Abstract
CD1e is a member of the CD1 family that participates in lipid antigen presentation without interacting with the T-cell receptor. It binds lipids in lysosomes and facilitates processing of complex glycolipids, thus promoting editing of lipid antigens. We find that CD1e may positively or negatively affect lipid presentation by CD1b, CD1c, and CD1d. This effect is caused by the capacity of CD1e to facilitate rapid formation of CD1–lipid complexes, as shown for CD1d, and also to accelerate their turnover. Similar results were obtained with antigen-presenting cells from CD1e transgenic mice in which lipid complexes are assembled more efficiently and show faster turnover than in WT antigen-presenting cells. These effects maximize and temporally narrow CD1-restricted responses, as shown by reactivity to Sphingomonas paucimobilis-derived lipid antigens. CD1e is therefore an important modulator of both group 1 and group 2 CD1-restricted responses influencing the lipid antigen availability as well as the generation and persistence of CD1–lipid complexes.
Keywords: CD1 restriction, lipid transfer proteins, natural killer T cells
Humans and some other species, but not rodents of the family Muridae (mice and rats), express CD1e. Similar to the other CD1 molecules, CD1e is associated with β2 microglobulin and binds lipid antigens. CD1e is the only CD1 molecule that becomes soluble and is never expressed on the plasma membrane, thus it cannot behave as an antigen-presenting molecule. Moreover, CD1e shows a very peculiar intracellular distribution, which in dendritic cells (DCs) correlates with their maturation stage (1). In immature DCs, CD1e prevalently accumulates in the trans-Golgi network (TGN), whereas, in mature DCs, it distributes in late endosomes and lysosomes (LYs) where it is cleaved into an active soluble form (2). In LYs of mature DCs, CD1e accumulates and persists because of increased protein stability, which is associated with a progressive shortening of the carbohydrate side chains (3).
Relatively little is known about the role of CD1e in immune responses. CD1e assists processing by the lysosomal α-mannosidase of mycobacterial hexamannosylated phosphatidyl-myo-inositols (PIM6), containing six α-d-Manp units, into dimannosylated forms (PIM2), containing only two α-d-Manp units (4). This form of PIM is stimulatory to specific CD1b-restricted T cells.
Whether CD1e also participates in presentation of lipid antigens by other CD1 molecules is matter of investigation. After the exit from the endoplasmic reticulum, CD1 molecules traffic to the cell surface via the secretory pathway before being reinternalized into the endosomal compartments. CD1a molecules undergo cycles of internalization into early/sorting endosomes followed by early/recycling endosomes (5, 6), whereas CD1c molecules traffic to early recycling endosomes and, to a lesser extent, to late endosomes and LYs (5, 7). In contrast, CD1b and human CD1d molecules recycle in late endosome/LYs compartments where they can colocalize with CD1e (4).
Because of the colocalization of CD1e and CD1b in LYs, where CD1c and CD1d molecules are also found, we investigated whether CD1e could also assist lipid antigen presentation by CD1 molecules other than CD1b. Our findings show that the activity of CD1e is broader than the one already reported and provide evidence that CD1e operates with multiple mechanisms to modulate the immune response to lipid antigens.
Results
CD1e Participates in the Presentation of CD1b- and CD1c-Restricted Antigens.
To study the possible functions of CD1e in CD1-restricted presentation of lipid antigens, THP-1 cells stably expressing CD1b, CD1c, or CD1d with or without CD1e were used as antigen-presenting cells (APCs) for human T-cell clones specific for self- or nonself-antigens. In each case, transfectants expressing equal levels of CD1 molecules were chosen (Fig. S1).
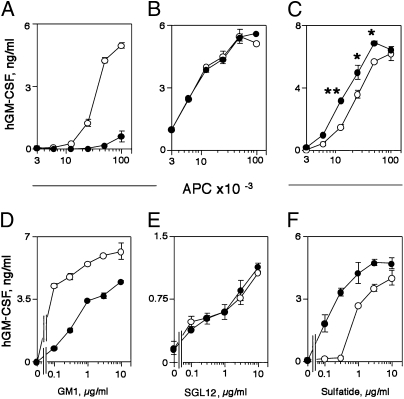
The effects of CD1e expression on CD1b- or CD1c-restricted T-cell clones reactive to self-antigens differed depending on the particular clone tested and the antigen specificity (Fig. 1 A–C). When APCs expressed CD1e, the autoreactive response of the CD1b-restricted and ganglioside GM1-specific T-cell clone GG123b was inhibited (Fig. 1A) and that of the CD1c-autoreactive (unknown antigen) K34B27.f clone was unaffected (Fig. 1B), whereas the response of the CD1c autoreactive (unknown antigen) DN4.99 clone was increased (Fig. 1C).
Fig. 1.
CD1e participates in the presentation of CD1b- and CD1c-restricted lipid antigens. (A–C) Self-antigens. (A) CD1b-restricted clone GG123b response to THP-1 CD1b and CD1e (●) and THP-1 CD1b only (○). (B and C) CD1c-restricted clones K34B27.f (B) and DN4.99 (C) response to THP-1 CD1c and CD1e (●) and THP-1 CD1c only (○). (D–F) CD1b-dependent exogenous antigens. GG33a clone response to GM1 (D), Z4B27 clone response to SGL12 (E), and DS1C9b clone response to sulfatide (F) presented by THP-1 CD1b and CD1e (●) and THP-1 CD1b only (○). Human GM-CSF (hGM-CSF) release is expressed as mean ng/mL ± SD (n = 3). *P < 0.05, **P < 0.01. Data shown are representative of at least three experiments.
CD1e also influenced, in a variable manner, the T-cell response to exogenously added antigens presented by CD1b. CD1e impaired the response of the GG33a clone to ganglioside GM1 (Fig. 1D), whereas it did not modify the response of Z4B27 cells to a mycobacterial diacylsulfoglycolipid synthetic analog (8) (Fig. 1E), and it facilitated the response of the DS1C9b clone to sulfatide (Fig. 1F). Thus, CD1e can modulate the response to lipid antigens presented by CD1b or CD1c in a variable manner.
Type 1 and Type 2 Natural Killer T (NKT) Clones Respond Differently to Self-Lipids in the Presence of CD1e.
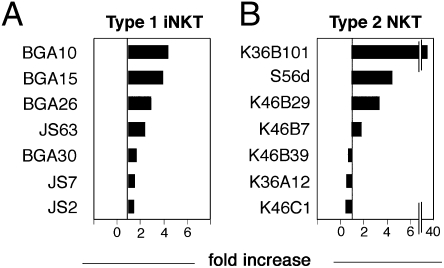
Autoreactive type 1 NKT (iNKT) and type 2 NKT cell clones (identified as CD1d-restricted, Vα24-Jα18−, and CD161+) were stimulated with APCs expressing only CD1d or both CD1d and CD1e without addition of exogenous lipid antigens. Different human iNKT clones were selected according to their T-cell receptor β (TCRβ) CDR3 sequences. All self-reactive iNKT clones showed increased activation in the presence of CD1e (between 1.5 and 4 times higher than the response in the absence of CD1e) and varied according to individual clones (Fig. 2A and Fig. S2A). In contrast, type 2 NKT clones showed a more variable responsiveness (Fig. 2B and Fig. S2 B and C). Some clones were positively influenced (1.8–38 times higher response in the presence of CD1e) (Fig. 2B), whereas other clones were negatively influenced by CD1e (30–60% inhibition) (Fig. 2B and Fig. S2C). One clone was not affected (Fig. S2B). Similar findings were observed when IL-4 and IFN-γ were investigated and when C1R CD1-transfected cells were used as APCs (Fig. S2 D and E), thus confirming general effects on T-cell activation.
Fig. 2.
Autoreactive CD1d-restricted clones are influenced by CD1e. Type 1 (A) and type 2 (B) NKT cell clones were incubated with single-transfected (CD1D only) and double-transfected (CD1E and CD1D) THP-1 cells. The difference in activation is shown as fold increase (ratio of cytokine release in response to double- and single-transfected cells). In total, 5 × 104 T cells were stimulated with 105 THP-1 cells. Released hGM-CSF is expressed as mean ng/mL ± SD (n = 3). Data shown are representative of at least three experiments.
In most cases, CD1e significantly influenced T-cell response, indicating that the human type 2 NKT cells studied here probably recognize antigens loaded within late endosomes, where CD1e is localized. Antigen loading in late endosomes was excluded when a single mouse type 2 NKT hybridoma was studied (9), suggesting that different antigens may stimulate type 2 NKT cells.
These results suggested that CD1e also regulates the response of CD1d-restricted T cells. The observed positive, negative, or neutral effects could be related to the nature and relative abundance of individual antigens that determine the final net effect of CD1e on CD1-restricted presentation.
CD1e Effects on Exogenous Glycolipid Antigen Presentation to iNKT Cells.
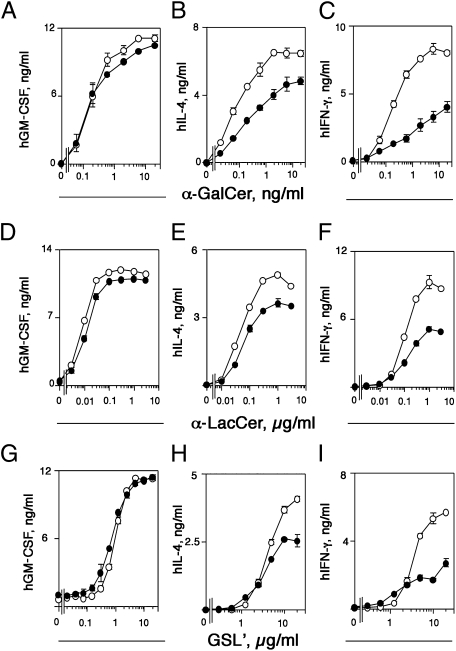
To study insight into the mechanisms of CD1e modulation of lipid antigen presentation, we focused on iNKT cells. Nonself-reactive human iNKT clones were chosen to avoid confusing effects of self-antigens. The effects of CD1e were studied on presentation of α-galactosylceramide (α-GalCer), a potent iNKT TCR agonist; α-lactosylceramide (α-LacCer), an agonist with intermediate potency (10); and α-galacturonosylceramide (GSL′), a weaker agonist (11). In these experiments, the antigens were present during the entire period of T-cell stimulation. Surprisingly, the expression of CD1e did not change the response to all three antigens when GM-CSF release was measured (Fig. 3 A, D, and G), whereas it caused reduction of IL-4 (Fig. 3 B, E, and H) and IFN-γ (Fig. 3 C, F, and I) release. Similar results were observed when C1R transfectants were used as APCs (Fig. S2 F, G, and H). This reduction was observed at all doses of α-GalCer and at high doses of GSL′, whereas α-LacCer produced intermediate results. These differential effects, according to type of released cytokine and antigen dose, excluded the possibility that CD1E-transfected cells are per se impaired in antigen presentation and suggested that CD1e influenced both loading and unloading of other CD1 molecules.
Fig. 3.
CD1e influences the type of cytokines secreted in response to the presentation of exogenously added antigen. THP-1 cells transfected with the CD1D gene (○) or with the CD1D and CD1E genes (●) were incubated for 2 h with different concentrations of α-GalCer (A–C), α-LacCer (D–F), or GSL′ (G–I) before addition of the human iNKT clone VM-D5. Released cytokines are expressed as mean ng/mL ± SD (n = 3). Data shown are representative of at least three experiments.
CD1e Affects the Kinetics of CD1d–Antigen Complex Formation and Duration.
CD1e might directly influence the number of CD1d–antigen complexes available over time, which has been shown to affect the type of cytokines released (12–14). To test this possibility, antigen-pulse experiments were performed to compare the time required for the generation of CD1d–antigen stimulatory complexes. CD1e expression induced a fast IL-4 release at 4 h after pulsing with α-GalCer (10 ng/mL). Without CD1e, cytokine release was observed only after 8 h (Fig. 4A). Similar kinetics were observed when releases of GM-CSF or IFN-γ were measured. These results suggest that CD1e assisted the immediate presentation of lipid antigens by accelerating the formation of CD1–antigen complexes. At later time points, when the antigen was available to APCs for 8–24 h, the CD1e effects were no longer detected, in agreement with the results described for GM-CSF release in Fig. 3A. These results apparently differed from those observed when IL-4 and IFN-γ were detected (Fig. 3 B and C) and might be ascribed to important differences in the experimental setup. The data shown in Fig. 3 were obtained with living APCs and with the antigen and T cells always present during the assay. In this case, T-cell activation integrated the response to available CD1d–antigen complexes over time. Instead, the data of Fig. 4A were obtained with fixed APCs with T cells added after fixation at the end of the antigen pulse. In this second experimental setup, T-cell activation reflected the response to the complexes available only at the time point when APCs were fixed. A second important difference was that fixed APCs are less efficient than living APCs in T-cell stimulation.
Fig. 4.
CD1e facilitates antigen loading and unloading on CD1d. (A) CD1e promotes α-GalCer presentation after a short time pulse. APCs were pulsed for different lengths of time with α-GalCer (10 ng/mL) and fixed before addition of human iNKT cells. (B) CD1e shortens the half-life of CD1d–α-GalCer complexes. APCs were pulsed for 2 h with α-GalCer (2 ng/mL) and chased for different lengths of time before the addition of human iNKT cells. (A and B) THP-1 cells transfected with the CD1D gene (○) or with the CD1D and CD1E genes (●) were used. Mean release of IL-4 is shown (±SD) (n = 3). (C) Plate-bound CD1d was pulsed with α-GalCer (1 μg/mL) in the presence of different soluble LTPs, BSA, or CD1e or with PBS before addition of human iNKT cells. hGM-CSF release in the absence of α-GalCer was below detection limits of the assay. (D). Plate-bound CD1d was loaded with α-GalCer (1 μg/mL), washed, then incubated overnight with different LTPs or PBS before addition of human iNKT cells. (C and D) Bars show mean hGM-CSF release (+SD) (n = 3). *P < 0.05, **P < 0.01. Data shown are representative of at least three experiments. (E and F) CD1e mediates the transfer of anionic lipids to CD1d (E) and lipid unloading from CD1d–GD3 complexes (F). (E) IEF gel of the products after incubation of CD1d (10 μM) for 1 h at pH 5.0 with phosphatidylcholine/phosphatidylethanolamine/sphingomyelin/cholesterol liposomes incorporating the indicated anionic lipid (15% molar ratio, 200 μM final concentration) in the presence or absence of CD1e (10 μM). The first two lanes show a control experiment using liposomes devoid of anionic lipids. (F) IEF gel of the products when purified CD1d–GD3 complexes were incubated with or without CD1e and the indicated lipids (15% molar ratio in liposomes). The lipids used were sulfatide (SLF), GD3, bis-(monoacylglycero)phosphate (BMP), and phosphatidylserine (PS).
To investigate whether CD1e also influenced the unloading of CD1d and the persistence of the stimulatory complexes, pulse–chase experiments were performed. APCs expressing CD1d only or both CD1d and CD1e were pulsed with α-GalCer for 2 h, extensively washed, and chased for different lengths of time before fixation and use in T-cell activation assays. The 2-h time point was chosen because it was sufficient to load enough antigen capable of stimulating iNKT cells (when living APCs were used), without allowing antigen overloading of APCs.
The presence of CD1e negatively affected T-cell responses, which had already declined when CD1e+ APCs were chased for 18 h, whereas the response to CD1e− APCs at this time point was still maximal (Fig. 4B). The response declined in both APCs at later points and dropped below 50% at 36 h. Similar kinetics were observed when releases of GM-CSF or of IFN-γ were measured.
CD1e Facilitates Loading and Unloading of Lipid Antigens on CD1d.
These data suggested that CD1e might facilitate antigen loading and unloading onto other CD1 molecules, thus acting as a lipid transfer protein (LTP). To investigate whether CD1e directly facilitates lipid binding to CD1d, we performed plate-bound CD1d-based lipid-presentation assays. Recombinant soluble human CD1d coated onto culture plates was loaded with fixed amounts of α-GalCer in the presence or absence of recombinant CD1e. Plates were washed, and the response of iNKT cells was tested after 18 h. Recombinant soluble saposins A, B, or C were used as controls (15, 16). CD1e significantly enhanced T-cell activation and was more efficient than saposins B and C (Fig. 4C).
We tested whether CD1e also facilitates unloading of soluble CD1d molecules previously loaded with α-GalCer. A second type of plate-bound assay was established in which soluble CD1d was coated to the plastic, optimally loaded with α-GalCer, then washed and incubated with soluble CD1e or saposin A, B, or C before addition of iNKT cells (Fig. 4D). CD1e significantly decreased GM-CSF release (>50%), whereas saposins had no effect in this in vitro assay. Even greater inhibition was observed when releases of IL-4 or IFN-γ were measured.
The CD1e activity on CD1d loading and unloading was also investigated by isoelectrofocusing (IEF). CD1e facilitated loading of soluble CD1d with the anionic lipids sulfatide and ganglioside GD3 (GD3). On the contrary, no effect was observed when bis-(monoacylglycero)phosphate and phosphatidylserine were used (Fig. 4E). CD1e also facilitated unloading of preformed CD1d–GD3 complexes in the presence of acceptor liposomes (Fig. 4F, lanes 1 and 2). Addition of α-GalCer, sulfatide, and bis-(monoacylglycero)phosphate increased the unloading effect of CD1e, using these in vitro experimental conditions (Fig. 4F, lanes 3–8). Similar unloading effects were observed when CD1d–GM1 or CD1d–sulfatide complexes were used. The capacity of CD1e to influence CD1d unloading was much more evident in the iNKT cell activation assay that is closer to physiological conditions, indicating that the IEF assay is less sensitive and probably influenced by several in vitro conditions. Altogether, these experiments support the hypothesis that CD1e facilitates both antigen loading and unloading of CD1d.
Facilitated Presentation of Lipid Antigens in CD1e Transgenic (Tg) Mice.
To further dissect the mechanisms by which CD1e facilitates CD1d-restricted antigen presentation, we generated Tg mice expressing the human CD1E cDNA under the H-2 Eα promoter directing expression on APCs. In Tg mice, the expression of CD1e was found in B cells, peritoneal macrophages, and bone marrow-derived DCs (BMDCs), but not in T lymphocytes (Fig. S3A) or thymocytes. Soluble CD1e molecules were detected by pulse–chase labeling experiments followed by immunoprecipitation in DCs of Tg mice but not non-Tg littermates (Fig. S4) and partially colocalized with the Golgi marker TGN-38 and with MHC class II compartment (Fig. S3B), thus showing a distribution similar to the one occurring in human cells (1). Number and phenotype of iNKT cells in thymus and periphery as well as of other lymphocyte populations and monocytes were comparable in Tg and WT mice (Figs. S5–S7). Eα-CD1e Tg mice therefore represent a unique animal model in which to study the role of CD1e in CD1d-restricted antigen presentation.
To investigate the effects of CD1e on CD1d-restricted presentation of endogenous lipid antigens, DCs from Tg and WT mice were used to stimulate human autoreactive iNKT cells (Fig. S8A). In control experiments, WT or Tg thymocytes (which do not express CD1e) were used to stimulate the same iNKT cells (Fig. S8B). CD1e presence in DCs positively affected the response of iNKT cells, whereas Tg and WT thymocytes were equally stimulatory. Additional control experiments showed that the presentation of α-GalCer was identical when using the same setup described for the four APC types and an iNKT cell hybridoma (Fig. S8 C and D). CD1e may thus affect the presentation of endogenous lipid antigens in mouse APCs also.
We next investigated whether CD1d–antigen stimulatory complexes were formed with faster kinetics in Tg DCs than in WT DCs, as previously found in the human system. The presence of CD1e resulted in stronger iNKT responses after 30-min α-GalCer pulse, and this effect remained at later time points (Fig. 5A).
Fig. 5.
CD1e expressed in Tg DCs influences presentation of self-lipids. (A) DCs from Tg mice (●) or non-Tg littermates (○) were incubated for different lengths of time with α-GalCer (10 ng/mL) and then fixed before addition of iNKT mouse hybridoma cells. (B) The half-life of CD1d–α-GalCer complexes is reduced in Tg DCs. DCs from Tg mice (●) or non-Tg littermates (○) were pulsed for 2 h with α-GalCer (2 ng/mL) and chased for different lengths of time before addition of iNKT mouse hybridoma cells. (A and B) Released mIL-2 (ng/mL ± SD) was determined in triplicate. (C) Detection of CD1d–α-GalCer complexes by L363 antibody staining on the surface of Tg (●) or WT (○) DCs incubated for 24 h with α-GalCer. (D) Detection of CD1d–α-GalCer complexes by L363 antibody on Tg (●) or WT (○) DCs pulsed for 24 h with different doses of α-GalCer and chased for 24 h before staining. Data shown are representative of three independent experiments.
We also tested whether CD1e reduced the persistence of CD1d–antigen complexes. Tg or WT DCs were loaded with α-GalCer and chased for different lengths of time before fixation and presentation to iNKT hybridoma cells. Similar to the behavior observed with human APCs, 50% reduction of maximal stimulation was observed after 24-h chase of CD1e Tg APCs and after 30-h chase with WT APCs (Fig. 5B). Staining with the L363 mAb, which detects CD1d–α-GalCer complexes (14, 17), confirmed the increased appearance of complexes on the plasma membrane at very early time points (Fig. 5C) and their reduced persistence at late time points (24 h) (Fig. 5D) despite equal levels of surface CD1d. This effect was dose-dependent and was no longer observed at the highest α-GalCer dose (Fig. 5D).
Hence, CD1e participated in efficient lipid exchange from CD1d in both mouse and human APCs, facilitating immediate loading of lipid antigens but also reducing the half-life of stimulatory CD1d–lipid antigen complexes.
CD1e Tunes the Response of iNKT Cells to Infection.
We asked whether CD1e plays a role in promoting iNKT cell response to Sphingomonas paucimobilis, which encodes iNKT-stimulatory antigens (18, 19). When WT and Tg DCs were pulsed with different numbers of heat-killed S. paucimobilis bacteria and used to stimulate iNKT hybridoma cells for 24 h, both APCs showed equal stimulatory capacity (Fig. 6A). However, when APCs were incubated for different lengths of time and immediately fixed, the presence of CD1e facilitated the generation of stimulatory CD1d complexes, as previously observed with α-GalCer (Fig. 5E). Tg DCs already stimulated iNKT hybridoma cells at 2 h after pulsing and induced a persistently higher cytokine release (Fig. 6B). These findings show that APCs expressing or lacking CD1e have similar presentation capacity when the antigen remains in the culture and that CD1e promotes a faster response immediately after lipid antigens become available. The effects of CD1e during infection were investigated by using freshly isolated spleen cells stimulated with S. paucimobilis-infected Tg or WT DCs. The presence of CD1e induced more efficient activation of iNKT cells as shown by increased CD69 up-regulation (Fig. 6C) and increased percentage of CD69+ iNKT cells (Fig. 6D). A final series of experiments assessed the capacity of infected DCs to activate iNKT cells after different infection times. Tg or WT DCs were infected for differing times, then treated with gentamicin, washed, and used to stimulate spleen cells for 24 h. Tg DCs already induced maximal iNKT cell activation at 12 h after infection, whereas WT DCs only induced maximal activation at 48 h after infection (Fig. 6E). In conclusion, CD1e also enhanced iNKT cell response during S. paucimobilis infection.
Fig. 6.
DCs from Tg mice induce a better response after stimulation with S. paucimobilis. (A) DCs from Tg mice (●) or non-Tg littermates (○) were incubated for 2 h with heat-killed S. paucimobilis at different bacteria:APC ratios and then used to stimulate iNKT hybridoma cells. mIL-2 (ng/mL ± SD) was determined by ELISA. (B) DCs from Tg (●) or non-Tg littermates (○) were incubated for different lengths of time with heat-killed S. paucimobilis (bacteria:APC 200:1), washed, and then fixed before addition of iNKT hybridoma cells. After 24 h, mIL-2 (ng/mL ± SD) was determined. (C and D) DCs from Tg or non-Tg littermates were infected at a multiplicity of infection (MOI) of 1:1 for 2 h with S. paucimobilis or were not infected, then washed and incubated with gentamicin for 1 h, and finally cocultured with freshly isolated WT mouse splenocytes. CD69 mean fluorescence intensity (MFI) (C) and percentage (D) of CD69+ CD1d–α-GalCer dimer+ iNKT cells as measured by flow cytometry. *P < 0.05. (E) Tg (●) or WT (○) DCs were infected for different lengths of time with S. paucimobilis at a low MOI (1:1), incubated for 1 h with gentamicin, and then used to stimulate freshly isolated WT iNKT cells. Up-regulation of CD69 on CD1d–α-GalCer dimer+ iNKT cells was measured by flow cytometry. ■ indicates CD69 expression on iNKT cells incubated with noninfected DCs. Data shown are representative of at least three experiments.
Discussion
We have found that CD1e has evolved a unique capacity to complement the antigen-presentation functions of other CD1 family members. The presence of CD1e may influence presentation of lipid antigens by CD1b, CD1c, and CD1d, the three molecules that recycle within late endosomes/LYs where soluble CD1e is also localized. The effects on antigen presentation differ according to the presented antigen and not to the restricting CD1 molecule. In some instances, presentation is enhanced; in others, it is reduced. This finding might reflect a preference of CD1e for binding some lipids more efficiently than others. In some other instances, CD1e presence did not influence antigen presentation, suggesting that not all lipid antigens can bind CD1e or be unloaded from other CD1 molecules. These findings suggest a mechanism whereby a low binding affinity of a lipid antigen to CD1e might facilitate antigen presentation by CD1 molecules, whereas a high binding affinity to CD1e might reduce loading of CD1 molecules. This latter case could have two outcomes: a detrimental one such as impaired immune response to certain microbial lipid antigens or a favorable one upon sequestration of self-lipid antigens involved in autoimmunity. This function can be exerted in different cell types where CD1e is expressed: thymocytes, DCs, and Langerhans cells, which also express group 1 and group 2 CD1 molecules.
CD1e also influenced the presentation of self-antigens to type 1 (iNKT) and type 2 NKT cells. All autoreactive iNKT cells tested were positively influenced by the presence of CD1e, suggesting that CD1e facilitated formation of CD1d–lipid complexes with the endogenous unknown lipid antigen(s) that stimulate the autoreactive iNKT cells tested in this study.
A set of human autoreactive iNKT cell clones is activated by tail-deleted CD1d molecules that do not recycle through LYs (20), indirectly suggesting that the antigen stimulating these cells is not loaded within LYs. Our clones might represent a different type of autoreactive iNKT cells that recognize self-antigens loaded within endosomal compartments where CD1e colocalizes with CD1d. This possibility is supported by our findings that these iNKT cells do not recognize soluble CD1d molecules, which are secreted without trafficking through endosomal stations.
In contrast to the positive effects on autoreactive iNKT cells, CD1e may have opposite effects on type 2 NKT cells, which are also CD1d-restricted. We found that CD1e facilitated the activation of four type 2 NKT cell clones, inhibited the response of three clones, and was irrelevant for one clone. Both enhancing and inhibitory effects were very pronounced (1.8–30 times observed enhancement and 30–60% inhibition). We interpret these findings with the fact that type 2 autoreactive NKT cells may recognize a set of self-lipids that are not identical to those stimulating self-reactive iNKT cells. Thus, in this case, the type of lipid antigens and not the type of CD1 restriction also determines the effects of CD1e.
The expression of CD1e also influenced the cytokines released by T cells because iNKT cells stimulated with CD1e-expressing APCs and three different exogenous antigens showed a marked reduction of IFN-γ, a moderate reduction of IL-4, and almost no change in released GM-CSF. These effects were observed with two different types of transfectants (THP-1 and C1R), excluding unique behavior associated with the nature of APCs. The differential influence on the type of released cytokines probably reflects the reported different signal strength required for secretion of GM-CSF versus IL-4 and IFN-γ (12). The presence of CD1e might influence the number and nature of CD1–antigen complexes, leading to a reduced TCR signal strength that might affect IFN-γ production more readily than that of GM-CSF. Indeed, the most pronounced inhibitory effects occurred at high antigen doses, suggesting that CD1e modulated the lipid antigen presentation capacity of APCs. The impact of CD1e on CD1-restricted T-cell responses might be the outcome of a CD1e-mediated unloading of the antigen from other CD1 molecules.
This hypothesis is supported by the effect of CD1e on loading and unloading kinetics of CD1d–lipid complexes. CD1e already facilitated the formation of stimulatory α-GalCer complexes with CD1d at 4 h after antigen pulsing, but it also induced a faster reduction of the stimulatory capacity at later time points, which was confirmed by using two types of in vitro CD1d loading and unloading assays (plate-bound T-cell activation and IEF). In addition, CD1e behaved similarly in Tg mice and human cells. The mouse model confirmed that CD1e accelerates the formation of CD1d–lipid complexes and their rapid turnover when the antigen is limiting, as shown by iNKT cell activation experiments and by staining with a mAb specific for mouse CD1d–α-GalCer complexes. The increased turnover of stimulatory ligands may represent an efficient way to dampen T-cell responses of iNKT and group 1 CD1-restricted T cells at later time points.
Finally, CD1e also changed the early response of iNKT cells to bacterial-derived antigens, suggesting a modulation of iNKT cell responsiveness during infection. A significant increase of activated iNKT cells was observed when infection lasted between 12 and 24 h, whereas the enhancing effect was no longer visible after 48 h. Because this initial time frame is the most important for an innate immune response to infectious agents, CD1e can be considered to be a molecule actively participating in innate immune functions of iNKT cells.
In conclusion, CD1e may fine-tune the response to lipid antigens, influencing loading and unloading of other CD1 molecules as well as having major consequences on the temporal availability of stimulatory CD1–lipid complexes. These unexpected functions of CD1e have disclosed unique mechanisms whereby the immune response to lipid antigens is regulated.
Materials and Methods
Cells.
Human CD1d-restricted type 1 and type 2 NKT, CD1b-restricted and CD1c-restricted T-cell clones, and mouse iNKT hybridomas were derived as described (21–23) and maintained according to standard procedures. Human monocytic THP-1 and C1R B cells were transfected with human CD1B, CD1C, and CD1D cDNAs alone or in combination with human CD1E cDNA, using the BCMGS-Neo and BCMGS-Hygro vectors (4). Transfected cells expressing similar levels of CD1 molecules were selected by cell sorting to avoid a biased stimulation of responder cells.
Lipid Antigens.
Mycobacterial lipids were purified as described (24). SGL12 (8), α-GalCer (25), and α-LacCer (10) were synthesized as previously published. GM1 and sulfatide were purchased from Sigma. GSL′ was provided by P. Seeberger (The Max Planck Institute of Colloids and Interfaces, Berlin, Germany).
Flow Cytometry.
Human CD1 molecules were detected with BCD1b3.1 (anti-CD1b), 10C3 (anti-CD1c), and CD1d42 (anti-CD1d) mAbs, and human MHCI was detected with W6/32 mAb. Intracellular CD1e was detected in fixed and permeabilized cells with 20.6 mAb (4) and FITC-conjugated goat anti-mouse IgG1 (Southern Biotech). Samples were acquired on a CyAn ADP flow cytometer (Dako). Nonviable cells were excluded on the basis of light scatter, and incorporation of propidium iodide, doublets were excluded by pulse-width parameter. Live cells were analyzed with FloJo software (Tristar).
Antigen Presentation Assays.
Autoreactive T cells (105 cells per well) were stimulated with increasing numbers of APCs without addition of exogenous antigen. For exogenous antigen assessments, transfectants (0.5 × 105 cells per well), mouse thymocytes (5 × 105 cells per well), or DCs (105 cells per well) were preincubated for 2 h at 37 °C with antigens before addition of human T cells (105 cells per well) or mouse iNKT FF13 hybridoma cells (0.5 × 105 cells per well). For pulse experiments, transfectants or DCs (2 × 106 cells per well) were pulsed with α-GalCer (10 ng/mL), washed, fixed as described (24), and plated at 105 cells per well in a 1:1 ratio with T cells. For chase experiments, transfectants or DCs (5 × 105 cells per well) were pulsed for 2 h with 2 ng/mL of α-GalCer, washed, and chased before addition of human T cells or FF13.
CD1d Loading and Unloading Experiments.
Plates were coated with 10 μg/mL Bir1.4 mAb (26). Soluble recombinant human CD1d was purified by IEF and added overnight at room temperature at twofold molar excess over Bir1.4. In loading assays, α-GalCer was added with or without recombinant LTPs (4 μg/mL) or recombinant CD1e (10 μg/mL). In unloading assays, α-GalCer was added overnight, washed, and then CD1e or LTPs were added for an additional 24 h. After washing, T cells (1.5 × 105 cells per well) were added. In antigen presentation assays, released cytokines were measured in supernatants taken after 24 h [GM-CSF, IL-4, and mouse IL-2 (mIL-2)] or 48 h (human IFN-γ) by ELISA (BD Biosciences).
For IEF analysis of CD1e effects on CD1d loading and unloading, see SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean ± SD and analyzed with the unpaired Student's t test with Welch's correction. P ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank J. Mattner and P. Seeberger for S. paucimobilis antigens, D. Nebenius for the generation of Tg mice, M. Lepore for discussion, and P. Cullen for reading the manuscript. This work was supported by Swiss National Science Foundation Grant 3100A0 122464/1, European Union Seventh Framework Programme Tuberculosis Vaccine (TBVAC), University Hospital Basel, French National Research Agency Grant ANR-05-MIIM-006, and Etablissement Français du Sang-Alsace.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108809108/-/DCSupplemental.
References
- 1.Angenieux C, et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem. 2000;275:37757–37764. doi: 10.1074/jbc.M007082200. [DOI] [PubMed] [Google Scholar]
- 2.Angénieux C, et al. The cellular pathway of CD1e in immature and maturing dendritic cells. Traffic. 2005;6:286–302. doi: 10.1111/j.1600-0854.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 3.Maître B, et al. Control of the intracellular pathway of CD1e. Traffic. 2008;9:431–445. doi: 10.1111/j.1600-0854.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- 4.de la Salle H, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 5.Sugita M, et al. Separate pathways for antigen presentation by CD1 molecules. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- 6.Salamero J, et al. CD1a molecules traffic through the early recycling endosomal pathway in human Langerhans cells. J Invest Dermatol. 2001;116:401–408. doi: 10.1046/j.1523-1747.2001.01264.x. [DOI] [PubMed] [Google Scholar]
- 7.Sugita M, Brenner MB. T lymphocyte recognition of human group 1 CD1 molecules: Implications for innate and acquired immunity. Semin Immunol. 2000;12:511–516. doi: 10.1006/smim.2000.0277. [DOI] [PubMed] [Google Scholar]
- 8.Guiard J, et al. Fatty acyl structures of Mycobacterium tuberculosis sulfoglycolipid govern T cell response. J Immunol. 2009;182:7030–7037. doi: 10.4049/jimmunol.0804044. [DOI] [PubMed] [Google Scholar]
- 9.Roy KC, et al. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, et al. α-Lactosylceramide as a novel “sugar-capped” CD1d ligand for natural killer T cells: Biased cytokine profile and therapeutic activities. ChemBioChem. 2008;9:1423–1430. doi: 10.1002/cbic.200700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, et al. Design of natural killer T cell activators: Structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan BA, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im JS, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan W, et al. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci USA. 2007;104:5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu KO, et al. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand α-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 19.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, et al. Distinct endosomal trafficking requirements for presentation of autoantigens and exogenous lipids by human CD1d molecules. J Immunol. 2007;178:6181–6190. doi: 10.4049/jimmunol.178.10.6181. [DOI] [PubMed] [Google Scholar]
- 21.Schümann J, et al. Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism. Eur J Immunol. 2007;37:1431–1441. doi: 10.1002/eji.200737160. [DOI] [PubMed] [Google Scholar]
- 22.Shamshiev A, et al. Presentation of the same glycolipid by different CD1 molecules. J Exp Med. 2002;195:1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lalla C, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. 2011;41:602–610. doi: 10.1002/eji.201041211. [DOI] [PubMed] [Google Scholar]
- 24.Gilleron M, et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michieletti M, et al. Synthesis of α-galactosyl ceramide (KRN7000) and analogues thereof via a common precursor and their preliminary biological assessment. J Org Chem. 2008;73:9192–9195. doi: 10.1021/jo8019994. [DOI] [PubMed] [Google Scholar]
- 26.Nowbakht P, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.