differential associations of orphanage rearing and childhood abuse with amygdala volumes in children suggest that neglectful care might have greater impact than abuse on amygdala volumes. At this point, it is unclear whether less-severe disturbances in maternal care, such as those observed in mothers suffering from depressive symptomatology, are similarly associated with hippocampus and amygdala volumes, as reported for severely disturbed infant care (e.g., orphanage rearing).
In humans, well-documented disturbances in caregiving behavior have been reported in mothers with depressive symptomatology. Maternal depressive symptomatology (MDS) has been associated with overall lack of sensitivity to infant needs, and with increased rates of withdrawn, disengaged behaviors (27–29). Maternal depression often interferes with sensitive and supportive care of infants and young children, and there is increasing evidence that the offspring of mothers with depressive symptomatology, especially those who were clinically depressed during their child's early years, present increased activity of the HPA axis in childhood and adolescence (30–33). Given the increased sensitivity of the stress system reported in children of depressed mothers, and given that maternal depression has been shown to be associated with an overall lack of sensitivity to infant needs (27–29), we used the presence of MDS since birth as a human model to study the association between poor maternal care and hippocampus and amygdala volumes in children.
In the present study, we assessed both hippocampal and amygdala volumes in 10-y-old children exposed to MDS since birth. This unique population of children allowed us to assess whether poor maternal care, related to MDS, has a similar pattern of association to the volumes of the hippocampus and amygdala in children, as is the case for severely disturbed infant care induced by orphanage rearing. Children (n = 38) participating in this study were selected from an ongoing longitudinal community study (n = 572) of infant development (34) on the basis that they were exposed or not to MDS since birth. In all children, MDS was assessed at 5, 17, 30, 42, 60, 84, and 156 mo using the Center for Epidemiologic Studies Depression Scale (CES-D) (35). Joint developmental trajectories were estimated on the total sample of children from 5 to 84 mo to specifically select children from the longitudinal sample who were continually exposed to MDS since birth (n = 17; 7 boys and 10 girls) and those who were not exposed to MDS since birth (n = 21; 10 boys and 11 girls). All of the children were born in 1996 and were 10 y old (120 mo) at the time of this imaging study. The maternal CES-D score when the child was 156 mo (13 y) old was obtained to confirm the continuous exposure of children to low versus high MDS throughout development. The correlation between MDS and income was negative (Spearman r = −0.29, P < 0.001). Given that low income has been shown to modulate maternal care in association with MDS (24), children were further selected on the basis of income and were distributed equally across the two MDS groups (P > 0.05). The final sample of children in the two groups did not differ with regard to weight, height, body mass index, waist, brachial circumference, and income (all P > 0.05).
Children were scanned at the Montreal Neurological Institute (MNI) and volumes of the amygdala and hippocampus were analyzed manually using a well-validated protocol (36), controlling for individual differences in head and brain size by registering volumes into standard stereotaxic space. To determine whether children exposed or not to MDS since birth differed with regard to levels of glucocorticoids, we measured salivary glucocorticoid levels in all children upon arrival to the scanning session, right before entry into the scanner, and right after the scan. Exposure to a scanning environment has been shown to lead to a significant increase in glucocorticoid levels in adults (37) and teenagers (38), so glucorticoid levels in response to the scanning environment allowed us to explore potential group differences in stress responsivity.
Results
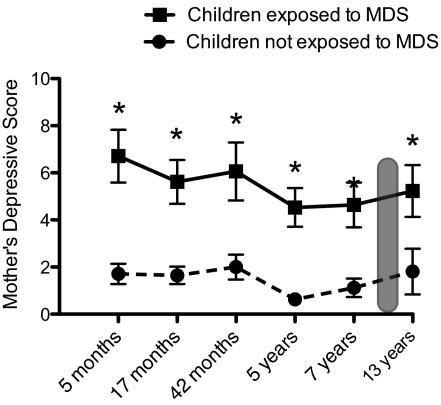
Preliminary analyses performed on maternal depressive scores at 5, 17, 30, 42, 60, 84, and 156 mo confirmed the continuous exposure or not of children to MDS since birth. A two-way repeated-measure ANOVA was performed, with MDS group and sex as between-subjects factors, and time (5, 17, 30, 42, 60, 84, 156 mo) as the within-subject factor. Results revealed a significant main effect of group [F(1,34) = 66.64; P < 0.0001], with no effect of sex or time (main effects or interactions; P > 0.05). As shown in Fig. 1, children from the exposed group had mothers who presented high scores of MDS from 5 mo to 13 y of age, confirming continuous exposure of these children to maternal MDS throughout development.
Fig. 1.
Scores of mothers on the CES-D at child's age 5, 17, 30, 42, 60 (5 y), 84 (7 y), and 156 mo (13 y). Mothers of children exposed to MDS presented significantly higher MDS at all timepoints compared with mothers of children not exposed to MDS (*P < 0.001). The gray zone represents time of scanning at 120 mo (10 y). Error bars represent SEMs.
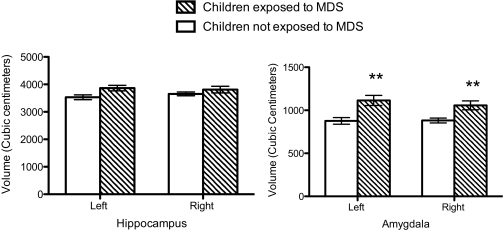
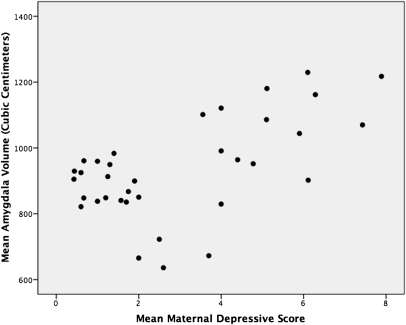
Two-way repeated-measure ANOVAs were performed on amygdala and hippocampal volumes using MDS group and sex as the between-subject factors and hemisphere (left versus right volume) as the within-subject factor. Analyses of brain volumes were corrected for multiple comparisons using a Bonferroni correction (0.05/2 = 0.025). One outlier was found on descriptive analyses of amygdala and hippocampal volumes (same individual) and data from this individual was removed from the analyses. As shown in Fig. 2 (Left), we found no group difference on hippocampal volumes (Ps above 0.05). Furthermore, neither sex nor hemisphere effects (main effects or interactions) were significant (P > 0.05). However, we found a significant main effect of group on amygdala volumes [F(1,34) = 11.78; P < 0.002], revealing larger left and right amygdala volumes in children exposed to MDS since birth compared with children not exposed to MDS since birth (Fig. 2, Right). Groups did not differ with regard to sex or hemisphere (P > 0.05) and no interaction effect was found. Given the main effect of MDS on amygdala volumes in children, we tested whether the mean maternal depression scores assessed over 7 y were associated with amygdala volumes in children. The results revealed a significant positive association between the mean depression score of the mother over the first 7 y of the child's life and her own child's mean amygdala volume (r = 0.59; P < 0.0001) (Fig. 3).
Fig. 2.
Left and right hippocampal (Left) and amygdala (Right) volumes (cubic centimeters) in children exposed to MDS since birth and children not exposed to MDS since birth. Error bars represent SEMs. **P < 0.01 for left and right amygdala volume.
Fig. 3.
Correlation between 7-y mean depression score in the mother and mean (left and right) amygdala volumes (r = 0.59; P < 0.0001) in her own child. Amygdala volume is in cubic centimeters.
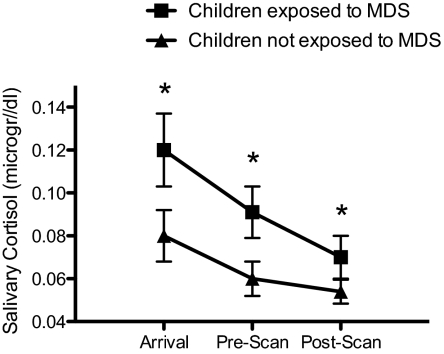
Finally, a two-way repeated-measure ANOVA was performed on salivary glucocorticoid levels using MDS group and sex as the between-subject factors, time (arrival versus prescan versus postscan) as the within-subject factor, and time of sampling as a covariate. We found a significant main effect of MDS group on total glucocorticoid levels [F(1,30)= 4.86; P = 0.035], with no effect of time or sex (main or interaction effects; P > 0.05). As shown in Fig. 4, children exposed to MDS since birth presented increased levels of salivary glucocorticoids when in the testing environment compared with children not exposed to MDS since birth.
Fig. 4.
Salivary cortisol levels in children exposed to MDS since birth and in children not exposed to MDS since birth at the time of arrival to the scanning session, as well as pre- and postscan. *significant at P < 0.05. Error bars represent SEMs.
Discussion
Our data demonstrate that children from a community study exposed to MDS since birth present significantly larger amygdala volumes compared with children not exposed to MDS, whereas the two groups did not differ with regard to hippocampal volumes. Our results are strikingly similar to those of two independent studies that reported enlarged amygdala volumes and no hippocampal difference in children previously reared in orphanages (25, 26). These results are also consistent with animal data showing that poor caregiving in rodents is associated with acceleration of amygdala development in offspring (9, 10), and with others showing that maternal deprivation in rodents (8) and primates (39) is not associated with changes in hippocampal volumes during infancy. Extending these previous findings, our results further show a linear relationship between the mean maternal depressive score of the mothers and the amygdala volumes in their 10-y-old offspring.
The absence of difference in hippocampal volumes in children as a function of MDS is in accordance with recent results showing the absence of reduced hippocampal volumes in children exposed to abuse/maltreatment (22–24) and children reared in orphanages (25, 26). The present study extends this finding by showing that variations in normal childhood experiences, as evidenced by exposure to MDS, is not associated with changes in hippocampal volumes when measured during childhood. According to recent studies (21, 40, 41), if exposure to early adversity has an impact on the hippocampus, this effect may not be apparent until adolescence or adulthood, reflecting the presence of an incubation period for the effects of early adversity on hippocampal volume in humans. Scanning this population of children after puberty or during adulthood should provide important data to further test this hypothesis.
The most important finding of this study is that poor maternal care, generally associated with the presence of MDS, leads to similar effects on amygdala volumes as those reported when the mother is absent, as reflected in data from orphan children (9, 10). These results are in accordance with animal data showing similar effects of maternal deprivation and poor maternal care on the amygdala (9–13, 42), and suggest that the amygdala may be particularly sensitive to variations in the quantity and the quality of maternal care during infancy.
The amygdala has been implicated in learning about the emotional significance of stimuli (43), a mechanism that is subserved by close interactions between glucocorticoids and corticotropin-releasing factor (CRF) in the amygdala (44). Alterations of glucocorticoids and CRF have been reported in anxious and depressed disorders (45, 46), and glucocorticoid regulation of CRF appears to be essential for responding to environmental salience and for maintaining behavioral responses to potential threats (44). The capacity to determine the relative safety or danger of situations is adaptive at any age. However, in early life, when less is known about the environment, the need to learn about the safety or danger of novel environments may be greater (47). In humans, the amygdala exhibits a period of development extending from year one to late childhood (47, 48), and a recent longitudinal study in primates showed that the most rapid rate of amygdala development occurs during the early postnatal period (49). Based on these findings, it has recently been suggested that this rapid rate of change may heighten the vulnerability of the amygdala to environmental influence early in life. Given that maternal care is an important regulator of response to environmental stress in early life, this finding suggests that the amygdala may show increased sensitivity to quality of maternal care during development.
An increased sensitivity of the amygdala in response to different amounts of maternal care has recently been observed in a rodent study by Endelmann and Auger (50). These authors examined the consequences of simulated maternal grooming on estrogen receptor-α promoter methylation and mRNA expression in the amygdala of females, based on previous observations that neonatal males tend to receive more maternal grooming than females (51). The authors found that providing females with somatosensory stimulation mimicking increased maternal grooming resulted in male-typical patterns of estrogen receptor-α mRNA levels and methylation within the developing amygdala. As the amygdala is important for numerous socioemotional behaviors, these data suggest that quantitative differences in mother–infant interactions may program lasting changes in gene expression that impact social or emotional processes occurring within the amygdala.
Quality of maternal care also has important effects on stress responsiveness and behavior in developing rodents. Strong maternal behavior, involving licking and grooming of the offspring, produces a “neophilic” animal that is more exploratory of novel environments, less emotionally reactive, and that produces a lower glucocorticoid stress response in novel situations. In contrast, poor maternal care leads to a “neophobic” phenotype that is less exploratory of a novel situation and that shows a greater glucocorticoid response in novel situations (52). In the present study, we found that children of mothers who present depressive symptomatology since birth produced a greater glucococorticoid response to a novel environment, a finding that goes along with animal studies measuring stress responses in rat pups as a function of quality of maternal care.
The long duration of exposure to MDS may be the key factor in explaining the effect of MDS on amygdala volumes in the children tested in the present study. Results obtained with previously institutionalized orphan children show that children who have been adopted late by high-income families present enlarged amygdala volumes, but children who have been adopted early by high-income families do not present enlarged amygdala volumes (25). The absence of recovery in the amygdala in cases of long duration of exposure to low-quality care could be a way by which the organism, once placed in an adverse environment, ensures that it is prepared for future adversity, thus increasing probability of survival. Future studies assessing amygdala volumes in children exposed to MDS at different ages (and consequently for different durations) should provide highly valuable data on this important question.
The larger amygdala volumes reported in children of mothers presenting depressive symptomatology since birth could also be a result of genetics. Enlarged amygdala volumes have been reported in depressed patients (53, 54), and the intriguing significant association we observed between long-term depressive scores in mothers and amygdala volumes in their children could be the result of genetic transmission of enlarged amygdala volumes from the mother to the child. If this is the case, children's enlarged amygdala volumes could reflect a propensity toward depression, this state being transmitted pre- or postnatally from mothers to children. Clearly, studies assessing amygdala and hippocampal volumes in siblings/twins exposed to maternal depression could allow clarification of genetic and environmental effects of maternal depression on amygdala and hippocampal volumes in children.
Finally, it is important to note that the endocrine and structural changes observed in children of mothers presenting depressive symptomatology since birth could be related to a combination of paternal and maternal factors. Contrary to most rodents, human children benefit from biparental care, and it might be that the observed associations between MDS and amygdala volumes in children are modulated by variations in paternal care associated with the presence of MDS. It has recently been shown that among children experiencing low father involvement in infancy, behavioral, autonomic, and adrenocortical reactivity become risk factors for mental health symptoms at age 9 (55). More importantly, the most severe symptoms were found for children with high autonomic reactivity who, as infants, had experienced low father involvement and mothers with symptoms of depression (55). Taken together, these results suggest that low paternal caretaking in infancy may also be an important predisposing factor for the emergence of variations in amygdala volumes in middle childhood, a contribution that may exacerbate the consequences of a poor care experience associated with maternal depression.
As discussed by previous authors (47, 56), it will be extremely difficult to test the effects of variations in quantity and quality of maternal and paternal care on brain development in human children, as it is almost impossible to manipulate the human environment in a way that would allow us to test the predictive value of our models. However, the fact that the brain may be highly responsive to environmental conditions during early development is in agreement with research agendas aimed at assessing the impact of early interventions on the pattern of brain development during childhood. In children facing early adversity, provisions of help to the family unit, such as prenatal and infancy nurse home visits (57), video-feedback interventions to promote maternal sensitivity and infant attachment security (58), or provision of environmental enrichment, such as enriched daycare/school environment (59) and social support from members of the community (60), could induce a heterotypic reorganization of synaptic development, programming of neurotrophic factors, or changes in gene expression that could lead to resilience later in life. If this is the case, then it may be that any type of intervention performed during the early years could have a tremendous effect in preventing the deleterious impact of poor or absent parental care on the developing brain.
Materials and Methods
Selection of Participants.
In all children participating in the Quebec Longitudinal Study of Child Development (n = 572), we estimated joint developmental trajectories from ages 5 to 84 mo using a censored normal density procedure for MDS to specifically select children who were continually or never exposed to MDS since birth (see SI Text for the method used for estimating developmental trajectories). Results of the developmental trajectories analysis showed that 86 participants from the longitudinal study were eligible to take part in the study at age 10 y based on the developmental trajectories of exposure to high (21% of the sample) versus low (79% of the sample) MDS since birth when matching for income. From the 86 participants who were eligible to participate in the study, 48 refused to participate or did not meet the criteria to go into the scanner, leading to a total of 38 participants, of which 17 were in the high MDS group (7 boys and 10 girls) and 21 were in the low MDS group (10 boys and 11 girls). Children were free of medication. The brain imaging study was approved by the Research Ethics Committee of Sainte-Justine Hospital, the Douglas Mental Health Institute, and Louis H. Lafontaine Hospital in Montreal. All parents signed a consent form, and all children signed an assent form.
MRI Volumetric Analyses.
Children were scanned at the MNI using a 1.5T Siemens Vision MRI Scanner (Siemens), with scanning parameters from the National Institute of Mental Health (NIMH) MRI study of brain development (61). High-resolution T1-weighted image scans (isotropic 1 mm) were acquired using a 3D spoiled gradient-echo acquisition protocol with sagittal volume excitation to guarantee high contrast and sufficient resolution for structural analysis. All images were transferred to a MacIntosh computer (Apple Inc.). The raw images were corrected for nonuniformity (62), registered into MNI space (63), and normalized for signal intensity to harmonize gray-white matter contrast across subjects. With regard to the brain-imaging analyses performed, the results were automatically corrected for total brain and head volumes, as all images were registered into standard stereotaxic space based on the pediatric NIMH protocol (61) before any of the analyses were performed. This step automatically corrects for any interindividual differences in head and brain size so that later comparisons are unaffected by variations in head/brain size existing between the individuals. For the amygdala and hippocampal segmentation, the structure was hand drawn using a well-validated (36) segmentation protocol developed by one of the coauthors (J.C.P.) of the article. Another coauthor (V.C.) segmented all volumes and this person was blind with regard to participant's grouping variable (see SI Text for the manual segmentation procedure for analysis of hippocampus and amygdala volumes).
Assessment of Glucocorticoid Levels.
We measured salivary glucocorticoid levels upon arrival to the scanning environment, right before entry into the scanner, and right after the scan. For saliva sampling, participants were asked to fill a small plastic vial with 10 mm of pure saliva (i.e., passive drool). Saliva samples were maintained at −20 °C until the time of glucocorticoid concentration determination. Analyses were performed at the Douglas Mental Health Institute in Montreal, using an Enzyme Immunoassay (EIA) kit from Salimetrics LLC.
Procedure.
All children were scanned at the MNI during a morning weekend day to avoid conflicts with school curriculum and to control for the circadian rhythm of glucocorticoids. To ensure that children were not moving during the MRI procedure, they were presented with various children movies and had to choose a movie they would like to watch in the scanner. They were told that if they liked the movie, they would be able to keep it after the scan. The movie was then presented to the children inside the scanner using headphones and a projector. Children were further told that upon completion of the scan, they would receive by mail a picture of their brain. All children received a color copy of their brain after the procedure.
Supplementary Material
Acknowledgments
We thank the anonymous reviewers who provided highly valuable comments on this manuscript. This study was supported by grants from the John D. and Catherine T. MacArthur Foundation, the Canadian Institutes for Health Research (Grant MOP-44072), and the Fonds de Recherche en Santé du Québec. S.J.L. is supported by a Senior Investigator Chair from the Canadian Institutes of Health Research, Institute of Gender and Health. J.R.S. was supported by a Fonds de Recherche en Santé du Québec research-scientist award.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105371108/-/DCSupplemental.
References
- 1.Suomi SJ, van der Horst FC, van der Veer R. Rigorous experiments on monkey love: An account of Harry F. Harlow's role in the history of attachment theory. Integr Psychol Behav Sci. 2008;42:354–369. doi: 10.1007/s12124-008-9072-9. [DOI] [PubMed] [Google Scholar]
- 2.Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology. 1988;13(1):143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 3.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 5.Gruss M, Braun K, Frey JU, Korz V. Maternal separation during a specific postnatal time window prevents reinforcement of hippocampal long-term potentiation in adolescent rats. Neuroscience. 2008;152(1):1–7. doi: 10.1016/j.neuroscience.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 8.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 9.Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. J Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- 10.Ono M, et al. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156:1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 11.Becker K, Abraham A, Kindler J, Helmeke C, Braun K. Exposure to neonatal separation stress alters exploratory behavior and corticotropin releasing factor expression in neurons in the amygdala and hippocampus. Dev Neurobiol. 2007;67:617–629. doi: 10.1002/dneu.20372. [DOI] [PubMed] [Google Scholar]
- 12.Salzberg M, et al. Early postnatal stress confers enduring vulnerability to limbic epileptogenesis. Epilepsia. 2007;48:2079–2085. doi: 10.1111/j.1528-1167.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabatini MJ, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosom Med. 1998;60:765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buss C, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremner JD, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 20.Vythilingam M, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.De Bellis MD, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 23.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 24.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 25.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 27.Field T. The effects of mother's physical and emotional unavailability on emotion regulation. In: Fox NA, editor. The Development of Emotion Regulation. Chicago: University of Chicago Press; 1994. pp. 208–227. [PubMed] [Google Scholar]
- 28.Field T. Early interactions between infants and their postpartum depressed mothers. Infant Behav Dev. 1984;7:537–540. [Google Scholar]
- 29.Murray L, Cooper P. Intergenerational transmission of affective and cognitive processes asociated with depression: Infancy and the pre-school years. In: Goodyer IM, editor. Unipolar Depression: A Lifespan Perspective. Oxford: Oxford University Press; 2003. pp. 17–46. [Google Scholar]
- 30.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 31.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 32.Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry. 2007;62(1):40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- 34.Santé Québec QC, Jetté M, Desrosiers H, Tremblay RE. “In 2001… I'll be 5 years old ”Survey of 5 months old infants: Preliminary report from the Québec Longitudinal Study of Childhood Development (QLSCD). (Article in French) In: Guide Québec., editor. Canada: Ministère de la Santé et des Services Sociaux, Montréal; 1997. [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychometric Measure. 1977;1:385–401. [Google Scholar]
- 36.Pruessner JC, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 37.Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp. 2006;27:889–895. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinelli S, et al. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 41.Rao H, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez DM, et al. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: Effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 44.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- 46.Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: Focus on monoamine transporters and the HPA axis. CNS Spectr. 2004;9(6, Suppl 4):23–31. doi: 10.1017/s1092852900025475. [DOI] [PubMed] [Google Scholar]
- 47.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci. 2009;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 49.Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 52.Champagne DL, et al. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frodl T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 54.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 55.Boyce WT, et al. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J Am Acad Child Adolesc Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- 56.Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olds DL, et al. Effects of nurse home visiting on maternal and child functioning: Age-9 follow-up of a randomized trial. Pediatrics. 2007;120:e832–e845. doi: 10.1542/peds.2006-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rusconi-Serpa S, Sancho Rossignol A, McDonough SC. Video feedback in parent-infant treatments. Child Adolesc Psychiatr Clin N Am. 2009;18:735–751. doi: 10.1016/j.chc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Geoffroy MC, Côté SM, Parent S, Séguin JR. Daycare attendance, stress, and mental health. Can J Psychiatry. 2006;51:607–615. doi: 10.1177/070674370605100909. [DOI] [PubMed] [Google Scholar]
- 60.Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Soc Sci Med. 2009;68:2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans AC, Brain Development Cooperative Group The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 62.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 63.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.