Synchronous activity is ubiquitous in natural systems and has fascinated the likes of engineers, mathematicians, and biologists (1). Although coordinated oscillations have been described among atoms, molecules, cells, and organisms on timescales from fractions of a second to years, our understanding of the mechanisms underlying synchrony is often limited. A study in PNAS (2) provides insights into how diffusible signals organize the daily oscillations among cells in the brain. In this system, three neuropeptides released from distinct groups of neurons can coordinate cycles of gene expression across the larger population of cells (Fig. 1A).
Fig. 1.
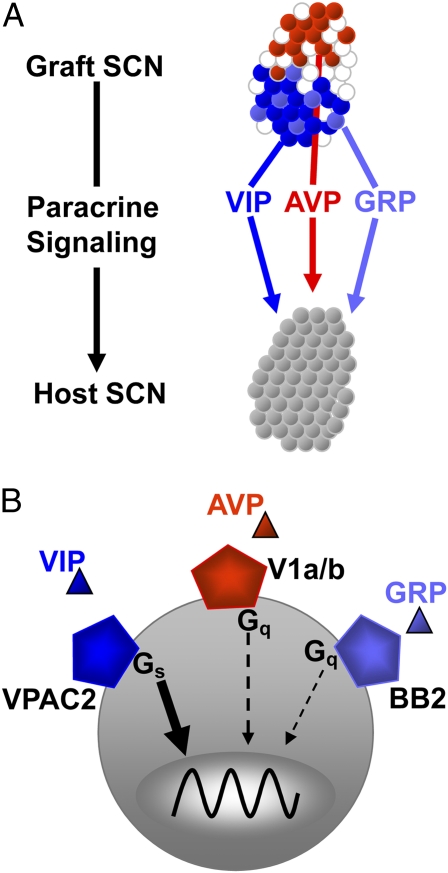
Multiple signals contribute to drive and maintain circadian rhythmicity. (A) As described by Maywood et al. (2), a unique coculture system reveals how neuropeptides released by neurons within a circadian SCN graft can diffuse and restore daily rhythms to an arrhythmic host SCN. Three neuropeptides endogenous to the SCN differ in their potency as paracrine coordinators of circadian rhythms. (B) VIP signaling through its receptor, VPAC2, and Gαs dominates, whereas AVP and GRP can partially compensate for the loss of VIP signaling to sustain synchronous circadian rhythms in gene expression across the SCN.
In mammals, the suprachiasmatic nucleus (SCN) is responsible for generating near-24 h (circadian) cycling in a vast array of physiological and behavioral events, including sleep–wake, hormone release, metabolism, and gene expression (3). The nearly 20,000 SCN neurons spontaneously synchronize to each other early in development. A burning question in the field has been: How do these cells synchronize to each other to coordinate daily rhythms in behavior?
Maywood et al. took full advantage of the features that make the SCN an excellent model for understanding communication among networked oscillators. The SCN can be isolated and its daily cycling studied in the controlled environment of a Petri dish for weeks (4). The SCN can be transplanted to rescue lost rhythms (5). The periodicity of the SCN can be manipulated through changes in key genes; more than 20 transcription factors, kinases, phosphatases, and their regulators have been shown to determine its cycle length (6, 7). Finally, Maywood et al. followed on evidence that SCN neurons secrete factors capable of coordinating rhythms in the brain and body. Early studies revealed that transplants of SCN inside a dialysis membrane can restore locomotor rhythms in the absence of synaptic connections to the host brain (5). More recently, cocultures of immortalized SCN cell lines (8) or SCN explants (9) have been found to sustain rhythmicity in target cells several millimeters away. In vivo, the SCN can impose circadian rhythms on embryonic fibroblasts implanted under the skin (10). Taken together, these results have led to the hypothesis that the SCN can communicate timing information through at least one, as yet unidentified, diffusible factor.
The breakthrough came when Maywood et al. invented a system whereby they could reversibly appose an SCN slice onto another SCN. Using real-time bioluminescence imaging, they measured the influence of the grafted SCN on the circadian gene expression in the cells of the host SCN. They started by asking whether the SCN taken from a wild-type mouse is capable of rescuing daily rhythms in the SCN taken from an arrhythmic mouse. Previous studies had demonstrated that loss of vasoactive intestinal polypeptide (VIP) or its cognate receptor, VPAC2R (encoded by the Vipr2 gene) abolished synchronized circadian rhythms in the SCN and in locomotor behavior (11, 12). The new study (2) shows that wild-type SCN can quickly restore rhythms to SCN lacking VIP. By changing the circadian genotype of the graft SCN, the authors cleverly reveal they can drive the host SCN at either much shorter or longer periods. SCN lacking VIP do not rescue rhythms in the host, indicating that VIP is critical. However, is VIP the signal that passes across the divide to the other SCN? The answer was a resounding “Yes, but….” Wild-type grafts could slowly rescue weak rhythms in SCN lacking the VPAC2 receptor, indicating that VIP signaling is the major pathway to coordinated rhythms in the SCN, but leaving room for additional, less potent synchronizing agents. To test whether other signals can organize rhythms in the SCN, the authors took aim at two other candidate secreted peptides. They found that the weak rescue of rhythms in Vipr2−/− SCN could be abolished by blockers of receptors for gastrin-releasing peptide (GRP) (BB2r) or arginine vasopressin (AVP) (V1a and V1b). Interestingly, the rescue of Vip-deficient SCN by wild-type grafts was weakened by blockers of AVP receptors but less affected by blockers of GRP receptors. This leads to a model whereby coupling among SCN cells depends primarily on VIP–VPAC2R signaling, modestly on V1a/V1b signaling, and weakly on BB2r signaling (Fig. 1B).
How do these extracellular, paracrine signals impinge on the intracellular circadian gene network to synchronize rhythms across the population of cells? A prevailing view holds that these neuropeptides act through their G protein-coupled receptors to modulate cAMP levels to up-regulate expression of clock genes and, ultimately, adjust the circadian phase of each cell (13). Here, Maywood et al. provided a surprising insight. As an alternative to rescuing rhythms in Vip−/− SCN, they examined SCN from mice lacking the two cryptochrome (Cry) genes. Although these mice show no circadian rhythms in behavior, and their SCN have been reported to express no daily rhythms in neuronal firing rates (14), they found that Cry-null SCN express weak circadian rhythms. This was unexpected but is similar to the recent discovery that the SCN from other arrhythmic mice can show weak circadian cycling (15). The bigger surprise came when they found that these weak rhythms were slow to respond to grafted SCN. The authors argue that this implicates intercellular signaling in sustaining the weak rhythms of the Cry-null SCN and slowing their response to signals from a grafted SCN.
What is the significance of this hierarchical arrangement of three neuropeptides coordinating gene expression across thousands of cells? The conclusion here that VIP–VPAC2R signaling dominates is consistent with the observation that loss of VIP or VPAC2R abolishes circadian rhythms in most behaviors and physiological processes in most mice (11, 12). That GRP and AVP can, in the absence of VIP, suffice to sustain daily cycling is consistent with the observation that some or all VIP/VPAC2R-deficient mice can still show synchronized circadian rhythms under some conditions (16) and that GRP can induce rhythms in VPAC2R-null SCN (17). This is strikingly reminiscent of the modulatory role of neuropeptides in other neural networks like the central pattern generators underlying faster oscillatory behaviors like locomotion, respiration, and chewing (18).
Notably, there may be more secreted factors that can mediate or modulate circadian synchrony under the right environmental or developmental conditions. For example, a functional screen for genes encoding secreted and membrane-bound proteins in the SCN identified more than 100 peptides, including neuropeptide precursors, growth factors, cytokines, chemotrophins, and transmembrane proteins, that signal after cleavage (19). More recently, methods for directly sequestering and sequencing peptides secreted from SCN explants identified more than 100 peptides derived from 27 precursor proteins (20). Some of these are likely to be secreted rhythmically within the SCN or SCN target tissues and, thus, carry timing information. How far can these signals travel? VIP, AVP, and GRP, at least, can go the distance.
Acknowledgments
This work is supported by National Institutes of Health Grants NIMH63109 (to E.D.H.) and F30NS070376 (to G.M.F.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 14306.
References
- 1.Strogatz SH. Sync: The Emerging Science of Spontaneous Order. New York: Hyperion; 2003. [Google Scholar]
- 2.Maywood ES, Chesham JE, O'Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 5.LeSauter J, Silver R. Output signals of the SCN. Chronobiol Int. 1998;15:535–550. doi: 10.3109/07420529808998706. [DOI] [PubMed] [Google Scholar]
- 6.Nolan PM, Parsons MJ. Clocks go forward: Progress in the molecular genetic analysis of rhythmic behaviour. Mamm Genome. 2009;20:67–70. doi: 10.1007/s00335-008-9166-1. [DOI] [PubMed] [Google Scholar]
- 7.Ukai H, Ueda HR. Systems biology of mammalian circadian clocks. Annu Rev Physiol. 2010;72:579–603. doi: 10.1146/annurev-physiol-073109-130051. [DOI] [PubMed] [Google Scholar]
- 8.Allen G, Rappe J, Earnest DJ, Cassone VM. Oscillating on borrowed time: Diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. J Neurosci. 2001;21:7937–7943. doi: 10.1523/JNEUROSCI.21-20-07937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pando MP, Morse D, Cermakian N, Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell. 2002;110:107–117. doi: 10.1016/s0092-8674(02)00803-6. [DOI] [PubMed] [Google Scholar]
- 11.Aton SJ, Herzog ED. Come together, right…now: Synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An S, Irwin RP, Allen CN, Tsai CA, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–2296. doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albus H, et al. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12:1130–1133. doi: 10.1016/s0960-9822(02)00923-5. [DOI] [PubMed] [Google Scholar]
- 15.Ko CH, et al. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 2010;8:e1000513. doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power A, Hughes AT, Samuels RE, Piggins HD. Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J Biol Rhythms. 2010;25:235–246. doi: 10.1177/0748730410374446. [DOI] [PubMed] [Google Scholar]
- 17.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Kramer A, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 20.Lee JE, et al. Endogenous peptide discovery of the rat circadian clock: A focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol Cell Proteomics. 2010;9:285–297. doi: 10.1074/mcp.M900362-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]